Abstract
To develop a novel type of biocontrol agent, we focus on bacteria that are characterized by both chitinase activity and biofilm development. Chitinolytic bacteria were isolated from sediments and chitin flakes immersed in the water of a sand dune lake, Sakata, in Niigata, Japan. Thirty-one isolates from more than 5100 isolated strains were examined chitinase activity and biofilm formation. Phylogenetic analysis of these isolates based on the 16S rRNA gene sequences revealed that most isolates belonged to the family Aeromonadaceae, followed by Paenibacillaceae, Enterobacteriaceae, and Neisseriaceae. The specific activity of chitinase of four selected strains was higher than that of a reference strain. The molecular size of one chitinase produced by Andreprevotia was greater than that of typical bacterial chitinases. The dialyzed culture supernatant containing chitinases of the four strains suppressed hyphal growth of Trichoderma reesei. These results indicate that these four strains are good candidates for biocontrol agents.
The dialyzed culture supernatant containing chitinases of the four isolated strains suppressed hyphal growth of Trichoderma reesei.
Abbreviations:
Chitin, an insoluble linear β-1,4-linked homopolymer of N-acetylglucosamine (GlcNAc), is the second most abundant polysaccharide in nature after cellulose, with an annual production of 100 billion tons [Citation1], and is a common constituent of fungal cell walls, exoskeletons of insects, and shells of crustaceans. Chitinases (EC 3.2.1.14) are glycoside hydrolases (GHs) that degrade chitin by hydrolyzing β-1,4-glycosidic linkages. These enzymes occur in a variety of organisms. On the basis of amino acid sequence similarity in the catalytic domain, most chitinases are classified into two different families of glycoside hydrolases, families 18 and 19 [Citation2,3]. Family 18 chitinases are distributed in a wide range of organisms including bacteria, fungi, viruses, plants, and animals, whereas family 19 chitinases are mostly found in plants and a relatively limited group of prokaryotic organisms [Citation4–10].
Chitinase genes from various chitinolytic bacteria have been cloned, analyzed, and their biochemical properties have been examined in detail [Citation11–15]. A large number of studies have reported that chitinases and chitinolytic bacteria play an important role in inhibiting the mycelial extension of various pathogenic fungi [Citation16–19]. Therefore, bacterial chitinases play a critical role in the digestion of chitin in fungal cell walls, and chitinolytic bacteria could be widely applied as environmentally friendly agents for the biocontrol of agricultural phytopathogens [Citation18,20–23]. Of the bacterial chitinases, family 19 chitinases have been found to be primary enzymes involved in inhibitory activity against fungi [Citation4,6,8,9,24,25], while only a few family 18 chitinases have been reported to exhibit such activity [Citation19,26]. Most of the chitinolytic bacteria and their chitinases that have been studied in the context of phytopathogenic control have been isolated from soil and marine environments, while only a few of them have been isolated from freshwater environments [Citation6]. Thus, analyses of chitinolytic bacteria isolated from freshwater environments and characterization of their chitinases are important for understanding their function and efficiency against pathogenic fungi and nematodes, among others, in agriculture.
Microbial cells attach to biotic or abiotic surfaces and develop biofilms. A biofilm can be formed by a single bacterial species or can contain numerous species of bacteria, fungi, algae, and/or protozoa. Biofilms have been shown to protect microorganisms against environmental stresses [Citation27]. Chitinolytic bacteria that form biofilms can stably attach to the mycelia of fungi. Chitin in fungal cell walls can induce chitinase expression in chitinolytic bacteria, and then the expressed chitinases can degrade such chitin as a source of nutrition, resulting in fungal death. Chitinolytic bacteria that form biofilms could be more efficient at degrading chitin in fungal cell walls than bacteria with no or low ability to form biofilms [Citation28,29]. However, to the best of our knowledge, no studies on prokaryotic organisms that form biofilms and produce chitinases and their applications in agricultural fungal control have been reported. The attachment of some bacteria to fungi and the role of this process in destroying fungi has been reported. For example, Pseudomonas aeruginosa has been shown to be a bacterium that attaches to, spreads on, and kills the dimorphic pathogenic fungus Candida albicans via its filamentous form [Citation30]. In addition, Salmonella enterica Typhimurium SL1344 has the ability to form biofilms on the hyphae of Aspergillus niger [Citation31]. Moreover, recently, Hover et al. [Citation32] demonstrated that a chitinolytic soil bacterium, Serratia marcescens 1, bound to, migrated along, formed biofilms on, and killed the hyphae of several zygomycete molds, such as Absidia, Rhizopus sp, Rhizopus oryzae, Rhizopus microspores, and Mucor circinelloides. Unfortunately, these bacteria are causative of diseases in animals including humans, so it has been difficult to apply them for fungal biocontrol in crop production.
Against this background, in this report, we describe the screening of freshwater bacteria for those that possess high chitinase activity and generate biofilms from Sakata, a sand dune lake, in Niigata, Japan, and we analyzed the chitinase and antifungal activities of the selected bacteria and the culture supernatant. Sakata has no inflow, with the lake water instead being provided by groundwater. Hence, Sakata is thought to be a particularly promising place to identify new species or different types of chitinolytic bacteria.
Materials and methods
Sampling and isolation of chitinolytic bacteria
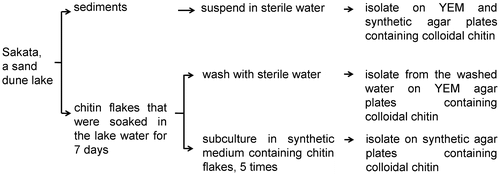
Sakata is a sand dune lake in Niigata, Japan. It is located at 37°49′N, 138°52′E, at 5 m above sea level, and has an area of 0.76 km2. The lake water is mainly provided by groundwater running under the dunes. Sakata has only one small stream, along which the lake water flows into a river, which does not freeze in the winter. To isolate chitinolytic bacteria, the sediments from five sites in the lake were collected. Two nylon nets containing crab shell chitin flakes (Tokyo Chemical Industry, Tokyo, Japan) and two nylon nets containing shrimp shell chitin flakes (Sigma-Aldrich, USA), 10 g per bag, were placed in the lake water at two different sites in Sakata. Seven days later, the chitin flakes were recovered and any bacteria bound to them were isolated.
