Abstract
AVCP cytochrome c′ from mesophilic Allochromatium vinosum exhibits lower stability than a thermophilic counterpart, Hydrogenophilus thermoluteolus cytochrome c′ (PHCP), in which the six specific amino acid residues that are not conserved in AVCP are responsible for its stability. Here we measured the stability of AVCP variants carrying these specific residues instead of the original AVCP ones. Among the six single AVCP variants, all of which formed a dimeric structure similar to that of the wild-type, three were successfully stabilized compared with the wild-type, while one showed lower stability than the wild-type. In addition, the most stabilized and destabilized AVCP variants could bind CO, similar to the wild-type. These results indicated that mesophilic AVCP could be stabilized through specific three mutations modeled by the thermophilic counterpart, PHCP, without changing the CO binding ability.
Mesophilic AVCP could be stabilized through mutations.
Cytochromes c′ are heme proteins found in a variety of Gram-negative bacteria. They are class II cytochromes c [Citation1] and usually form a homo dimeric structure. The single subunit comprises a four-helix bundle protein consisting of about 130 amino acid residues with a pentacoordinate heme [Citation2]. In the aspect of in vitro function, cytochromes c′ are able to bind CO or NO through the heme [Citation3,4].
Previously, we showed that cytochrome c′ (PHCP) from thermophilic Hydrogenophilus thermoluteolus growing optimally at 52 °C had a denaturation temperature of 87 °C, which was higher than that of the mesophilic homologue (AVCP, 53 °C) from Allochromatium vinosum growing optimally at 25 °C [Citation5–8]. X-ray crystal structure and analytical centrifugation analysis indicated that PHCP (PDB ID; 5B3I) was a dimeric protein similar to AVCP (PDB ID; 1BBH) [Citation9,10].
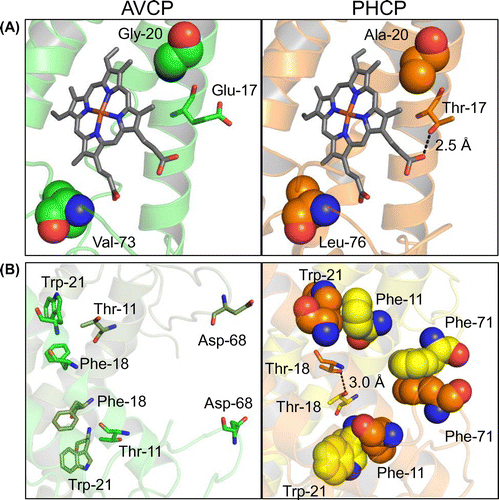
Although the main chain structures of AVCP and PHCP were almost superimposable, some specific interactions around the heme and at the subunit-subunit interface occurred in PHCP, but not in AVCP [Citation9]. Around the heme of PHCP, the Thr-17 side chain formed a hydrogen bond with the heme propionate, and Ala-20 and Leu-76 hydrophobically interacted with the heme in the same subunit (Figure (A)). At the subunit-subunit interface in PHCP, the Phe-11 residue on one subunit interacted with the Trp-21 residue on the other subunit, and the Thr-18 residues in the two subunits formed a hydrogen bond. In addition, the Phe-71 residues in the two subunits formed a π-π stacking structure (Figure (B)). These six specific residues in PHCP (Phe-11, Thr-17, Thr-18, Ala-20, Phe-71, and Leu-76) were separately substituted with the corresponding amino acid residues found in AVCP, Thr-11, Glu-17, Phe-18, Gly-20, Asp-68, and Val-73, respectively (original AVCP numbering system, as deposited in PDB ID; 1BBH, was adopted). All the resulting PHCP variants exhibited decreased stability compared with the wild-type, experimentally proving that these six residues were responsible for the PHCP stability, as indicated by a crystal structure comparison [Citation9,10].
Figure 1. Structure comparison between the AVCP and PHCP wild-type proteins. (A) The structure difference between AVCP and PHCP around the heme. (B) The structure difference between AVCP and PHCP at the subunit-subunit interface. Hydrogen bond formation is illustrated by a dotted line and distance in both panels.
In this study, AVCP variants carrying the six PHCP-specific residues instead of the original ones were examined as to their stability and CO binding ability. The results were compared with those for the wild-type AVCP protein in order to see whether the AVCP protein can be stabilized while retaining its original CO binding ability.
