Abstract
Although rice bran consumption is reportedly has numerous beneficial effects on human health, the relationship between rice bran and the prevention of photoaging has not been investigated in detail. We sought to investigate whether consumption of rice bran supplement (RBS) can elicit preventive effects against UVB-induced photoaging in vivo. Dorsal skin sections of hairless mice were exposed to UVB over 16 weeks. RBS consumption suppressed UVB-induced wrinkle formation and inhibited the loss of water content and epidermal thickening in the mouse skin. Western blot and immunohistochemical analyses revealed that repeated exposure to UVB upregulated matrix metalloproteinase-13 (MMP-13) and cyclooxygenase-2 (COX-2) expression, while consumption of RBS suppressed MMP-13 and COX-2 expression, as well as mitogen-activated protein kinase (MAPK) signaling pathways. These findings suggest that RBS could be a potential bioactive ingredient in nutricosmetics to inhibit wrinkle formation and water content loss via the suppression of COX-2 and MMP-13 expression.
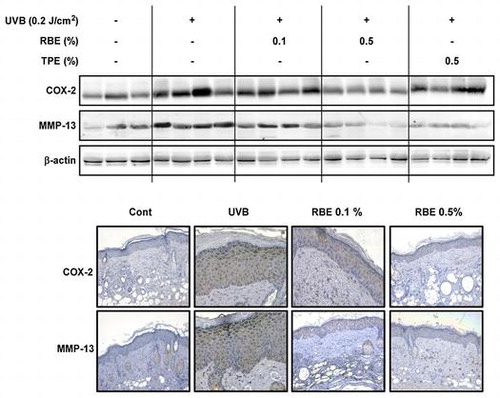
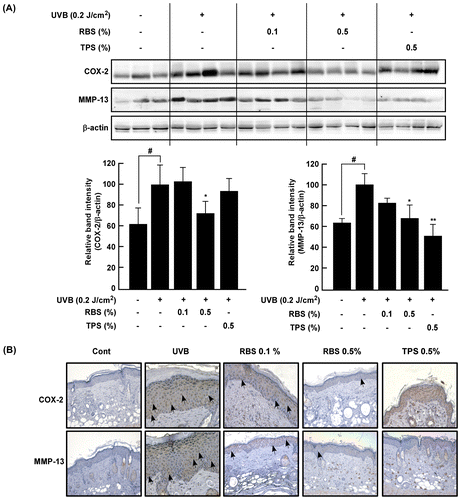
Effect of RBS on UVB-irradiated COX-2 and MMP-13 expression in SKH-1 hairless mouse skin.
Rice bran refers to the germ layer of Oryza sativa seeds and is a major by-product that is generated during the milling of rice [Citation1]. Rice oil, as well as the bran and hulls harbor significant concentrations of bioactive compounds, and brans that are pigmented tend to contain greater quantities of such compounds than white bran [Citation2]. Rice bran has been shown to inhibit tumor promotion, inactivate macrophages, inhibit angiogenesis and cause reductions in serum cholesterol levels [Citation3,4].
Approximately half a million tons of rice bran are generated annually, with only 30% used for the production of rice bran oil, edible enzymes, cosmetics materials, and feedstuffs, with the remainder comprising agricultural waste [Citation5,6].
As society ages in the developed world and economic prosperity increases, greater interest is turning to functional foods that promote health benefits and reduce the risk of developing chronic diseases [Citation7]. Recent interest in healthy lifestyles and wellness products has been growing rapidly, and functional food markets for weight control, stress management, and beauty products are expected to increase [Citation8,9]. Similarly, the popularity of beauty trends that pursue improved appearance from internal health has been causing an increase in demand for products that promote skin health. Poorer skin health has been linked with a higher risk of depression. Additionally, it is widely believed that quality of life can change due to one’s appearance [Citation10].