For isolating bacteria from the sediments, 1 g (wet weight) of each sediment was suspended in 9.0 mL of sterile water. One hundred microliters of each suspension at an appropriate dilution was spread on yeast extract-supplemented minimal (YEM) agar medium (w/v, 0.05% yeast extract, 0.1% (NH4)2SO4, 0.136% KH2PO4, 0.03% MgSO4·7H2O, pH 8.5, 1.5% agar) and synthetic agar medium (w/v, 0.5% (NH4)2SO4, 0.085% KH2PO4, 0.015% K2HPO4, 0.05% MgSO4, 0.01% NaCl, 0.01% CaCl2, pH 6.1, 1.5% agar) containing 0.2% (w/v) colloidal chitin and incubated at 30 °C for 2 days. Then, chitinolytic bacteria grown on the plates were isolated.
To isolate chitinolytic bacteria strongly adhering to the chitin flakes, a portion of the chitin flakes was inoculated into fresh synthetic medium containing chitin and subcultured several times, as previously described [Citation33]. This work involves a sub-screening step to select the dominant bacteria that have high chitinase activity after subculturing. Briefly, 0.2 g of chitin flakes was inoculated in 100 mL of synthetic medium containing 0.2% chitin flakes as the sole carbon source and incubated at 30 °C and 150 rpm until the culture showed significant turbidity caused by bacterial growth. Then, 100 μL of the culture was transferred into fresh synthetic medium. After five cycles of cultivation, chitinase-producing bacteria in the culture medium at appropriate dilutions were spread and purified on synthetic agar plates containing 0.2% colloidal chitin at 30 °C.
In addition, to collect a different type of chitinolytic bacteria forming biofilms on the chitin flakes, 1 g of chitin flakes was vigorously washed with 9.0 mL of sterile water, and bacterial cells from the water were inoculated and purified on the YEM solid medium supplemented with 0.2% colloidal chitin at 30 °C. The isolation procedures are illustrated in Figure .
Chitinase activity in the culture supernatant
Isolated chitinolytic bacteria were grown in YEM medium containing 0.2% colloidal chitin for 3 days (30 °C, 150 rpm). Cells were separated by centrifugation (8000 × g, 5 min, 4 °C) and the supernatant was dialyzed against 20 mM sodium phosphate buffer (pH 6.0) overnight at 4 °C. The dialyzed protein solution was used for measuring chitinase activity and protein concentration. The chitinase activity assay was conducted in a reaction mixture (total volume, 600 μL) containing 0.1% colloidal chitin as the substrate and an appropriate volume of crude enzyme in 20 mM sodium phosphate buffer (pH 6.0). The reaction mixture was incubated for 15 min at 37 °C, and the amount of reducing sugars released in the reaction was then measured by a modified version of Schales’ procedure using N-acetyl-D-glucosamine (GlcNAc) as a standard [Citation34]. One unit of chitinase activity was defined as the amount of enzyme that released 1 μmol of reducing sugar per min.
Protein concentration in the culture supernatants was measured using a BCA Protein Assay Kit (Thermal Scientific, USA) with bovine serum albumin as a standard.
Quantitative biofilm assay
The biofilm formation of the isolates was estimated using a 96-well microtiter plate, in accordance with a previously described procedure [Citation35]. Overnight cultures of bacterial strains were inoculated at 1:100 in 200 μL of Luria-Bertani (LB) medium. Inoculated cultures were grown in the 96-well polystyrene microtiter plate for a further 24 h at 26 °C, without shaking. After cultivation, the unbound cells were removed by discarding the medium and rinsing the wells with water three times, after which the bound cells were stained with 1.0% (w/v) crystal violet for at least 1 min and then rinsed three times with water. Then, the dye was solubilized with 33% (v/v) acetic acid. Finally, the biofilm formation was quantified by measuring the absorbance at 630 nm using a microtiter plate reader (Model 680; Bio-Rad, USA).
PCR amplification, sequencing, and phylogenetic analysis of the 16S rRNA gene
Genomic DNA from an overnight culture of each isolate was extracted by boiling for 5 min, followed by centrifugation (13,000 rpm, 1 min, 4 °C) to remove debris and unbroken cells. The genomic DNA in the supernatant was used as a template for amplification by PCR. A nearly full-length segment of 16S rRNA gene nucleotides was amplified in a 50-μL reaction tube using universal primers, 27f-YM and 1492r (Table ), and a KOD-Plus-Neo Kit (Toyobo Co., Ltd., Osaka, Japan), in accordance with the manufacturer’s instructions. The reaction mixtures were incubated in an iCycler thermal cycler (Bio-Rad, USA) under a schedule consisting of predenaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 48 °C for 30 s, and extension at 68 °C for 1 min. The amplified products were then separated by electrophoresis on agarose (1.5%, w/v) gel. The target bands in the agarose gel were cut out and purified using a Wizard SV Gel and Clean-Up Kit (Promega Co., USA). Sequencing reactions were conducted in a CEQ8000 Genetic Analysis System (Beckman Coulter Inc., USA) by using a CEQ Dye Terminator Cycle Sequencing Kit (Beckman Coulter Inc., USA) based on the supplier’s instructions.
Table 1. Oligonucleotides used in this study.
The nucleotide sequences of the 16S rRNA genes obtained by the sequencing were compared with the known 16S rRNA gene sequences available in the DDBJ/Genbank/EMBL databases using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the taxonomic positions of the isolates. A phylogenetic tree was produced by using the MEGA version 6.0 software [Citation36] after multiple alignments of data by CLUSTAL W [Citation37]. The tree was constructed using the neighbor-joining method [Citation38], and evolutionary distances were computed using the Kimura two-parameter method [Citation39]. A bootstrap analysis (1000 replications) was carried out to evaluate the topology of the resulting tree [Citation40].