Materials and methods
Protein preparation
Plasmids carrying genes for AVCP variants were constructed using Escherichia coli strain DH5α. Thr-11, Glu-17, Phe-18, Gly-20, Asp-68, and Val-73 in AVCP were substituted with Phe-11, Thr-17, Thr-18, Ala-20, Phe-68, and Leu-73, respectively (T11F, E17T, F18T, G20A, D68F, and V73L), using wild-type AVCP expression plasmid [Citation6], PrimeSTAR Mutagenesis Kit (TaKaRa Bio, Japan), and primers (Table ). These resulting plasmids were transformed into E. coli strain JCB387 harboring the pEC86 plasmid encoding cytochrome c maturation proteins for overproduction [Citation11]. The transformed E. coli JCB387 cells were grown aerobically and a periplasmic protein fraction containing recombinant AVCP variant protein was recovered by a cold osmotic shock method as described previously [Citation12].
Table 1. Sequences of oligonucleotide primers used for mutagenesis. The nucleic acid residues in upper case are those that were mutated.
The periplasmic protein fractions were dialyzed against 10 mM Tris-HCl buffer (pH 7.5) at 4 °C and then loaded onto a DEAE anion-exchange column (diameter 4 cm, height 10 cm; Tosoh, Tokyo) that had been equilibrated with 10 mM Tris-HCl buffer (pH 7.5) at 4 °C. The fractions containing AVCP variants were eluted with the same buffer containing 0.15 M NaCl. This fraction was further dialyzed against 10 mM Tris-HCl buffer (pH 7.5) at 4 °C and loaded onto a HiTrap Q anion-exchange column (diameter 1.4 cm, height 3 cm; GE Healthcare), which had been equilibrated with the same buffer. The proteins were eluted with a linear gradient of NaCl (0–0.3 M). The resulting brown fraction was dialyzed against 25 mM sodium acetate buffer (pH 5.0) at 4 °C and loaded onto a HiTrap SP cation-exchange column (diameter 1.4 cm, height 3 cm; GE Healthcare) equilibrated with the same buffer. The proteins were eluted with a linear gradient of NaCl (0–0.3 M). The fractions containing the AVCP variants were finally separated on a gel filtration column of a Superdex 75 (diameter 1.6 cm, height 60 cm; GE Healthcare) equilibrated with 10 mM Tris-HCl buffer (pH 7.5) containing 0.15 M NaCl at 4 °C. The concentrations of the purified proteins were determined with a Bradford protein assay kit (Bio-Rad, Tokyo), using bovine serum albumin as a standard. The protein purity was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Analytical ultracentrifugation
Sedimentation velocity analysis of the AVCP variants was performed with a Beckman Optima XL-I analytical ultracentrifuge (Beckman-Coulter, Fullerton, CA) equipped with a 4-hole An60 Ti rotor at 20 °C using 12 mm double-sector charcoal-filled Epon centerpieces with quartz windows. Five μM each protein solution was dissolved in 10 mM Tris-HCl buffer (pH 7.5) containing 0.1 M NaCl. Data were collected using absorbance optics at 390–400 nm with a radial increment of 0.003 cm at a rotor speed of 42,000 rpm.
The distribution of sedimentation coefficients (s) was analyzed using the continuous c(s) distribution model in the program SEDFIT (version 14.1 Feb 2013) [Citation13], as previously reported [Citation9]. The partial specific volume of each protein, calculated from the amino acid composition, buffer density (ρ0 = 1.00260 g/cm3), and buffer viscosity (η0 = 0.01014 P), was estimated using the program SEDNTERP (http://sednterp.unh.edu).
Thermal stability measurement
The stability of the AVCP variants was measured by monitoring circular dichroism (CD) spectra using a Jasco J-820 CD spectrometer (Jasco, Japan). A 20 μM protein solution dialyzed against 20 mM potassium phosphate buffer (pH 7.0) was analyzed. The CD ellipticity change at 222 nm was monitored in a cuvette of a path length of 1 mm. The CD values were recorded from 25 to 100 °C at intervals of 0.5 °C at a heating rate of 1.0 °C/min. The raw data were subjected to nonlinear least-squares fitting, as described previously [Citation14]. The data points were corrected for the slope of the base lines for the native and denatured forms, and were normalized to calculate the fraction of protein denatured.