The aging of the skin is a multisystem degenerative process influenced by a combination of intrinsic and extrinsic factors [Citation11]. These factors together lead to structural and physiological changes and that become progressive in each layer, resulting in overall changes in appearance, particularly to areas heavily exposed to the sun [Citation12]. Photoaging is commonly characterized by increased roughness and melanin production, as well as laxity, sagging, and wrinkling, and results from the degradation of two factors [Citation13,14]. The dermis acts as a structural support within the skin, while extended solar UV exposure leads to fragmentation and disruption of the major structural proteins collagen and elastin [Citation15].
Matrix metalloproteinases (MMPs) are critical role players in tissue remodeling and have also been implicated in various pathological processes [Citation16]. The relationship between collagen fibrils, cell surfaces and MMPs has been postulated to allow cells to migrate along collagen, referred to as keratinocyte migration. The irradiation of skin with UVB increases the development of benign papillomas, hyperplasia, and carcinomas, associated with increased levels of cyclooxygenase-2 (COX-2) expression [Citation17]. UV stimulation of cell membrane receptors causes the concomitant activation of multiple receptor-coupled signal transduction pathways, involving mitogen-activated protein kinases (MAPKs) and is a major cause of upregulated angiogenic factors, like COX-2 and MMPs, contributing to angiogenesis during tumor invasion and carcinogenesis [Citation18].
In this study, we investigated the inhibitory effects of rice bran supplement (RBS) on UVB-induced photoaging using the SKH-1 hairless mouse model, by examining COX-2 and MMP-13 expression, MAPK pathway signaling, and epidermal thickening.
Materials and methods
Materials
Chemical reagents were obtained from Sigma–Aldrich (MO, USA). The antibodies against β-actin, MMP-1, and MMP-13 were obtained from Santa Cruz Biotech (CA, USA). The antibodies against p44/42 MAP Kinase, COX-2, p38 MAPK, SAPK/JNK, phospho-p44/42 MAPK (Erk 1/2) (Thr202/Tyr204), and phospho-SAPK/JNK (Thr183/Tyr185) were obtained from Cell Signaling Biotechnology (Beverly, MA, USA). The antibody against Phosphorylated p38 MAPK (pT180/pY182) was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Sample preparation
The standardized rice bran supplement (RBS, lot No. SD-RB-002) was purchased from S&D Co., Ltd. (Cheongju, Republic of Korea) and used for experimental diet. According to the manufacturing method provided by the manufacturer, rice (Oryza sativa L.) bran was extracted in 20% EtOH for 8 h at 40 °C. In preparation of RBS, rice bran extract is mixed with American Institute of Nutrition-93G (AIN-93G) diet and other additives such as polysorbate 20, d-α-tocopherol, sodium caseinate, and dextrin. The same amount of additives was added to the normal and UVB diet. RBS was standardized to contain 4.5 mg/g of γ-oryzanol. Taraxacum platycarpum was extracted by ethanol for 24 h and Taraxacum platycarpum supplement (TPS) prepared by the same method as RBS. The experimental diets were based on the AIN-93G diet formula. The composition of experimental diets is listed in Table .
Table 1. Composition of experimental diet (unit: g/kg diet).
Animal experiments
Six-week-old female SKH-1 hairless mice (20–22 g) were purchased from Central Laboratory Animal Inc. (Seoul, Korea). The animals were housed in a temperature-controlled room (at 23 ± 2 °C) with a 12-h light/dark cycle and permitted free access to food and water. All animals were treated humanely, and the study design (KFRI-M-17014) was approved by an ethics committee and conducted according to the guidelines for animal care and use of Korea Food Research Institute. Forty mice were allocated randomly to each group (eight mice per group, five groups in total) consisting of (i) normal diet and no treatment (normal), (ii) normal diet and UVB-irradiated group (UVB), (iii) UVB-irradiated with 0.1% (w/w) RBS diet group and (iv) UVB-irradiated and 0.5% (w/w) RBS diet group and (v) UVB-irradiated and 0.5% (w/w) TPS diet group.