Chitinase production in culture supernatant and chitinase detection by SDS–PAGE and zymography
S. marcescens 2170 was used as a reference strain to compare the production of chitinases of the isolates because S. marcescens is well-known to be a high-chitinase-producing bacterium and is one of the most extensively studied chitinolytic bacteria [Citation14,41]. Each isolate was aerobically cultivated in YEM medium containing 0.5% chitin powder (Junsei Chemical Co., Tokyo, Japan) at 30 °C and 150 rpm. At each time point, a portion of the culture was sampled. After centrifugation (8000 × g, 5 min, 4 °C) to remove the cells and debris, the supernatant was dialyzed against 20 mM sodium phosphate buffer (pH 6.0) at 4 °C overnight. The chitinase activity and protein concentration of the dialyzed protein solution were measured and the protein solution was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and zymography.
SDS–PAGE was carried out as described by Laemmli [Citation42] using 12.5% polyacrylamide gels. Sample proteins were suspended in the loading buffer solution (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 5% β-mercaptoethanol, 10% glycerol, and 0.5% bromophenol blue), boiled for 3 min, and applied to SDS–PAGE analysis. The renaturation of the enzymes in polyacrylamide gels after SDS–PAGE and the detection of chitinase activity were performed as previously described [Citation15] using an agarose gel sheet containing 0.03% glycol chitin as the substrate.
Antifungal activity assay
The antifungal activity of the isolated bacteria was determined by measuring the inhibition of the growth of Trichoderma reesei IFO 31329 using a dual-culture plate assay, by a modified version of a previously described method [Citation20]. A single colony (at day 2 of incubation) of the SWCS-3.14 isolate was streaked in a straight line (2.0 cm in length) at a distance of 2.5 cm from the center of agar plates containing a 1:1 (v/v) ratio of potato dextrose agar (PDA) and YEM supplemented with 0.2% glycol chitin. The plates were incubated at 30 °C for 24 h to grow the bacteria. Then, each single bacterial colony (at day 2 of incubation) of the isolates and the reference strain was inoculated in a straight line as described above. After 24 h of incubation, a mycelial plug (0.5 cm in diameter) of T. reesei previously grown on the plate containing the same medium components was placed on the plate center. Plates without the inoculation of bacterial cells were employed as a control. The plates were then incubated at 30 °C for 3 days and the antagonistic activity of the isolates was evaluated by visual inspection.
Crude proteins prepared from a culture supernatant of the bacteria were also analyzed for their inhibition of the extension of T. reesei mycelia, via a modified version of a previously described procedure [Citation25]. The selected isolates were individually cultured in YEM medium containing 0.2% colloidal chitin for 3 days (30 °C, 150 rpm). After removing the cells and debris by centrifugation (9000 rpm, 4 °C, 20 min), ammonium sulfate was added to the supernatants to achieve 80% saturation. The precipitates formed were dialyzed in 20 mM sodium phosphate buffer (pH 6.0) at 4 °C overnight to remove low-molecular-mass substances such as antibiotics. Protein concentration and chitinase activity in the dialyzed sample were measured. A blank paper disk (8 mm in diameter; Toyo Roshi Kaisha, Ltd., Tokyo, Japan) was placed in the center of a PDA plate (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan), onto which 40 μL of fungal conidial suspension (2.5–5 × 105 conidia/mL) was then inoculated. After 24 h of incubation at 25 °C, wells (5.5 mm in diameter) were subsequently punched into the agar at a distance of 15 mm from the plate center. A solution containing 0.4 mg of protein was applied to a well. As a control, sterile water was added to the well. To confirm the inhibition of hyphal growth caused by the crude chitinases or other components, the protein solutions (0.4 mg of protein per well) were boiled for 10 min to inactivate all chitinases. Then, the protein solutions were applied to the wells as described above. Finally, the plates were incubated for 1–3 days at 25 °C and the inhibition of mycelial extension was determined by visual inspection.
Nucleotide sequence accession numbers
The sequences of the 16S rRNA gene of the 16 isolates determined in this study have been deposited in the DNA Data Bank of Japan (DDBJ) database under accession numbers LC326488–LC326503.
Chemicals
Colloidal chitin and glycol chitin were prepared from powdered chitin (code 038–13635) purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), following the methods described by Yamada & Imoto [Citation43] and Jeuniaux [Citation44], respectively.
Results
Isolation of chitinolytic bacteria
More than 5100 isolates formed clearing zones caused by colloidal chitin degradation by chitinases on the agar plates. Based on the size of the clearing zones and the morphological characteristics of their colonies, 31 isolates were selected for further examination. Most colonies of these isolates were circular or irregular, smooth or slotted, translucent, umbonal, and entire when grown on the plates containing colloidal chitin (Figure , Table S1). As shown in Table S1, seven bacteria isolated from sediments formed smaller clearing zones than the other isolates. Eleven strains, which were isolated from the chitin flakes after subculturing several times, showed large clearing zones on the colloidal chitin plate. In particular, among the 31 isolates, 13 bacteria isolated from the chitin flakes without subculturing formed larger clearing zones than the other strains.
Figure 2. The clearing zone formed by the isolates and references on the YEM agar plates containing colloidal chitin.
Chitinase activity and biofilm formation
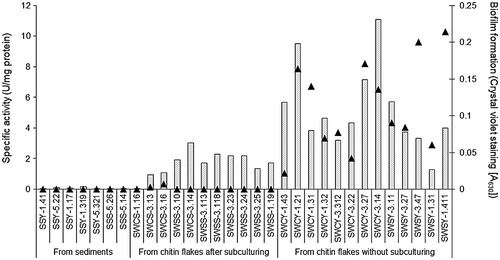
The chitinase activity in the culture supernatant when the isolates were grown in the presence of 0.2% colloidal chitin was examined, and biofilms formed in the polystyrene microtiter plate containing LB medium were quantified. As shown in Figure , all strains isolated from the sediments had low chitinase activity and did not form biofilms. The strains isolated from chitin flakes after subculturing several times showed high chitinase activity. However, these isolates exhibited slight or no biofilm formation. Interestingly, the chitinolytic bacteria isolated from the chitin flakes without subculturing exhibited higher chitinase activity than the other isolates, and all of them formed biofilms in the polystyrene microtiter plate containing LB medium. These isolates also formed larger clearing zones on colloidal chitin agar plates than the other isolates (Table S1). Based on these results, the 13 strains isolated from the chitin flakes without subculturing were selected for the next examination. In addition, three strains (SWCS-3.14, SWSS-3.23, and SWSS-3.24) isolated from the chitin flakes after subculturing were also chosen for further study because of their high chitinase activity among their group (Figure ). Although these three isolates did not form biofilms in the polystyrene microtiter plate containing LB medium, they formed biofilms in the plate containing YEM medium with 0.25% glucose (data not shown).