The fraction of protein denatured was plotted as a function of temperature, and the resulting denaturation curves were used to determine the thermodynamic parameters. The calculation was performed by the curve fitting of the CD values versus the temperature by van’t Hoff analysis, and then the temperature at the midpoint of the transition (Tm) and the enthalpy change during protein denaturation at Tm (∆H) were determined. The entropy change during protein denaturation at Tm (∆S) of the AVCP wild-type protein was calculated with the equation ∆S = ∆H/Tm. The fitting curves obtained in the temperature range of 25–100 °C were used to calculate thermodynamic parameters in order to reflect protein denaturation precisely. The differences in the free energy changes of denaturation between the variant proteins and the wild-type at the wild-type Tm (∆∆G) were calculated using the equation given by Becktel and Schellman [Citation15], ∆∆G = ∆Tm*∆S (wild-type), where ∆Tm is the difference in Tm value between the AVCP variant and wild-type proteins, and ∆S (wild-type) the entropy change of the wild-type protein at Tm.
Structure simulation analysis
Three-dimensional model structures of the AVCP variant proteins were simulated by using the crystal structure of the wild-type AVCP (PDB ID; 1BBH) as a template using the SWISS-MODEL workplace [Citation16].
CO binding assay
The AVCP wild-type and variant proteins (final 6 μM) in 10 mM Tris-HCl buffer (pH 7.5) were reduced with a grain of sodium dithionite. The reduced protein solutions were bubbled with CO (99.9%; GL Science, Japan). Visible absorption spectra of these preparations were obtained with a JASCO V-730-Bio spectrophotometer (Jasco, Japan) at 25 °C.
Results and discussion
Subunit compositions of the AVCP variant proteins
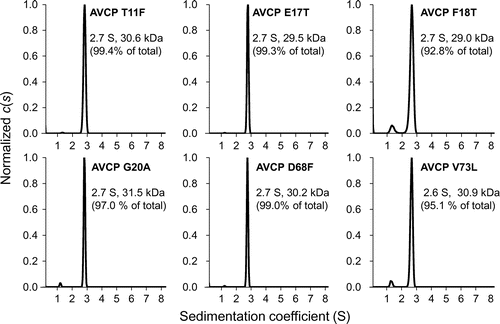
Consistent with the dimeric structure determined on crystal structure analysis and gel filtration chromatography [Citation10,17], the AVCP wild-type protein showed an s value of 2.8 S with a 98.9% population on analysis by means of the ultracentrifugation method (data not shown), the calculated molecular weight being 30.1 kDa, indicating a dimeric association state. The subunit compositions of the AVCP variant proteins were also analyzed by the same method (Figure ). The three single AVCP variants, T11F, E17T, and D68F showed dimeric structures with a population of more than 99%, exhibiting similar S values (~2.6–2.7 S) to that of the wild-type, indicating dimeric compositions. On the other hand, the remaining three single AVCP variants, F18T, G20A, and V73L showed dimeric structures with populations of 92–97% of total with values (~2.6–2.7 S), and the parts of the populations showed monomeric structures, exhibited by smaller S values (~1.1–1.3 S) than that of dimeric structures, indicating that these three variant proteins existed predominantly as a dimer with small quantities of monomer. The Phe-18 residue of the AVCP protein located at subunit-subunit interface (Figure (B)), and thus may affect the subunit composition directly thorough the mutation. In contrast, the Gly-20 and Val-73 residues of the AVCP protein did not locate at subunit-subunit interface (Figure (A)), and may affect the subunit compositions indirectly thorough the mutations.
Thermal stability experiments
Denaturation curves obtained on CD measurement for the AVCP wild-type and its variant proteins were normalized (Figure ). All these denaturation curves were S-shaped, indicating a two-state denaturation transition. After completion of the heating process up to each Tm, the protein solutions were cooled and then kept at 30 °C. From the CD spectra obtained at 30 °C before and after the heating, it was confirmed that nearly 100% of the native state was retained (data not shown), suggesting a reversible denaturation process. These results indicated that the denaturation observed in the present study correctly provided equilibrium thermodynamic parameters (Table ).
Figure 3. Thermal denaturation curves of the AVCP variants obtained with CD spectra. Representative normalized raw data points are shown at a temperature interval of 2.5 °C. A fitting curve for each data-set is also shown.
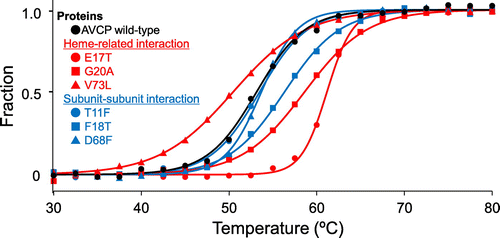
Table 2. Parameters characterizing the thermal denaturation detected in CD spectra for AVCP variants.
