Abstract
Filamentous fungi are used to produce fermented foods, organic acids, beneficial secondary metabolites and various enzymes. During such processes, these fungi balance cellular NAD+:NADH ratios to adapt to environmental redox stimuli. Cellular NAD(H) status in fungal cells is a trigger of changes in metabolic pathways including those of glycolysis, fermentation, and the production of organic acids, amino acids and secondary metabolites. Under hypoxic conditions, high NADH:NAD+ ratios lead to the inactivation of various dehydrogenases, and the metabolic flow involving NAD+ is down-regulated compared with normoxic conditions. This review provides an overview of the metabolic mechanisms of filamentous fungi under hypoxic conditions that alter the cellular NADH:NAD+ balance. We also discuss the relationship between the intracellular redox balance (NAD/NADH ratio) and the production of beneficial secondary metabolites that arise from repressing the HDAC activity of sirtuin A via Nudix hydrolase A (NdxA)-dependent NAD+ degradation.
Fungal metabolic changes in the pathways such as glycolysis, fermentation, and the production of organic acids, amino acids and secondary metabolites are induced by environmental redox stimuli.
Filamentous fungi are used to produce fermented foods, organic acids, beneficial secondary metabolites and various enzymes such as amylases, proteases, cellulases and β-1,4-mannanases [Citation1–6]. The filamentous fungus Aspergillus oryzae (koji mold) has served for over 1000 years in the production of traditional foods such as sake (rice wine), shoyu (soy sauce), miso and other fermented foods in Japan and elsewhere around the world. The Food and Drug Administration in the USA lists Koji mold as a “generally recognized as safe” (GRAS) species and the World Health Organization has also confirmed its safety [Citation5,6]. Aspergillus oryzae can produce large quantities of enzymes and beneficial secondary metabolites, such as kojic acid and WYK-1 [Citation7,8]. Filamentous fungi can also produce secondary metabolites, including pharmacologically important antibiotics and lethal mycotoxins. The Ascomycetes genus Aspergillus includes many strains that produce secondary metabolites and the opportunistic pathogen Aspergillus fumigatus produces those that are associated with its virulence [Citation9]. Aspergilli also produce the antibiotic penicillin G as well as toxic and carcinogenic aflatoxin and its derivatives, which are related to agricultural contaminant mycotoxins [Citation10,11]. Mycotoxins produced by filamentous fungi are serious pollutants of stored cereals, and they are harmful to human health if consumed.
The importance of filamentous fungi to the food, biochemical and pharmaceutical industries has long been realized, but relationships between energy conservation and the intracellular redox balance (NAD/NADH ratio) during the growth of fungal cells and their production of organic acids and secondary metabolites are not fully understood.
Oxygen (O2) serves as a substrate for the biosynthesis of essential compounds such as sterols, unsaturated fatty acids and heme [Citation12]. It is also required for a mitochondrial O2 respiration mechanism that conserves energy for growth. Hence, respiration generates reactive oxygen species (ROS) such as singlet oxygen, peroxide, superoxide and hydroxyl radicals that cause oxidative damage to cells. Filamentous fungi possess proteins that protect against oxidative damage, and fungal cells adapt to oxidative stress by producing antioxidants such as superoxide dismutase, thioredoxin (Trx), reduced glutathione (GSH), and their reductases. Thioredoxin is the most likely candidate producer of GSH because the combination of NADPH, Trx reductase (TR) and Trx reduces oxidized GSSG in fungi [Citation13]. Reduced glutathione and Trx reductases comprise a protein containing FAD that reduces GSSG and Trx to GSH and reduced Trx using NAD(P)H as an electron donor [Citation14]. These facts imply that the reduction of NAD+ to NADH is important under oxidative stress.