Figure 3. Chitinase activity and biofilm formation of the isolates.
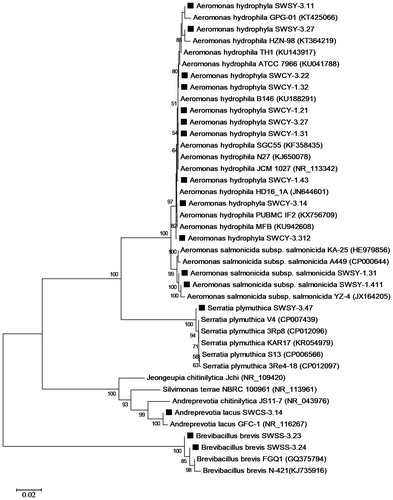
Phylogenetic analysis of the isolates
Sixteen isolates that showed high chitinase activity and biofilm formation were selected to determine the nucleotide sequence of nearly the entire length of the 16S rRNA gene. The results of phylogenetic analysis of the 16S rRNA gene of the isolates are shown in Table and Figure . Ten strains were estimated to be Aeromonas hydrophila; two strains to be Aeromonas salmonicida subsp. salmonicida, family Aeromonadaceae, class Gammaproteobacteria; and one strain to be Serratia plymuthica, family Enterobacteriaceae, class Gammaproteobacteria. These strains were isolated from the chitin flakes without subculturing. Among three strains isolated from the chitin flakes after subculturing, one strain were estimated to be Andreprevotia lacus, family Neisseriaceae, class Betaproteobacteria; and the other two isolates to be Brevibacillus brevis, family Paenibacillaceae, class Bacilli.
Table 2. Sequence analysis of partial 16S rRNA genes from the isolated strains.
Figure 4. Phylogenetic analysis of the isolates (filled rectangle) based on the 16S rRNA gene sequences.
Chitinase production in the culture supernatant and chitinase detection by SDS–PAGE and zymography
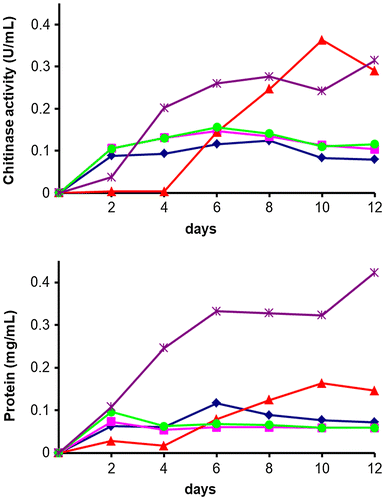
From the results of chitinase activity, biofilm formation, and phylogenetic analysis, four bacterial strains (SWCS-3.14, SWSY-3.47, SWSY-1.31, SWSY-1.411), which showed different characteristics for chitinase activity and biofilm formation, and were not or rare human pathogenic bacteria [Citation45,46], were selected and examined the production of chitinases in the YEM medium containing chitin powder. Of the four selected isolates, S. plymuthica SWSY-3.47 showed the highest chitinase activity, followed by two strains of A. salmonicida SWSY-1.411 and SWSY-1.31. A. lacus SWCS-3.14 showed the lowest chitinase activity (Figure ). However, these bacteria produced a low level of the overall protein in the culture medium compared with S. marcescens 2170. Hence, the strains showed higher specific activity of chitinases than S. marcescens 2170. S. marcescens 2170 was used as a reference strain to evaluate the level of production of chitinases; this bacterium is well-known as a high-chitinase-producing bacterium and is one of the most extensively studied chitinolytic bacteria [Citation14,41]. For example, at day 8 of cultivation, the specific activities of the chitinase produced by A. lacus SWCS-3.14, S. plymuthica SWSY-3.47, A. salmonicida SWSY-1.411, and SWSY-1.31 were 1.4, 2.0, 2.2, and 2.1 U/mg protein, respectively, while the specific activity of chitinases from the reference was 0.8 U/mg protein. This indicates that the four selected strains produce a considerable amount of chitinases as a proportion of total proteins in the presence of chitin.
Figure 5. Time course of chitinase production in the culture supernatant.
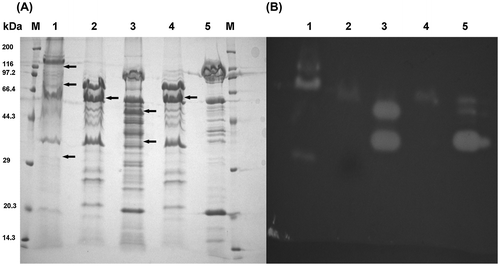
The production of chitinases was also detected in the agarose gel sheet containing glycol chitin after SDS–PAGE (Figure ). The zymography analysis (Figure (B)) showed that A. lacus SWCS-3.14 had at least three active chitinase bands, S. plymuthica SWSY-3.47 had at least two active chitinase bands, and the two strains of A. salmonicida, SWSY-1.411 and SWSY-1.31, had a single active chitinase band.
Figure 6. SDS–PAGE and zymography analyses of the chitinase production in the culture supernatant.
Antifungal activity
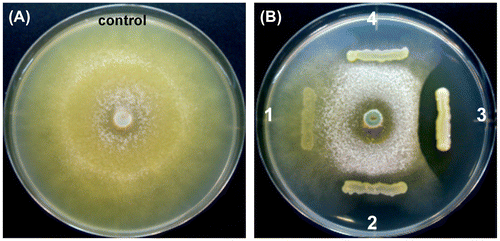
The antagonistic activity of the selected four strains against T. reesei was examined by using a dual-culture assay. As presented in Figure , S. plymuthica SWSY-3.47 formed the largest inhibition zone against T. reesei, implying the strongest antagonistic activity, followed by the two strains of A. salmonicida, SWSY-1.411 and SWSY-1.31. However, A. lacus SWCS-3.14 showed almost no activity. This indicates that three out of the four strains have antagonistic activity against T. reesei.