UVB irradiation
UVB irradiation was administered with a bank of 4 Westinghouse F520 lamps (National Biological, OH, USA) generating light at 6 J/sec/m within the UVB range. The UVB irradiation source (Bio-Link; Vilber Torcy, France) produced wavelengths of 254, 312, and 365 nm, with a peak emission at 312 nm. The UVB chamber was equipped with a Kodak Kodacel K6808 filter. Mouse dorsal regions were exposed to UVB irradiation three times weekly, and increased each week by 1 minimal erythema dose (1 MED = 50 mJ/cm2) until 4 MED and then held at 4 MED until 16 weeks had passed. At the end of the experiment, the mice were sacrificed and the skin samples were harvested and assessed for the expression of biomarkers related to photoaging via Western blot and immunohistochemical analyses.
Evaluation of water content
The water content within the mouse dorsal skin was assessed with skin capacitance measurements (Skin-O-Mat, Cosmomed, Germany) and held at 50% (±5%) humidity, 20 °C (±2 °C) and for an hour on the day prior to euthanasia for further experiments. When standard deviation for five or six consecutively measured values was less than 0.1, the average value for the previous five values was defined as the water content value for each animal.
Wrinkle formation assessment
After 12 weeks of UVB irradiation, the skin of the animals was molded with silicone rubber to measure the degree of wrinkle formation. Following anesthetization, silicone polymer (Silfo, Flexico Developments LDT, England) reagent was mixed and spread thinly on the dorsal area, completely dried, and then carefully removed from the replica. The simulated boards were analyzed by Daegu Technopark (Daegu, Korea). Skin wrinkle curves were evaluated using the replica with a skin wrinkle measuring device (Visioline® VL650, Courage-Khazaka Electronic) and various skin characteristics were analyzed using a light source with a b/w CMOS camera.
Western blot analyses
The mouse skin tissue was collected in 2 mL tubes containing lysis buffer and steel beads and then homogenized twice for 2 min at 20 Hz in a TissueLyser II (Qiagen, CA, USA). The liver lysates were also centrifuged at 12,000 rpm for 20 min. The proteins were washed via centrifugation and the concentration assessed with a DC Protein Assay kit (Bio-Rad) following the manufacturer’s instructions. The skin tissue extract was used for 10% SDS-PAGE and transferred onto PVDF membranes. The lysate was subjected to 10% SDS-PAGE and transferred to a PVDF membrane (Immobilon®-P transfer membrane). Following the transfer, membranes were incubated with primary antibodies overnight at 4 °C. Expression levels were then visualized with a chemiluminescence detection kit (ATTO, Tokyo, Japan) following hybridization with a horseradish peroxidase (HRP)-conjugated secondary antibody.
Histological analysis
5-μm thick sections of 10% formalin solution-fixed, paraffin-embedded skin tissues were sliced and mounted on silane-coated slides. The tissue samples were then washed with water, and dehydrated with an ethanol gradient, cleared using xylene, and paraffin wax embedded. The tissue sections were then prepared for staining with hematoxylin and eosin (H&E). The deparaffinized sections were warmed in 10 mM citrate buffer (pH 6.0) for 15 min in a microwave for antigen retrieval. To assess the target proteins, the slides were incubated with primary antibodies at 4C overnight in 1% BSA solution and developed with SignalStain® Boost IHC Detection Reagent (HPR, rabbit) antibodies (Cell Signaling). The sites that bound with peroxidase were detected with a SignalStain® DAB Substrate Kit (Cell Signaling). Finally, counterstaining was performed using Harris hematoxylin solution (Sigma–Aldrich). The thickness of the epidermis and MMP-13 and COX-2 expression were visualized by fluorescence microscopy (Nikon Eclipse Ti-S, Japan) and the images were analyzed with Metamorph (Molecular Devices, PA, USA) software.