Hypoxia (O2 depletion) imposes a challenge on most eukaryotes and induces several adaptation mechanisms to ensure survival. Such mechanisms include a metabolic shift from oxidative phosphorylation to fermentation, which contributes to NADH re-oxidation and ATP production, along with decreased translation and cell growth [Citation15–17]. Environments with limited O2 availability impose defective electron acceptors on eukaryotic mitochondrial respiration. Under such conditions, alcohol fermentation is significant in providing an alternative electron acceptor (pyruvate) to re-oxidize NADH to NAD+, and in supporting the growth of the ascomycetes Saccharomyces cerevisiae, Schizosaccharomyces pombe and Candida albicans [Citation18–20]. This accompanies alterations in global transcription [Citation21–23] and cellular proteins [Citation24,25] for energy conservation and for the biosynthesis of cellular components. For example, S. cerevisiae down-regulates the genes for respiratory complexes and the tricarboxylic acid (TCA) cycle, and up-regulates those for glycolysis and ethanol production in response to anoxia (O2 depletion) [Citation24]. Oxygen sensing and the regulation of O2-responsive genes by heme and sterols in yeast have been reported [Citation26–28]. The filamentous fungus Aspergillus nidulans belongs to the same phylum as these yeasts, and uses nitrate as an alternative electron acceptor to O2 under O2-limited conditions [Citation29,30]. The fungus Fusarium oxysporum reduces nitrate to ammonium under hypoxic conditions and simultaneously oxidizes ethanol to acetate. This reaction generates ATP through substrate-level phosphorylation, and is referred to as ammonia fermentation [Citation31,32]. Takasaki et al. found that the fungus Aspergillus nidulans produces a similar reaction and identified nitrate and nitrite reductases encoded by niaD and niiA, as the enzymes responsible for ammonia production [Citation29,33]. Takasaki et al. also found that the acetogenic reaction is catalyzed by alcohol dehydrogenase, coenzyme A (CoA)-acylating aldehyde dehydrogenase and acetate kinase (Ack). The last step of the reaction catalyzed by Ack produces ATP. The production of Ack requires a functional facA gene encoding acetyl-CoA synthetase (Acs) that is widely conserved in prokaryotes and eukaryotes [Citation29]. Global changes in protein production and transcription profiles for hypoxic adaptation occur in Aspergillus nidulans when cultured under O2-limitation [Citation34].
Both NAD(P)+ and NAD(P)H function as hydride-transferring co-enzymes of cellular dehydrogenases. Organisms balance cellular NADH:NAD+ ratios to adapt to environmental redox stimuli. Under hypoxic conditions, high NADH:NAD+ ratios lead to the inactivation of various dehydrogenases, and metabolic flow using NAD+ is more down-regulated compared with normoxic conditions. This review provides an overview of current knowledge regarding fungal NAD(H) homeostasis as well as NAD(H) generation and degradation. We also discuss the relationship between the intracellular redox balance (NAD/NADH ratio) under hypoxia and the production of organic acids, amino acids, and beneficial secondary metabolites by filamentous fungi.
Adaptation to hypoxia by fungal denitrification and ammonia fermentation
Some bacteria and filamentous fungi reduce nitrate () through four steps to generate nitrite (
), nitric oxide (NO), and nitrous oxide (N2O) as intermediates, and finally evolve nitrogen gas (N2) under hypoxia [Citation35]. This process is called denitrification, and the mechanism has been characterized (Figure ). In the filamentous fungi Fusarium oxysporum and Aspergillus nidulans,
reductase (Nar),
reductase (Nir), NO reductase (Nor), and N2O reductase (Nos) are involved in steps that are coupled with the oxidation of electron donors such as NAD(P)H and/or ubiquinol, and ATP production. Fusarium oxysporum possesses Nir activity that is dependent upon ubiquinol as an electron shuttling compound in the mitochondrial respiratory chain. Some filamentous fungi reduce
for nitrogen assimilation. The first step of this process is the reduction of
to
, which is catalyzed by NAD(P)H-dependent Nar (NAD(P)H-Nar). The
is subsequently reduced by NAD(P)H-dependent Nir (NAD(P)H-Nir) to produce ammonium (
), which is incorporated into amino acids and other nitrogenous compounds as described below. Both NAD(P)H-Nar (niaD) and NAD(P)H-Nir (niiA) are conserved over a wide variety of filamentous fungi [Citation36]. These also indicate that filamentous fungi use NAD(P)H-Nar and ubiquinol-dependent Nar (ubiquinol-Nar) for denitrification (Figure ). The second step of denitrification is the reduction of
to NO that is catalyzed by Nir. The mitochondrial copper-containing
reductase, NirK, is responsible for fungal denitrification in F. oxysporum and A. oryzae [Citation37–39].