Single AVCP variants that were stabilized
Among the six single AVCP variants, three having the E17T, G20A, and V73L mutations were designed so as to increase the heme-related interaction, as predicted on structure comparison between the AVCP and PHCP wild-type proteins (Figure (A)). Of these, the E17T and G20A variants apparently exhibited higher Tm values (positive ΔTm values) than that of the wild-type (Table ). In addition, the ΔΔG values of these two variants were positive, indicating that these single mutations resulted in stabilization. Structure simulation analysis of the variants using the AVCP wild-type protein structure indicated that Thr-17 introduced formed a hydrogen bond with the heme propionate (Figure (A)). The ΔΔG value of the E17T variant, ~8 kJ/mol, was within the range for the theoretical hydrogen bond energy (~5–~10 kJ/mol), indicating that the hydrogen bond formation between Thr-17 and heme newly occurred in this variant, while the wild-type Glu-17 residue protruded from the protein surface without any interaction (Figure (A)).
Figure 4. Structure simulation for the AVCP variants using the SWISS-MODEL. (A) Interaction between Thr-17 and heme. Hydrogen bond formation is illustrated by a dotted line and distance. (B) Hydrophobic interaction between Ala-20 and heme. (C) Two Thr-18 residues at the subunit-subunit interface. (D) Leu-73 introduced. (E) Phe-11 introduced. (F) Phe-68 introduced.
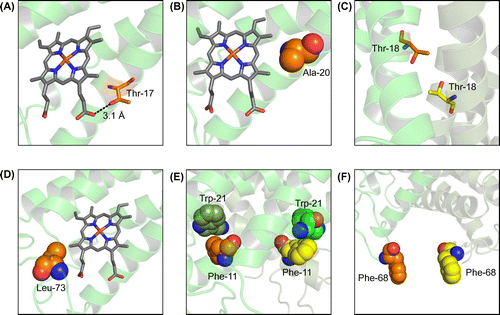
The Ala-20 residue introduced into AVCP filled a void space around the heme (Figure (B)). Ala-20 was also conserved in cytochrome c′ (TTCP) from another thermophile, Thermochromatium tepidum, showing 82% sequence identity with AVCP [Citation18]. The Tm value of the TTCP protein was 75 °C, which was significantly higher than that of the AVCP wild-type, although pH conditions for the stability measurements of these two proteins slightly differed. In the 3D structure of TTCP (PDB ID; 3VRC), the Ala-20 residue was faced with the heme and could contribute to the stabilization by hydrophobic interaction in the molecule. Thus the AVCP G20A variant may strengthen interaction between the Ala-20 side chain and the heme, as observed in the PHCP (Figure ) and TTCP structure.
The other three single variants having the T11F, F18T, and D68F mutations were designed so as to increase the subunit-subunit interaction, as predicted on structure comparison between the AVCP and PHCP wild-type proteins (Figure (B)). Of these, the F18T variant exhibited a higher Tm value (positive ΔTm value) than that of the wild-type (Table ). In addition, the ΔΔG value of this variant was positive, indicating that this single mutation resulted in stabilization. Structure simulation analysis indicated that the side chains of two Thr-18 residues located at the subunit-subunit interface were close, but did not form a definite hydrogen bond (Figure (C)), with having 4.5 Å distance between the side chain oxygen atoms, unlike in the structure of the PHCP wild-type protein, with having 3.0 Å distance between the corresponding atoms (Figure (B)). Therefore, it was plausible that the two Thr-18 residues formed an incomplete hydrogen bond, thus the AVCP F18T variant exhibited a smaller ΔΔG value than that of the theoretical hydrogen bond (Table ).
Overall, among the six single AVCP variants, three were successfully stabilized compared with the wild-type, although the Tm and ΔΔG values of these three AVCP variants did not reach to those of PHCP (Table ). However, our present results clearly indicate that the stability of mesophilic AVCP could be enhanced through specific mutations modeled by the thermophilic counterpart, PHCP.
Single AVCP variants that were not stabilized
The V73L variant exhibited negative values for ΔTm and ΔΔG, and the T11F and D68F variants nearly the same values for these thermodynamic parameters (Table ). Although these three mutations were introduced so as to increase stability of the AVCP protein, the residues introduced did not contribute to the stability enhancement. Structure simulation analysis indicated that the Leu-73 may cause steric hindrance with the heme (Figure (D)), and Phe-11 and Phe-68 did not undergo any specific subunit-subunit interaction (Figure (E), (F)), unlike in the PHCP structure (Figure ).