Statistical analysis
As needed, data are expressed as mean values ± S.D, with significant differences determined by one-way ANOVA (Analysis Of Variance). Statistical analysis was performed using the GraphPad Prism 5.01 (GraphPad Software, Inc.) for Windows. *p < 0.05, **p < 0.01 and ***p < 0.001 were used throughout the article to show statistical significance.
Results
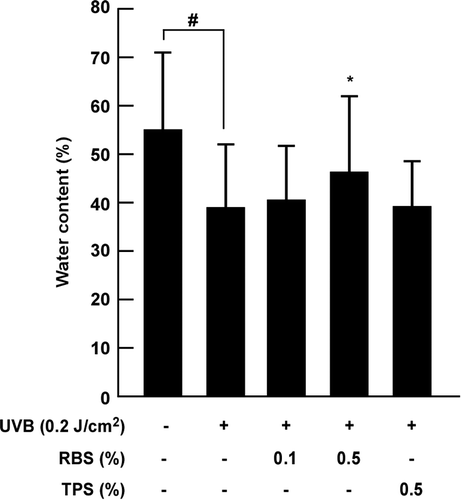
RBS inhibits UVB-induced loss of water content in mouse skin
UVB irradiation damages dermal tissue within the skin, and as a result, the barrier of the skin collapses resulting in imbalances between oil and moisture, and damage to the skin overall. We sought to investigate the effect of RBS on the water content of UVB-irradiated skin, as an indicator of skin moisture. Repeated exposure to UVB reduced the water content, although this occurred to a lower extent in the animals fed with high-dose RBS by the 12th week (Figure ). Throughout the experimental period, body weight did not differ significantly between the groups (data not shown).
Figure 1. Effect of RBS on UVB-irradiated water content in SKH-1 hairless mouse skin. Results are shown as means ± S.D. (n = 8). The hash symbol (#) indicates a significant difference (p < 0.05) between the control group and the UVB-irradiated group. Asterisks (*) indicate a significant difference of p < 0.05 between the RBS diet group and UVB-irradiated group.
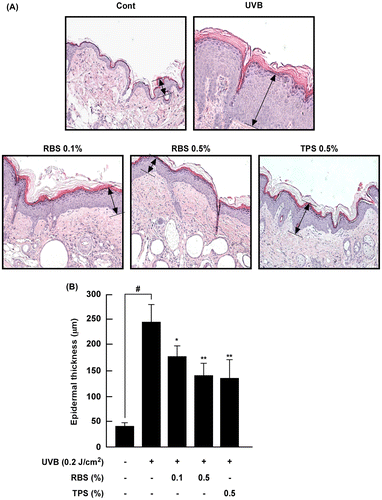
RBS inhibits UVB-dependent epidermal thickening of mouse skin
Change in epidermal thickness is regarded as a marker of skin damage. Epidermal thickness increased following repeated exposure to UVB irradiation, while RBS and TPS significantly inhibited these increases by 42.6 and 44.8%, respectively (Figure (A) and (B)).
Figure 2. Effect of RBS on UVB-irradiated epidermal thickening in SKH-1 hairless mouse skin. (A) Hematoxylin- and eosin-stained images of UVB-irradiated mouse skin. Images are representative of results from 5 tissue samples. (B) RBS prevents UVB-dependent increases in epidermal thickness. The dorsal skin was excised, sectioned, mounted onto slides, and stained with hematoxylin and eosin for assessment. Results are shown as means ± S.D. (n = 8). The hash symbol (#) indicates a significant difference (p < 0.05) between the control group and the UVB-irradiated group. Asterisks (* and **) indicate a significant difference of p < 0.05 or p < 0.01, respectively, between the RBS diet group and UVB-irradiated group.