Figure 1. Schematic representation of fungal denitrification and ammonia fermentation.
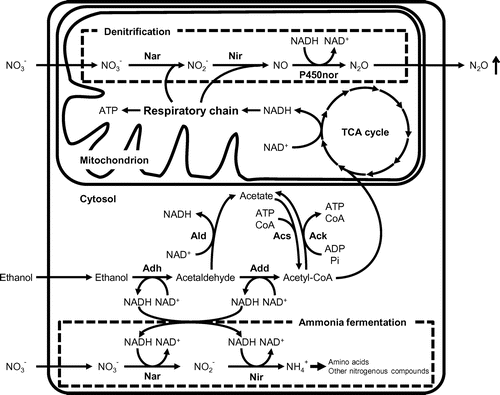
Among the enzymes involved in denitrification, NO reductases from F. oxysporum and A. oryzae have been characterized in detail. While bacterial Nor is associated with cytochrome bc, fungal Nor belongs to the cytochrome P450 superfamily (CYP55), namely P450nor [Citation40]. Typical proteins belonging to this superfamily are monooxygenases that catalyze carbon-atom hydroxylation using O2 as substrate, and require electron donor proteins such as cytochrome P450 reductase and cytochrome b5. However, the reaction of P450nor is NO reduction, which does not require additional proteinous components. Takaya et al. constructed an F. oxysporum strain with a deleted chromosomal gene for P450nor, and the strain accumulated NO in the culture medium and produced less N2O than the wild-type strain [Citation16]. These findings indicate that P450nor is essential for fungal denitrification.
Ammonia fermentation is another hypoxic process through which some fungi reduce nitrate () to ammonia (
) and simultaneously oxidize ethanol to acetate (Figure ). This phenomenon differs from denitrification in that
production does not accompany N2O production under hypoxia, and the mechanism has been characterized (Figure ). The first step of
assimilation is the reduction of
to
, which is catalyzed by NAD(P)H-Nar.
is subsequently reduced by NAD(P)H-Nir to generate ammonium (
), which is incorporated into glutamate and other nitrogenous compounds. Both enzymes reside in the cytosol and are distinct from the denitrifying types [Citation14] NAD(P)H-Nar and NAD(P)H-Nir are conserved across a wide variety of filamentous fungi. Aspergillus nidulans encodes niaD and niiA, which encode NAD(P)H-Nar and NAD(P)H-Nir, which are the enzymes responsible for ammonia production [Citation29,33]. The acetogenic reaction catalyzed by alcohol dehydrogenase (Adh), coenzyme A (CoA)-acylating aldehyde dehydrogenase (Add) and acetate kinase (Ack) cooperates with the reduction of
to
(Figure ). Ack uses ADP and acetyl-CoA as substrates to form ATP and acetate. Both Add and Ack activities are specifically induced only in hypoxic cells that ferment ammonia, indicating that ethanol is oxidized to acetate by Adh, Add and Ack coupled with ATP production, and the reduction of
to
(Figure ). The activity of Ack requires a functional facA gene that encodes acetyl-CoA synthetase, which is widely conserved in both prokaryotes and eukaryotes [Citation29]. Environments with limited O2 availability impose defective electron acceptors [on] eukaryotic mitochondrial respiration. Mechanisms such as denitrification and ammonia fermentation include a metabolic shift from oxidative phosphorylation to fermentation, and contribute to NAD(P)H re-oxidation and ATP production under hypoxia [Citation15–17].