Figure 7. The antagonistic ability of the isolates against the hyphal growth of T. reesei using the dual-culture assay.
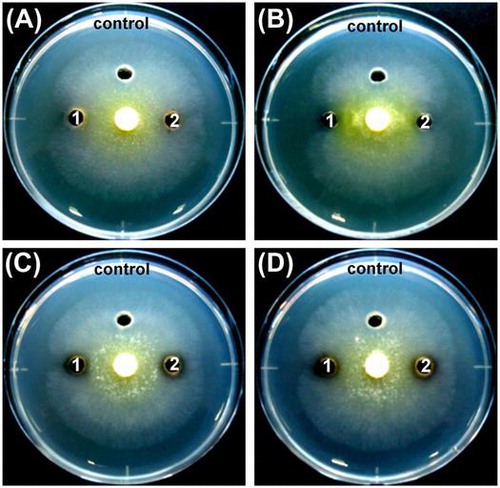
The crude proteins prepared from the culture supernatant of the selected four strains were tested for antifungal activity by inhibiting the mycelial extension of T. reesei. As shown in Figure and Table , crude proteins prepared from the strains clearly inhibited hyphal growth. Among these crude proteins, those from S. plymuthica SWSY-3.47 exhibited the strongest inhibition against T. reesei, followed by those from A. lacus SWCS-3.14, and then the two strains of A. salmonicida. In contrast, none of the boiled crude proteins after heat treatment at 100 °C for 10 min prepared from A. lacus SWCS-3.14, A. salmonicida SWSY-1.411, and SWSY-1.31 inhibited hyphal growth. Surprisingly, the boiled crude proteins prepared from S. plymuthica SWSY-3.47 still inhibited the growth of T. reesei (Figure (B), Table ). These results suggest that the crude proteins including chitinases from these strains have the ability to inhibit the growth of fungi.
Figure 8. The inhibition of hyphal growth of T. reesei treated by the crude proteins.
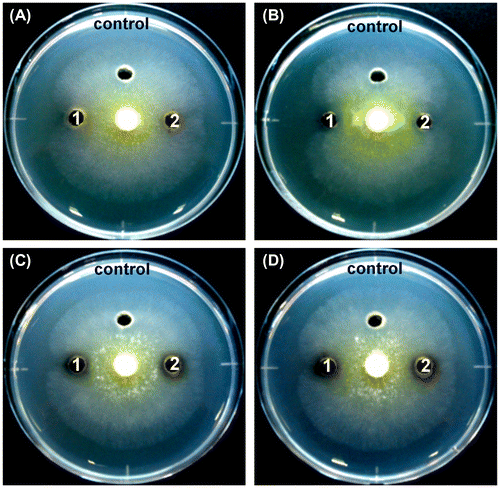
Table 3. The growth inhibition of T. reesei by using the crude proteins.
Discussion
The attachment of bacteria on the fungal surfaces is thought to be important for biocontrol [Citation29]. Bacteria that have an ability to form a biofilm can stably adhere to the fungal cell walls, grow and develop cell density, and secrete secondary metabolites as antifungal compounds. Moreover, the metabolites are covered by the biofilm, therefore those can be concentrated and then can enhance an antagonism against fungi compared with no biofilm-forming bacteria [Citation28,29]. Hence, biofilms may play a critical role for controlling phytopathogenic fungi [Citation28,47].
The aim of this study was to select chitinolytic bacteria that had high chitinase activity and also formed biofilms in order to develop a novel type of biological control agent. We chose a sand dune lake, Sakata, in Niigata, Japan, to isolate such bacteria. The water of this lake is mainly provided by groundwater running under the dunes. Sakata has only one small stream, along which the lake water flows into a river, which does not freeze in winter. It is a wintering site for waterfowl such as swans, geese, and ducks. Various aquatic species also live in the lake, such as shrimp, crabs, and fishes. Because no rivers flow into the lake, the chitin sources accumulated in Sakata are hardly altered by the action of flowing water, so different types of chitinolytic bacteria may remain at this site.
In this study, to obtain chitinolytic microorganisms from Sakata, the sediments were collected. Moreover, we assumed that a large number of chitinase-producing bacteria and biofilm-forming bacteria live in the lake. Therefore, chitin flakes were used and placed in the water to trap the biofilm-forming bacteria. We found that a large number of chitinolytic bacteria exhibiting high chitinase activity and biofilm formation were isolated from the chitin flakes, whereas the chitinolytic bacteria isolated from sediments showed poor chitinase activity and did not form biofilms (Figure ). Previously, Sato et al. [Citation33,48,49] reported the use of chitin flakes to collect chitinolytic organisms living in soil and freshwater; they found a novel bacterium, Chitiniphilus shinanonensis SAY3, from the moat water of Ueda Castle in Nagano Prefecture, Japan. Strain SAY3 produced one family 19 chitinase having antifungal activity [Citation6] and one family 18 chitinase reported as a novel endo-type chitinase [Citation50]. In this study, the use of chitin flakes were suggested to be useful to collect bacteria that have high chitinase activity and also form biofilms from freshwater environments.
To isolate bacteria that show high chitinase activity and form biofilm strongly attached to the chitin flakes, we hypothesized that subculturing step will be useful to screen such bacteria. However, the result clearly showed that the bacteria that displayed a higher specific activity and formed biofilms were obtained from the washed water without subculturing step (Figure ). A possible reason for this result may be attributed to differences in medium composition and pH, or inoculation of culture during the subculturing step. These conditions could affect bacterial population and enrichment in this step.