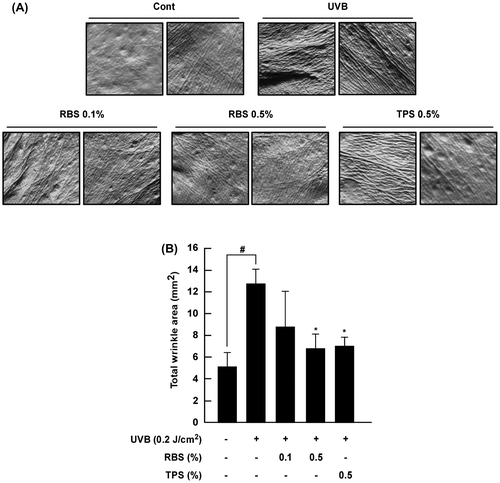
RBS inhibits UVB-dependent wrinkling in mouse skin
MMP-1 expression is known to increase following repeated exposure to ultraviolet light, which breaks down the collagen fibers that provide skin with elasticity and strength, resulting in the formation of thick wrinkles that are distinct from that expected with normal aging. Repeated UVB irradiation produced abnormal increases in wrinkling in the test animals. Conversely, SKH-1 hairless mice receiving the high-dose rice bran extract exhibited reduced wrinkle formation (Figure (A) and (B)).
Figure 3. Effect of RBS on UVB-irradiated wrinkle formation in SKH-1 hairless mouse skin. (A) External appearance of wrinkling. (B) RBS significantly inhibits UVB-irradiated wrinkle formation in SKH-1 hairless mice. The dorsal skin surface of the animals was exposed to UVB irradiation three times per week for 16 weeks. Prior to sacrifice, skin replica samples of the dorsal areas were taken. Wrinkle values were obtained using skin replica analysis. Results are shown as means ± S.D. (n = 8). The hash symbol (#) indicates a significant difference (p < 0.05) between the control group and the UVB-irradiated group. Asterisks (*) indicate a significant difference of p < 0.05 between the RBS diet group and UVB-irradiated group.
RBS inhibits UVB-induced MMP-13 and COX-2 upregulation in mouse skin
COX-2 and MMP-13 are two major enzymes involved in inflammation and photoaging, respectively. Western blot analyses of the mouse skin extracts revealed that RBS significantly inhibits UVB-irradiated COX-2 and MMP-13 expression (Figure (A)). These immunohistochemical analyses revealed that RBS inhibits UVB- induced upregulation of COX-2 and MMP-13 expression in mouse dorsal skin (Figure (B)). Interestingly, quantitative Real-Time RT-PCR analysis results showed that RBS significantly inhibited UVB-induced COX-2 and MMP-1 mRNA expression in immortalized human keratinocyte (HaCaT) cells (Figure S1A). We evaluated the inhibitory effect of RBS on UVB-induced pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α mRNA expression in immortalized human keratinocyte (HaCaT) cells. Quantitative Real-Time RT-PCR analysis results showed that RBS significantly inhibited UVB-induced IL-1β, IL-6, and TNF-α mRNA expression in HaCaT cells (Figure S1B).
Figure 4. Effect of RBS on UVB-irradiated COX-2 and MMP-13 expression in SKH-1 hairless mouse skin. (A) RBS inhibits UVB-irradiated COX-2 and MMP-13 expression in SKH-1 hairless mice. (B) Immunohistochemical staining for COX-2 and MMP-13 expression in the skin (magnification X200). Skin samples were stained for COX-2 and MMP-13 expression with a specific polyclonal antibody and assessed by microscopy. Results are shown as means ± S.D. (n = 8). The hash symbol (#) indicates a significant difference (p < 0.05) between the control group and the UVB-irradiated group. Asterisks (* and **) indicate a significant difference of p < 0.05 or p < 0.01 between the RBS diet group and UVB-irradiated group.