Difference in effects of mutations contributing to the heme-related and subunit-subunit interactions
As described above, Thr-17, Ala-20, and Thr-18 introduced contributed to the stability enhancement in the AVCP protein, the former two and last one being strengthening heme-related and subunit-subunit interactions, respectively. Judging from the ΔΔG values (Table ), the E17T and G20A mutations contributing to the heme-related interactions were more effective than the F18T mutation on the subunit-subunit interface. In addition, the T11F and D68F variants, the two AVCP variants out of three rationally designed to be increasing subunit-subunit interactions modeled with the thermophilic PHCP protein as a thermally stable benchmark, did not exhibit enhanced stability.
Possible reasons for these observations may be attributed to differences in interactions between heme-related and subunit-subunit ones. Generally heme environment is partially fixed due to the hydrophobic feature of heme, thus the effects of mutations are predictable. For example, monomeric cytochromes c could be rationally and successfully stabilized through strengthening heme-related interaction modeled by stable homologous counterparts [Citation11,19–21]. In contrast, subunit-subunit interface is generally formed with large number of amino acid residues, which are usually balanced. Therefore point mutations at subunit-subunit interface may easily result in imbalance and rearrangement of the structure. Effective stabilization at subunit-subunit interface by point mutations is difficult as observed in the present AVCP variants. Consistently, in fact, the PHCP reverse variants F11T, T18F, and F68D having altered subunit-subunit interactions were more severely destabilized than those having altered heme-related interactions (T17E, A20G, and L73V) [Citation9]. These considerations together may indicate stabilization strategy and difficulty in heme protein engineering.
CO-binding ability
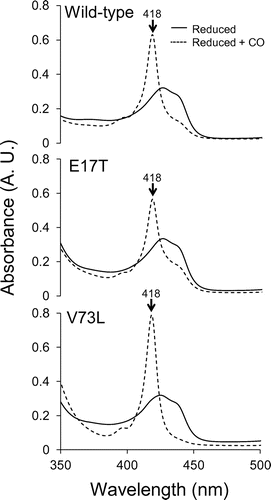
The heme environment often influences the functional properties of heme proteins. In order to confirm the CO-binding ability through the heme in the AVCP variants, visible absorption spectra of the E17T and V73L variants, which were most stabilized and destabilized, respectively, were obtained in the presence of CO. The spectra of the reduced AVCP wild-type, and E17T and V73L variants with CO contained a specific peak at 418 nm (Figure ), consistent with the previous published results for the AVCP wild-type under the same temperature and pH conditions [Citation22]. As previously discussed, this specific peak indicates that CO binds at the sixth position of the axial ligand of the heme. Therefore, CO could bind to the most stabilized E17T and destabilized V73L variants.
Conclusion
In this study, we showed that a mesophilic AVCP protein could be stabilized by three specific single mutations, which had been rationally modeled with the thermophilic PHCP protein as a thermally stable benchmark. The most stabilized and destabilized variants could bind CO, similar to the wild-type. The present study has deepened the understanding of the stability and functions of cytochromes c′, as comprehensively reviewed recently [Citation23].
Disclosure statement
No potential conflict of interest is reported by the authors.
Funding
This work was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan [numbers 26240045 and 16K07692 to Y.S.], and a grant from the Japan Society for the Promotion of Science [number 25-1446 to S.F.].
Author contributions
D.Y-K. conducted most of the experiments, analyzed the results with the aid of S.F., M.Y., and S.W., and wrote most of the paper. T.M. and Y.K. conducted the experiments involving analytical ultracentrifugation. M.Y., S.W., and Y.S. conceived the idea for the project, and wrote the paper with D.Y.-K. and S.F. All the authors reviewed the results and approved the final version of the manuscript.