The cells of A. lacus SWCS-3.14 did not form biofilms in the polystyrene microtiter plate containing LB medium (Figure ). By our observations, strain SWCS-3.14 grew very slightly compared to the other strains in LB medium. For example, an optical density at 630 nm of the maximum growth in the LB medium of strain SWCS-3.14 was only 0.11, while that of the other strains, SWSY-1.31, SWSY-1.411, and SWSY-3.47, was 1.18, 1.11, and 0.65, respectively. Therefore, YEM medium containing 0.25% glucose was used to examine biofilm formation. In this case, A. lacus SWCS-3.14 exhibited better growth and formed biofilms in the microtiter plate. The absorbance at 630 nm to measure biofilm formation of this strain was 0.04 and those of the other strains, SWSY-1.31, SWSY-1.411, and SWSY-3.47, were 0.08, 0.13, and 0.08. This indicates that strain SWCS-3.14 was capable of forming a biofilm. On the other hand, A. lacus SWCS-3.14 secreted several chitinases into the medium in the presence of chitin powder, one of which was a high-molecular-weight (~121 kDa) protein as estimated by SDS–PAGE (Figure ). In contrast, the molecular weight of known bacterial chitinases commonly ranges from 20 to 60 kDa [Citation21]. Only a limited number of prokaryotic chitinases with a high molecular weight have been reported so far from only a few chitinolytic bacteria. For example, Howard et al. [Citation12] revealed that Saccharophagus degradans 2-40 (formerly Microbulbifer degradans 2-40) possesses chitinase B with a molecular mass of 136.1 kDa, while Itoh et al. [Citation51,52] found a high-molecular-mass chitinase, ChiW (150 kDa), from Paenibacillus sp. strain FPU-7. Moreover, Tanaka et al. [Citation13] demonstrated that Thermococcus kodakaraensis KOD1 (previously reported as Pyrococcus kodakaraensis KOD1) possesses a 134-kDa chitinase, ChiA. These chitinases contain two catalytic domains belonging to the family 18 chitinases and various other domains, leading to diverse chitin degradation. At present, only one study on A. lacus GFC-1 has been reported, which just focused on the taxonomy [Citation53], and information on its genome, chitinase genes, as well as chitinase properties has not been clarified yet. It is thus important to characterize chitinases and biofilm formation in A. lacus SWCS-3.14.
The antifungal activity of A. lacus SWCS-3.14 was examined using its cells and crude proteins. Although the crude proteins distinctly inhibited the hyphal growth of T. reesei (Figure (A)), the cells did not suppress mycelial extension (Figure (B)). The antifungal activity of crude proteins could be due to other components such as antifungal proteins and/or antifungal antibiotics other than chitinases, that remained in the crude protein fraction after dialysis. However, such antifungal proteins and antifungal antibiotics have not been reported yet from this bacterium. In addition, our observations revealed that this isolate grew poorly and slowly on solid media such as YEM, synthetic medium, tryptone soya broth, nutrient broth, and LB. This strain exhibited better growth on media containing glucose, colloidal chitin, or glycol chitin, but the growth of the strain was still slower than that of the other isolates (data not shown). These observations imply that A. lacus SWCS-3.14 cells could not inhibit T. reesei, as shown in Figure (B), possibly due to weak growth, leading to only a tiny amount of chitinase being secreted into the agar medium, which was insufficient to inhibit hyphal extension.
The S. plymuthica species was reported to be a biological control agent of various phytopathogenic fungi [Citation16,23,54]. The antifungal activity of this species involves multiple factors including chitinase, antifungal antibiotics, and siderophores [Citation18,26]. Liu et al. reported that S. plymuthica HRO-C48 produced pyrrolnitrin that suppresses the growth of several fungal plant pathogens, and the production requires quorum-sensing signaling [Citation55]. In addition, two chitinolytic enzymes, CHIT60 and CHIT100, produced by the HRO-C48 showed an inhibitory effect on spore germination and germ tube elongation of Botrytis cinerea [Citation56]. One member of the S. plymuthica species, strain G3, is capable of forming biofilms, which is controlled by a quorum sensing system [Citation57]. The presence of a biofilm was reported to increase the survival of strain G3 under stress conditions [Citation58]. In the current study, S. plymuthica SWSY-3.47 exhibited significant antifungal activity against T. reesei by crude chitinases as well as its cells (Figures and ). This isolate also formed biofilms at a high level in the polystyrene microtiter plate containing LB medium (Figure ). In contrast, the protein solutions containing heat-inactivated chitinases still suppressed the mycelial extension with a lower inhibition zone compared with the untreated proteins (Figure (B)), although the chitinases in the boiled protein solutions did not show any hydrolytic activity, as examined by the Schales’ procedure (Table ). Thus, the inhibition must be caused by a heat-stable substance(s) produced by this bacterium. This substance(s) could be antifungal antibiotics. A large number of reports indicated that S. plymuthica produces a broad range of antibiotics that inhibit various plant-pathogenic fungi [Citation22,55,59]. Further studies are needed to identify and characterize this substance(s) concerning biocontrol.
In our isolates, a part of the nucleotide sequence of the 16S rRNA gene from two strains, A. salmonicida SWSY-1.411 and SWSY-1.31, showed the highest similarity (100%) to that of A. salmonicida subsp. salmonicida A449 (CP000644), and the partial 16S rRNA gene nucleotides of S. plymuthica SWSY-3.47 showed the highest similarity (99–100%) to those of S. plymuthica 3Re4-18 (CP012097), 3Rp8 (CP012096), S13 (CP006566), V4 (CP007439), 4Rx13 (CP006250), AS9 (CP002773), and PRI-2C (CP015613). In the CAZy database, these strains possess a number of GH18 chitinases, GH19 chitinases, and auxiliary activities family 10 (AA10) proteins. However, most these chitinases and AA10 proteins have not been characterized yet. Hence, cloning of the genes and characterization of the chitinases and AA10 proteins of the isolated bacteria, A. salmonicida SWSY-1.411 and SWSY-1.31 and S. plymuthica SWSY-3.47, are very important in next study.
In conclusion, the four chitinolytic bacteria isolated from chitin flakes placed into the lake water have potential for development as biocontrol agents. The strains show high chitinase activity, form biofilms, and have antifungal activity against T. reesei. In addition, A. lacus SWCS-3.14 possesses a high-molecular-weight chitinase, and S. plymuthica SWSY-3.47 produces not only chitinases but also a heat-stable substance(s) that inhibits the hyphal growth of T. reesei. We are planning to identify and characterize the chitinase molecules by applying gene cloning, and to clarify the relationship between chitinase activity and biofilm formation for the development of biocontrol agents.