Branched-chain amino acid fermentation and thiamine synthesis under hypoxia
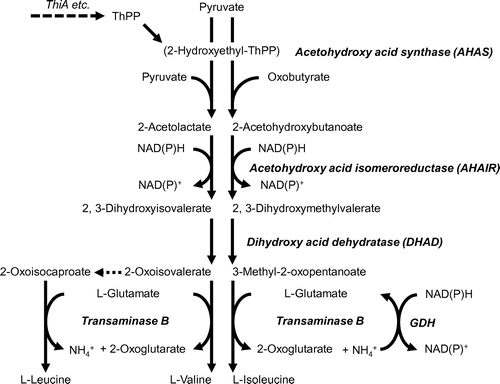
The limited electron acceptor (oxygen) availability afforded under hypoxic conditions causes cellular NADH accumulation. To adapt to such circumstances, A. nidulans activates the production of branched-chain amino acids (BCAA) [Citation41] as well as nitrate reduction, and alcohol and lactate production; these processes couple with re-oxidation from NAD(P)H to NAD(P)+ and support catabolic mechanisms. Metabolic disorders of essential BCAA can cause acidosis [Citation42]. Eukaryotic and prokaryotic microbes metabolize BCAA as carbon, nitrogen and energy sources. An evolutionarily conserved mechanism synthesizes BCAA from pyruvate in microbes via a pathway comprising four reactions catalyzed by acetohydroxy acid synthase (AHAS), which comprises the large catalytic subunit of acetohydroxy acid synthase (AHAS-L), the small subunit of acetohydroxy acid synthase (AHAS-S), acetohydroxy acid isomeroreductase (AHAIR), dihydroxy acid dehydratase (DHAD) and transaminase B (Figure ). These enzymes catalyze the biosynthesis of l-Ile from pyruvate and 2-oxobutyrate. An intermediate of l-Val synthesis, 2-oxovalerate, is a precursor of l-Leu synthesis. Although BCAA are synthesized as building blocks for proteins, A. nidulans cells incubated under hypoxic conditions do not use all BCAA synthesized de novo for building proteins but rather excrete a portion of them into the culture medium. The synthesis of BCAA de novo is up-regulated under hypoxia, which is consistent with their accumulation under such conditions. Disruptants of the genes for AHAS-S and AHAS-L (∆AHS-S and ∆AHS-L, respectively) that are involved in the initial step of BCAA synthesis respectively accumulate only 30% to 35% of BCAA compared with WT strain, and an amount that is below detection limits, respectively, under O2-limitation. Both AHAS-L and AHAS-S are involved in BCAA production under O2-limited conditions. The NAD(P)H:NADP+ ratios of hypoxic ∆AHS-L and ∆AHS-S cells were higher than that of WT. The extent of the increase in the ratio of NAD(P)H:NAD(P)+ corresponded to intracellular levels of AHAS activity. Thus, AHAS contributes to the re-oxidization of intracellular NAD(P)H to NAD(P)+ under hypoxic conditions. The mechanism of hypoxic BCAA synthesis re-oxidizes NAD(P)H and functions as an electron sink through which cells prevent an unbalanced NAD(P)H:NAD(P)+ ratio that in turn impairs cellular metabolism (Figure ).
Figure 2. Pathway for branched-chain amino acid synthesis.
Aspergillus nidulans possesses NADPH-glutamate dehydrogenase (GDH) that generates Glu from 2-oxoglutarate and ammonium (Figure ). This process is physiologically as significant as ammonia assimilation in fungi [Citation43]. Expression of the gene encoding GDH (gdhA) is up-regulated and intracellular GDH activity is 2.5-fold higher under hypoxic, than normoxic conditions. Compared with the WT, the gene disruptant of gdhA (∆GDH strain) accumulates 65% of Glu in the culture medium after 12 h of incubation under hypoxia. Comparable amounts of Ala, Thr, Pro, Asp and Phe are produced by hypoxic WT and ∆GDH, whereas about 30% of the BCAA produced by ∆GDH are detected in culture medium. The NAD(P)H:NAD(P)+ ratio of the ∆GDH strain is higher under hypoxic conditions than that of the WT. Under hypoxic conditions, GDH supplies Glu for BCAA production, oxidizes NADPH and contributes to maintaining the NAD(P)H:NAD(P)+ ratio (Figure ). Maintenance of the redox balance by the coordinated activity of Ala transaminase and GDH in fermenting Pyrococcus furiosus has been proposed [Citation44]. The Ala transaminase of higher plants participates in NAD+ regeneration under hypoxia, although this is dependent on Glu synthase instead of GDH [Citation45].