References
- Amber RP. Sequence variability in bacterial cytochromes c. Biochim Biophys Acta. 1991;1058:42–47.10.1016/S0005-2728(05)80266-X
- Moore GR. Bacterial 4-alpha-helical bundle cytochromes. Biochim Biophys Acta. 1991;1058:38–41.10.1016/S0005-2728(05)80265-8
- Antonyuk SV, Rustage N, Petersen CA, et al. Carbon monoxide poisoning is prevented by the energy costs of conformational changes in gas-binding haemproteins. Proc Natl Acad Sci U S A. 2011;108:15780–15785.10.1073/pnas.1109051108
- Andrew CR, George SJ, Lawson DM, et al. Six- to five-coordinate heme-nitrosyl conversion in cytochrome c′ and its relevance to guanylate cyclase. Biochemistry. 2002;41:2353–2360.10.1021/bi011419k
- Hayashi NR, Ishida T, Yokota A, et al. Hydrogenophilus thermoluteolus gen. nov., sp. nov., a thermophilic, facultatively chemolithoautotrophic, hydrogen-oxidizing bacterium. Int J Syst Bacteriol. 1999;49:783–786.10.1099/00207713-49-2-783
- Fujii S, Masanari M, Inoue H, et al. High thermal stability and unique trimer formation of cytochrome c′ from thermophilic Hydrogenophilus thermoluteolus. Biosci Biotechnol Biochem. 2013;77:1677–1681.10.1271/bbb.130226
- Evers TH, Merkx M. Successful recombinant production of Allochromatium vinosum cytochrome c′ requires coexpression of cmm genes in heme-rich Escherichia coli JCB712. Biochem Biophys Res Commun. 2005;327:668–674.10.1016/j.bbrc.2004.12.062
- Holm HW, Vennes JW. Occurrence of purple sulfur bacteria in a sewage treatment lagoon. Appl Microbiol. 1970;19:988–996.
- Fujii S, Oki H, Kawahara K, et al. Structural and functional insights into thermally stable cytochrome c′ from a thermophile. Protein Sci. 2017;26:737–748.10.1002/pro.3120
- Ren Z, Meyer T, McRee DE. Atomic structure of a cytochrome c′ with an unusual ligand-controlled dimer dissociation at 1.8 Å resolution. J Mol Biol. 1993;234:433–445.10.1006/jmbi.1993.1597
- Hasegawa J, Shimahara H, Mizutani M, et al. Stabilization of Pseudomonas aeruginosa cytochrome c551 by systematic amino acid substitutions based on the structure of thermophilic Hydrogenobacter thermophilus cytochrome c552. J Biol Chem. 1999;274:37533–37537.10.1074/jbc.274.53.37533
- Sambongi Y, Stoll R, Ferguson SJ. Alteration of haem-attachment and signal-cleavage sites for Paracoccus denitrificans cytochrome c550 probes pathway of c-type cytochrome biogenesis in Escherichia coli. Mol Microbiol. 1996;19:1193–1204.10.1111/mmi.1996.19.issue-6
- Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 2000;78:1606–1619.10.1016/S0006-3495(00)76713-0
- Uchiyama S, Ohshima A, Nakamura S, et al. Complete thermal-unfolding profiles of oxidized and reduced cytochromes c. J Am Chem Soc. 2004;126:14684–14685.10.1021/ja046667t
- Becktel WJ, Schellman JA. Protein stability curves. Biopolymers. 1987;26:1859–1877.10.1002/(ISSN)1097-0282
- Biasini M, Bienert S, Waterhouse A, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258.10.1093/nar/gku340
- Doyle ML, Gill SJ, Cusanovich MA. Ligand-controlled dissociation of Chromatium vinosum cytochrome c′. Biochemistry. 1986;25:2509–2516.10.1021/bi00357a034
- Hirano Y, Kimura Y, Suzuki H, et al. Structure analysis and comparative characterization of the cytochrome c′ and flavocytochrome c from thermophilic purple photosynthetic bacterium Thermochromatium tepidum. Biochemistry. 2012;51:6556–6567.10.1021/bi3005522
- Hasegawa J, Uchiyama S, Tanimoto Y, et al. Selected mutations in a mesophilic cytochrome c confer the stability of a thermophilic counterpart. J Biol Chem. 2000;275:37824–37828.10.1074/jbc.M005861200
- Yamanaka M, Masanari M, Sambongi Y. Conferment of folding ability to a naturally unfolded apocytochrome c through introduction of hydrophobic amino acid residues. Biochemistry. 2011;50:2313–2320.10.1021/bi101646m
- Masanari M, Fujii S, Kawahara K, et al. Comparative study on stabilization mechanism of monomeric cytochrome c5 from deep-sea piezophilic Shewanella violacea. Biosci Biotechnol Biochem. 2016;80:2365–2370.10.1080/09168451.2016.1232155
- Kassner RJ. Ligand binding properties of cytochromes c′. Biochim Biophys Acta. 1991;1058:8–12.10.1016/S0005-2728(05)80257-9
- Hough MA, Andrew CR. Cytochromes c′: structure, reactivity and relevance to haem-based gas sensing. Adv Microb Physiol. 2015;67:1–84.