Author contributions
K. Suzuki conceived the study. D.M. Tran and K. Suzuki designed, performed the experiments, analyzed the data, and wrote the manuscript. H. Sugimoto, D.A. Nguyen, and T. Watanabe involved in interpretation of the data and critical reading of the draft. All authors reviewed and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
The supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2017.1422969
Supplementary-Tran.doc
Download MS Word (80.5 KB)Acknowledgments
D. M. Tran is a Ph.D. student under a Japanese Government (Monbukagakusho: MEXT) scholarship. The authors wish to thank Sakata Waterfowl and Wetland Center, Niigata, Japan for generous assistance in the sample collection. We would also like to thank Dr. Md Muzahid E Rahman (Bangladesh Agriculture Research Institute, Bangladesh) for his guidance on the use of MEGA 6.0 software.
References
- Yu C, Bassler BL, Roseman S. Chemotaxis of the marine bacterium Vibrio furnissii to sugars. A potential mechanism for initiating the chitin catabolic cascade. J Biol Chem. 1993;268:9405–9409.
- Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1991;280:309–316.10.1042/bj2800309
- Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1993;293:781–788.10.1042/bj2930781
- García-Fraga B, Silva FA, López-Seijas J, et al. A novel family 19 chitinase from the marine-derived Pseudoalteromonas tunicata CCUG 44952T: heterzologous expression, characterization and antifungal activity. Biochem Eng J. 2015;93:84–93.10.1016/j.bej.2014.09.014
- Hoell IA, Dalhus B, Heggset EB, et al. Crystal structure and enzymatic properties of a bacterial family 19 chitinase reveal differences from plant enzymes. FEBS J. 2006;273:4889–4900.10.1111/ejb.2006.273.issue-21
- Huang L, Garbulewska E, Sato K, et al. Isolation of genes coding for chitin-degrading enzymes in the novel chitinolytic bacterium, Chitiniphilus shinanonensis, and characterization of a gene coding for a family 19 chitinase. J Biosci Bioeng. 2012;113:293–299.10.1016/j.jbiosc.2011.10.018
- Kong H, Shimosaka M, Ando Y, et al. Species-specific distribution of a modular family 19 chitinase gene in Burkholderia gladioli. FEMS Microbiol Ecol. 2001;37:135–141.10.1111/fem.2001.37.issue-2
- Ohno T, Armand S, Hata T, et al. A modular family 19 chitinase found in the prokaryotic organism Streptomyces griseus HUT 6037. J Bacteriol. 1996;178:5065–5070.10.1128/jb.178.17.5065-5070.1996
- Tsujibo H, Okamoto T, Hatano N, et al. Family 19 chitinases from Streptomyces thermoviolaceus OPC-520: molecular cloning and characterization. Biosci Biotech Biochem. 2000;64:2445–2453.10.1271/bbb.64.2445
- Ueda M, Kojima M, Yoshikawa T, et al. A novel type of family 19 chitinase from Aeromonas sp. No.10S-24. Cloning, sequence, expression, and the enzymatic properties. Eur J Biochem. 2003;270:2513–2520.10.1046/j.1432-1033.2003.03624.x
- Hashimoto M, Ikegami T, Seino S, et al. Expression and characterization of the chitin-binding domain of chitinase A1 from Bacillus circulans WL-12. J Bacteriol. 2000;182:3045–3054.10.1128/JB.182.11.3045-3054.2000
- Howard MB, Ekborg NA, Taylor LE 2nd, et al. Chitinase B of “Microbulbifer degradans” 2-40 contains two catalytic domains with different chitinolytic activities. J Bacteriol. 2004;186:1297–1303.10.1128/JB.186.5.1297-1303.2004
- Tanaka T, Fujiwara S, Nishikori S, et al. A unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic archaeon Pyrococcus kodakaraensis KOD1. Appl Environ Microbiol. 1999;65:5338–5344.
- Watanabe T, Kimura K, Sumiya T, et al. Genetic analysis of the chitinase system of Serratia marcescens 2170. J Bacteriol. 1997;179:7111–7117.10.1128/jb.179.22.7111-7117.1997
- Watanabe T, Oyanagi W, Suzuki K, et al. Chitinase system of Bacillus circulans WL-12 and importance of chitinase Al in chitin degradation. J Bacteriol. 1990;172:4017–4022.10.1128/jb.172.7.4017-4022.1990
- Chernin L, Ismailov Z, Haran S, et al. Chitinolytic Enterobacter agglomerans antagonistic to fungal plant pathogens. Appl Environ Microbiol. 1995;61:1720–1726.
- Itoh Y, Watanabe J, Fukada H, et al. Importance of Trp59 and Trp60 in chitin-binding, hydrolytic, and antifungal activities of Streptomyces griseus chitinase C. Appl Microbiol Biotechnol. 2006;72:1176–1184.10.1007/s00253-006-0405-7
- Kamensky M, Ovadis M, Chet I, et al. Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biol Biochem. 2003;35:323–331.10.1016/S0038-0717(02)00283-3
- Prasanna L, Eijsink VG, Meadow R, et al. A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl Microbiol Biotechnol. 2013;97:1601–1611.10.1007/s00253-012-4019-y
- Rahman MME, Hossain DM, Suzuki K, et al. Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. Aust. Plant Pathol. 2016;45:103–117.
- Bhattacharya D, Nagpure A, Gupta RK. Bacterial chitinase: properties and potential. Crit Rev Biotechnol. 2007;27:21–28.10.1080/07388550601168223
- Meziane H, Gavriel S, Ismailov Z, et al. Control of green and blue mould on orange fruit by Serratia plymuthica strains IC14 and IC1270 and putative modes of action. Postharvest Biol Technol. 2006;39:125–133.10.1016/j.postharvbio.2005.10.007
- Kurze S, Bahl H, Dahl R, et al. Biological control of fungal strawberry diseases by Serratia plymuthica HRO-C48. Plant Dis. 2001;85:529–534.10.1094/PDIS.2001.85.5.529
- Kawase T, Yokokawa S, Saito A, et al. Comparison of enzymatic and antifungal properties between family 18 and 19 chitinases from S. coelicolor A3(2). Biosci Biotechnol Biochem. 2006;70:988–998.10.1271/bbb.70.988
- Watanabe T, Kanai R, Kawase T, et al. Family 19 chitinases of Streptomyces species: characterization and distribution. Microbiology. 1999;145:3353–3363.10.1099/00221287-145-12-3353
- Chernin LS, De la Fuente L, Sobolev V, et al. Molecular cloning, structural analysis, and expression in Escherichia coli of a chitinase gene from Enterobacter agglomerans. Appl Environ Microbiol. 1997;63:834–839.