Thiamine (vitamin B1) is essential for all living systems, and it exists in free and phosphoester forms [Citation46–48]. Thiamine pyrophosphate (TPP) is a cofactor for carbohydrate and amino acid metabolic enzymes. Microorganisms that synthesize TPP produce thiazole and pyrimidine moieties that couple to form thiamine monophosphate [Citation49–51]. Bacterial ThiG initiates thiazole (5-(2-hydroxyethyl)-4-methylthiazole (HET) phosphate) synthesis via the oxidative condensation of 1-deoxy-D-xylulose-5-phosphate, glycine (or tyrosine) and cysteine [Citation49]. In contrast, S. cerevisiae Thi4p, which is called ThiA in A. nidulans and A. oryzae, is essential for HET phosphate synthesis and its substrate is NAD+ [Citation52–56]. The findings of proteomic analyses indicate that hypoxic A. nidulans cells upregulate enzymes containing TPP including pyruvate decarboxylase (PDC), transketolase, α-ketoglutarate dehydrogenase and AHAS, which are likely to participate in the hypoxic activation of ethanol fermentation, the pentose phosphate pathway (PPP), the tricarboxylate cycle, and dissimilatory BCAA fermentation, respectively [Citation34,41]. The expression of thiA and the content of thiamine in A. nidulans cells are hypoxically upregulated compared with normoxic cells [Citation34]. These phenomena are important for A. nidulans to ferment BCAA and ethanol by providing TPP for AHAS and PDC (Figure ). The fungal mechanisms of BCAA and ethanol fermentation are significant for regenerating NAD(P)+ under hypoxic conditions as described above [Citation41]. Thus, the hypoxic regulation of thiamine synthesis contributes to energy conservation by A. nidulans and constitutes an adaptive mechanism to decreased oxygen availability [Citation56].
The anabolic mechanism of BCAA synthesis is evolutionarily conserved beyond biological domains. This is significant because it highlights the catabolic (dissimilatory) role of the fungal mechanism of BCAA synthesis. The fungal nitrate-reducing system of ammonia fermentation consists of NAD(P)H-nitrate-and nitrite-reductases encoded by niaD and niiA, which were originally identified as anabolic enzymes for assimilating nitrate. The involvement of reductive anabolic enzymes in hypoxic catabolic mechanism is a common feature between hypoxic BCAA production and ammonia fermentation.
Cellular nucleotides and glycolytic mechanisms regulated by Nudix hydrolase A under hypoxia
Nucleoside diphosphates linked to moiety X (Nudix) hydrolases are characterized by having the conserved Nudix GX5EX7REVXEEXGU motif, where U is usually Ile, Leu or Val [Citation41], and these hydrolases are widely distributed across biological kingdoms ranging from bacteria to humans [Citation57,58]. Nudix hydrolases have been characterized into subfamilies based on their major substrates, dinucleoside polyphosphates, ADP-ribose, NADH, NAD+, and ribo-and deoxy-nucleoside triphosphates [Citation59–61]. Intracellular levels of these substrates are precisely regulated to avoid disrupting normal cell activities [Citation60,61]. Arabidopsis thaliana AtNUDX7 hydrolyzes NADH and modulates cellular NAD+ and NADH levels under normal conditions. We characterized the first fungal Nudix hydrolases (NdxA, NdxB, NdxC and NdxD) and indicated that cytosolic NdxA is the predominant NADH hydrolase that is up-regulated under hypoxia in A. nidulans cells [Citation62]. The ndxA gene disruptant (ΔNdxA) strain accumulates the same amount of NAD+ and 2.0-fold more NADH than the wild type strain (WT) under hypoxic conditions. These findings indicate that NdxA decreases NADH in WT and plays a distinct role in NADH maintenance in O2-limited cells [Citation12].