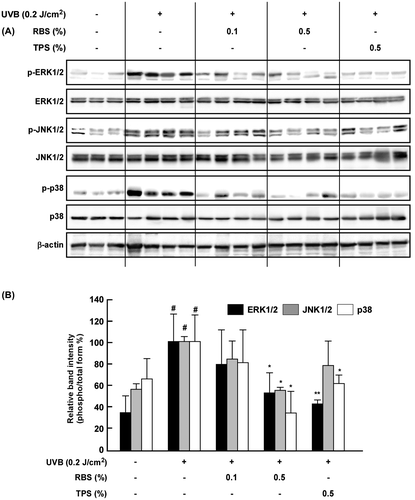
RBS inhibits UVB-induced phosphorylation of MAPK in mouse skin
Evidence has shown that MAPK signaling plays a central role in UV-induced MMP-1 and COX-2 upregulation. We therefore sought to investigate the impact of RBS on UVB-dependent activation of MAPK signaling in the mouse skin tissue. Western blot analyses revealed that RBS significantly inhibits UVB-induced phosphorylation of ERK1/2, JNK1/2, and p38 in mouse skin (Figure ).
Figure 5. Effect of RBS on UVB-irradiated phosphorylation of MAPKs in SKH-1 hairless mouse skin. RBS inhibits UVB-induced phosphorylation of ERK1/2, JNK1/2, and p-38 in SKH-1 hairless mice. Results are shown as means ± S.D. (n = 8). The hash symbol (#) indicates a significant difference (p < 0.05) between the control group and the UVB-irradiated group. Asterisks (* and **) indicate significant differences (of p < 0.05 or p < 0.01, respectively) between the RBS diet group and UVB-irradiated group.
Discussion
Functional food compounds have been reported to provide benefits beyond basic nutrition and likely play a role in preventing or reducing the risk of developing chronic diseases [Citation19]. As the demand for anti-aging skin care products continues to increase among consumers, functional agents that can improve skin health are being sought by the nutraceutical industry [Citation20]. A recent report by Global Industry Analysts Inc, suggests that the nutricosmetic market is showing a preference among consumers to address premature skin aging, and the greater use of safe, natural, and effective solutions. Skin physiology is known to be dependent on dietary patterns, and a higher intake of vegetables, fish, legumes, and olive oil is associated with a lower extent of skin wrinkling [Citation12]. The importance of dietary factors is, therefore, emerging with prominence under the term nutraceuticals, which include nutricosmetics.
The skin is a direct barrier to the environment and can be subject to accelerated aging due to environmental damage [Citation21]. Sun-induced aging of the skin (photoaging), like normal aging, is a cumulative process [Citation22]. There are several herbal extracts, minerals, and vitamins that are considered effective in preventing skin wrinkling as evidenced in clinical trials involving oral administration [Citation23]. A significant number of plant extracts have now been assessed for the potential to counteract UV-induced skin damage. Krameria triandra root extract has elicited protective effects against UVB-induced photodamage in human keratinocyte cells [Citation24]. It has been recently demonstrated that oral ingestion or topical application of rice wine suppresses disruption of the epidermal barrier that occurs in the presence of UV exposure [Citation25]. Rice wine application also reduced UV-dependent epidermal thickening in mice.
In the present study, we investigated the inhibitory effect of RBS on UVB-dependent skin inflammation and photoaging in SKH-1 hairless mice. The skin in this animal model can be easily damaged by UV exposure, following which the skin becomes dry, and the surface of the skin becomes cracked and fragile [Citation26].