- Singh R, Paul D, Jain RK. Biofilms: implications in bioremediation. Trends Microbiol. 2006;14:389–397.10.1016/j.tim.2006.07.001
- Kjelleberg S, Givskov M. The biofilm mode of life: mechanisms and adaptations. Wymondham: Horizon Bioscience; 2007.
- Seneviratne G, Zavahir JS, Bandara WMMS, et al. Fungal-bacterial biofilms: their development for novel biotechnological applications. World J Microbiol Biotechnol. 2008;24:739–743.10.1007/s11274-007-9539-8
- Hogan DA, Kolter R. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science. 2002;296:2229–2232.10.1126/science.1070784
- Brandl MT, Carter MQ, Parker CT, et al. Salmonella Biofilm formation on Aspergillus niger involves cellulose – chitin interactions. PLoS One. 2011;6:e25553.10.1371/journal.pone.0025553
- Hover T, Maya T, Ron S, et al. Mechanisms of bacterial (Serratia marcescens) attachment to, migration along, and killing of fungal hyphae. Appl Environ Microbiol. 2016;82:2585–2594.10.1128/AEM.04070-15
- Sato K, Kato Y, Taguchi G, et al. Chitiniphilus shinanonensis gen. nov., sp. nov., a novel chitin-degrading bacterium belonging to Betaproteobacteria. J Gen Appl Microbiol. 2009;55:147–153.10.2323/jgam.55.147
- Imoto T, Yagishita K. A simple activity measurement of lysozyme. Agric Biol Chem. 1971;35:1154–1156.10.1080/00021369.1971.10860050
- Jackson DW, Suzuki K, Oakford L, et al. Biofilm formation and dispersal under the influence of the global regulator CsrA of escherichia coli. J Bacteriol. 2002;184:290–301.10.1128/JB.184.1.290-301.2002
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.10.1093/molbev/mst197
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948.10.1093/bioinformatics/btm404
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120.10.1007/BF01731581
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791.10.1111/j.1558-5646.1985.tb00420.x
- Vaaje-Kolstad G, Horn SJ, Sørlie M, et al. The chitinolytic machinery of Serratia marcescens – a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J. 2013;280:3028–3049.10.1111/febs.12181
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Yamada H, Imoto T. A convenient synthesis of glycolchitin, a substrate of lysozyme. Carbohydr Res. 1981;92:160–162.10.1016/S0008-6215(00)85993-5
- Jeuniaux C. Chitinase. Methods Enzymol. 1966;8:644–650.10.1016/0076-6879(66)08117-5
- Carrero P, Garrote JA, Pacheco S, et al. Report of six cases of human infection by Serratia plymuthica. J Clin Microbiol. 1995;33:275–276.
- Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24:755–791.10.1128/CMR.00017-11
- Morikawa M. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J Biosci Bioeng. 2006;101:1–8.10.1263/jbb.101.1
- Sato K, Azama Y, Nogawa M, et al. Analysis of a change in bacterial community in different environments with addition of chitin or chitosan. J Biosci Bioeng. 2010;109:472–478.10.1016/j.jbiosc.2009.10.021
- Sato K, Kato Y, Fukamachi A, et al. Construction and analysis of a bacterial community exhibiting strong chitinolytic activity. Biosci Biotechnol Biochem. 2010;74:636–640.10.1271/bbb.90856
- Huang L, Shizume A, Nogawa M, et al. Heterologous expression and functional characterization of a novel chitinase from the chitinolytic bacterium chitiniphilus shinanonensis. Biosci Biotechnol Biochem. 2012;76:517–522.10.1271/bbb.110822
- Itoh T, Hibi T, Fujii Y, et al. Cooperative degradation of chitin by extracellular and cell surface-expressed chitinases from Paenibacillus sp. strain FPU-7. Appl Environ Microbiol. 2013;79:7482–7490.10.1128/AEM.02483-13
- Itoh T, Sugimoto I, Hibi T, et al. Overexpression, purification, and characterization of Paenibacillus cell surface-expressed chitinase ChiW with two catalytic domains. Biosci Biotechnol Biochem. 2014;78:624–634.10.1080/09168451.2014.891935
- Sheu SY, Chiu TF, Chou JH, et al. Andreprevotia lacus sp. nov., isolated from a fish-culture pond. Int J Syst Evol Microbiol. 2009;59:2482–2485.10.1099/ijs.0.009233-0
- Liu X, Jia J, Atkinson S, et al. Biocontrol potential of an endophytic Serratia sp. G3 and its mode of action. World J Microbiol Biotechnol. 2010;26:1465–1471.10.1007/s11274-010-0321-y
- Liu X, Bimerew M, Ma Y, et al. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiol Lett. 2007;270:299–305.10.1111/fml.2007.270.issue-2
- Frankowski J, Lorito M, Scala F, et al. Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch Microbiol. 2001;176:421–426.10.1007/s002030100347
- Liu X, Jia J, Popat R, et al. Characterisation of two quorum sensing systems in the endophytic Serratia plymuthica strain G3: differential control of motility and biofilm formation according to life-style. BMC Microbiol. 2011;11:26.10.1186/1471-2180-11-26
- Liu X, Wu Y, Chen Y, et al. RpoS differentially affects the general stress response and biofilm formation in the endophytic Serratia plymuthica G3. Res Microbiol. 2016;167:168–177.10.1016/j.resmic.2015.11.003
- Levenfors JJ, Hedman R, Thaning C, et al. Broad-spectrum antifungal metabolites produced by the soil bacterium Serratia plymuthica A 153. Soil Biol Biochem. 2004;36:4677–4685.
- Frank JA, Reich CI, Sharma S, et al. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74:2461–2470.10.1128/AEM.02272-07
- Youssef N, Sheik CS, Krumholz LR, et al. Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16S rRNA gene-based environmental surveys. Appl Environ Microbiol. 2009;75:5227–5236.10.1128/AEM.00592-09