Taraxacum platycarpum is a functional ingredient approved by the Ministry of Food and Drug Safety (MFDS) for skin moisturization and was used as a positive control. Previous studies have reported that extracts of Taraxacum platycarpum treatment can improve the severity and skin hydration in patients with mild atopic dermatitis [Citation27]. Also skin hydration mechanism of Taraxacum platycarpum extract is due to recovery of filaggrin in mice model and increased production of collagen with suppression of matrix MMP in vitro fibroblast cell line [Citation28]. RBS inhibited UVB-induced water content loss in comparison to the control group. The action of these enzymes including proteinases is critically influenced by intrinsic water content within skin tissue, while epidermal thickening is a core marker of inflammation and the progression of photoaging. We observed that RBS inhibited UVB-dependent epidermal thickening of mouse skin. Increases in cell division in keratinocytes following UV exposure can cause the accumulation of epidermal keratinocytes leading to direct increases in epidermal thickness. A previous study results reported that topical application of geland cream containing the semi-purified rice bran extracts entrapped in noisome improve hydration, skin lightening, and elasticity [Citation29]. However, there is no report on the effect of rice bran extract consumption on skin moisturizing, wrinkling, and inflammatory markers expression. COX-2 and MMP induction is known to be responsible for UV-induced damage to skin connective tissue, as well as inflammation [Citation30]. Previous studies have reported that the extract of Rhus javanica attenuates UVB-dependent photodamage and inflammation is known to affect the expression of MMPs and COX-2 in SKH-1 hairless mice [Citation31]. As expected, RBS prevented chronic UV-induced upregulation of COX-2 and MMP-13 levels in the mouse skin. Previous study results reported that α-tocopherol has protective effect on UVB-induced keratinocyte damages [Citation32]. Although we have added 0.015% α-tocopherol to the experimental diet as the antioxidant, we have confirmed that 0.015% α-tocopherol doesn’t affect cox-2 and mmp-1 mRNA expressions (Figure S2). We also examined the effect of RBS on UVB-dependent phosphorylation of MAPK family members. Western blot analyses of mouse skin extract revealed that RBS significantly inhibits UVB-induced MAPK phosphorylation in this model. Additionally, RBS has suppressive effect on UVB-induced IL-1β, IL-6, and TNF-α mRNA expression in human keratinocyte HaCaT cells (Figure S1B). Future studies are needed to clarify the anti-photoaging mechanisms responsible for these effects of rice bran components.
Taken together, our results show that RBS significantly inhibits UVB-induced water content loss, wrinkle formation, and epidermal thickening in vivo. This inhibition leads to the inhibition of MMP-13 and COX-2 expression by reducing MAPKs pathway signaling, and may have the potential to prevent photoaging in clinical settings.
Author contributions
Min Young Um, Suengmok Cho, and Sung Keun Jung conceived the project. Su Jeong Ha, Joon Park, and Jangho Lee designed and performed the experiments. All authors analyzed the data and discussed the result. Su Jeong Ha and Sung Keun Jung wrote the manuscript. All authors read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116036-03).
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/09168451.2017.1417021.
Supplementary-Materials-170473.pdf
Download PDF (236.9 KB)References
- Sharif MK, Butt MS, Anjum FM, et al. Rice bran: a novel functional ingredient. Crit Rev Food Sci Nutr. 2014;54(6):807–816.10.1080/10408398.2011.608586
- Slavin JL, Martini MC, Jacobs DR, et al. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr. 1999;70(3):459s–463s.
- Yasukawa K, Akihisa T, Kimura Y, et al. Inhibitory effect of cycloartenol ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin. Biol Pharm Bull. 1998;21(10):1072–1076.10.1248/bpb.21.1072
- Kahlon TS. Rice bran: production, composition, functionality and food applications, physiological benefits. Boca Raton (FL): CRC Press; 2009.10.1201/9781420043853
- Hanmoungjai P, Pyle D, Niranjan K. Enzymatic process for extracting oil and protein from rice bran. J Am Oil Chem Soc. 2001;78(8):817–821.10.1007/s11746-001-0348-2
- Suzuki R, Andrade A, Sousa J, et al. Preparation and characterization of activated carbon from rice bran. Bioresour Technol. 2007;98(10):1985–1991.10.1016/j.biortech.2006.08.001
- Niva M. “All foods affect health”: understandings of functional foods and healthy eating among health-oriented Finns. Appetite. 2007;48(3):384–393.10.1016/j.appet.2006.10.006
- Anscombe J, Thomas M, Stricker M, et al. Nutraceuticals: the front line of the battle for consumer health. Seoul: ATKearney; 2014.
- Kalra EK. Nutraceutical-definition and introduction. AAPS PharmSci. 2003;5(3):27–28.10.1208/ps050325
- Stice E, Hayward C, Cameron RP, et al. Body-image and eating disturbances predict onset of depression among female adolescents: a longitudinal study. J Abnormal Psychol. 2000;109(3):438–444.10.1037/0021-843X.109.3.438
- Sjerobabski-Masnec I, Šitum M. Skin aging. Acta Clin Croat. 2010;49(4):515–518.
- Binic I, Lazarevic V, Ljubenovic M, et al. Skin ageing: natural weapons and strategies. Evidence Based Complementary Altern Med. 2013;2013: 827248.
- D’Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248.10.3390/ijms140612222
- Xu Y, Fisher GJ. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J Dermatol Sci Suppl. 2005;1(2):S1–S8. DOI:10.1016/j.descs.2005.06.002
- Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470.
- Rabe JH, Mamelak AJ, McElgunn PJ, et al. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55(1):1–19.10.1016/j.jaad.2005.05.010
- Madson JG, Lynch DT, Svoboda J, et al. Erbb2 suppresses DNA damage-induced checkpoint activation and UV-induced mouse skin tumorigenesis. Am J Pathol. 2009;174(6):2357–2366.10.2353/ajpath.2009.080638
- Jung SK, Lee KW, Kim HY, et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010 May 15;79(10):1455–1461. DOI:10.1016/j.bcp.2010.01.004 PubMed PMID: ISI:000275811500009; English.
- Jiménez-Colmenero F, Carballo J, Cofrades S. Healthier meat and meat products: their role as functional foods. Meat Sci. 2001;59(1):5–13.10.1016/S0309-1740(01)00053-5
- Newburger AE. Cosmeceuticals: myths and misconceptions. Clin Dermatol. 2009;27(5):446–452.10.1016/j.clindermatol.2009.05.008
- Brodell LA, Rosenthal KS. Skin structure and function: the body’s primary defense against infection. Inf Dis Clin Pract. 2008;16(2):113–117.10.1097/IPC.0b013e3181660bf4
- Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermato Endocrinol. 2012;4(3):308–319.10.4161/derm.22804
- Korać RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. 2011;5(10):164.10.4103/0973-7847.91114
- Svobodová A, Psotová J, Walterová D. Natural phenolics in the prevention of uv-induced skin damage. a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147(2):137–145.10.5507/bp.2003.019
- Seo M-Y, Chung S-Y, Choi W-K, et al. Anti-aging effect of rice wine in cultured human fibroblasts and keratinocytes. J Biosci Bioeng. 2009;107(3):266–271.10.1016/j.jbiosc.2008.11.016
- Hashizume H. Skin aging and dry skin. J Dermatol. 2004;31(8):603–609.10.1111/jde.2004.31.issue-8
- Kim JH, Lee HI, Park JH, et al. The effect of the extracts of Taraxacum platycarpum (AF-343) in atopic dermatitis. Korean J Asthma Allergy Clin Immunol. 2010;30(1):36–42.
- Cho J-W, Y-s Jeong, Han J, et al. Skin hydration and collagen synthesis of AF-343 in HS68 cell line and NC/Nga mice by filaggrin expression and suppression of matrix metallopreteinase. Toxicol Res. 2011;27(4):225–229.10.5487/TR.2011.27.4.225
- Manosroi A, Chutoprapat R, Abe M, et al. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm Biol. 2012;50(2):208–224.10.3109/13880209.2011.596206
- Kim HH, Shin CM, Park C-H, et al. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J Lipid Res. 2005;46(8):1712–1720.10.1194/jlr.M500105-JLR200
- Ha SJ, Lee J, Kim H, et al. Preventive effect of Rhus javanica extract on UVB-induced skin inflammation and photoaging. J Funct Foods. 2016;27:589–599.10.1016/j.jff.2016.10.011
- Maalouf S, El-Sabban M, Darwiche N, et al. Protective effect of vitamin E on ultraviolet B light–induced damage in keratinocytes. Mol Carcinog. 2002;34(3):121–130.10.1002/(ISSN)1098-2744