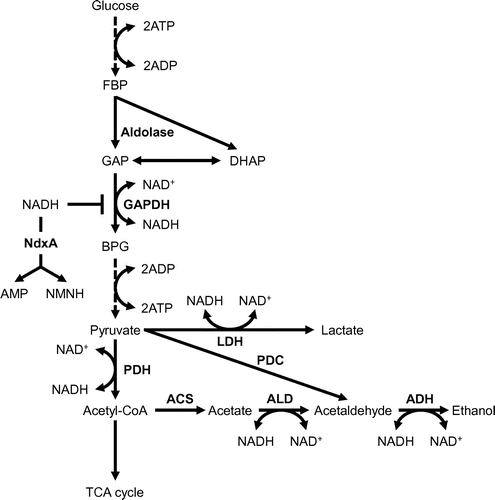
Aspergillus nidulans oxidizes exogenous glucose as a carbon source and generates ethanol as well as lactate (alcohol and lactate fermentation) under hypoxic conditions [Citation63]. The ΔNdxA cells produce less ethanol and lactate and consume glucose at a lower rate compared with the WT under hypoxic conditions, indicating reduced glycolytic flux in ΔNdxA (Figure ). The GAPDH reaction is inhibited by NADH that accumulates in hypoxic ΔNdxA cells (Figure ). Partly defective ethanol and lactate production is explained as glycolytic flux caused by the lack of NdxA during the GAPDH reaction. Cellular NADH levels regulate bacterial GAPDH [Citation9,11,58,64,65]. These indicate that the inhibition of GAPDH by NADH under O2-limited conditions in is similar between fungal and bacterial processes, whereas the involvement of NdxA in such inhibition among organisms illuminates a hitherto unknown role of NdxA in the regulation of glycolysis via NADH hydrolysis (Figure ). Nudix hydrolase functions in regulating the metabolism of primary carbon sources under hypoxia. Isozymes for NdxA are widely distributed in eukaryotes [Citation66]. In addition, eukaryotes across numerous phyla alter cellular NAD+ levels under hypoxia as well as under suitable nutrient conditions [Citation63,66]. The function of NdxA is conserved among eukaryotes; therefore, further investigation is required to understand the environmental responses of eukaryotes and how they metabolize carbon sources.
Figure 3. Glycolytic flow regulated by NdxA under hypoxia.
Nudix hydrolase A controls cellular NAD(H), sirtuin, and secondary metabolites during the stationary growth phase
Cellular levels of NAD+ and NADH are thought to be controlled by de novo and salvage mechanisms although evidence has not yet indicated that they are regulated by NAD+ degradation. We showed that NdxA is up-regulated during the stationary growth phase, and that it hydrolyzes and decreases cellular NAD+ and NADH in Aspergillus nidulans. During the early growth phase ΔNdxA produces as much NAD+ hydrolase activity as the WT strain, and the amount of NAD+ hydrolase activity is lower than that of the WT during the stationary phase. More NAD+ is accumulated during the stationary growth phase by ΔNdxA than by the WT, which coincides with decreased NAD+ hydrolase levels. Total amounts of NAD(H) increase 1.5-fold in the absence of NdxA, which maintains cellular NAD+/NADH homeostasis [Citation62].
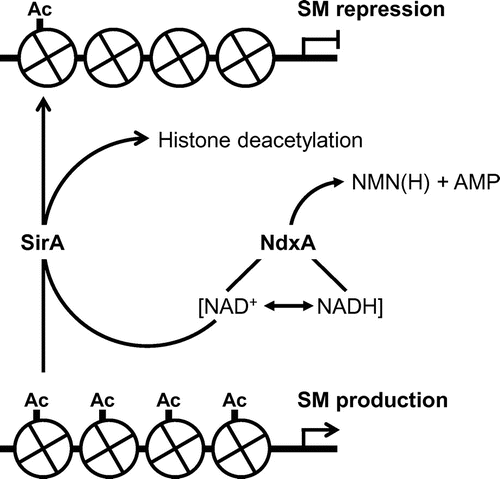
Penicillin G and sterigmatocystin are typical secondary metabolites of Aspergilli and the ΔNdxA strain produces less of both than the WT. In addition, ΔNdxA accumulates fewer transcripts of penicillin G cluster genes encoding isopenicillin-N synthetase (ipnA) and its acyltransferase (penDE) [Citation10,11], and of aflR and stcU encoding a transcription factor and an enzyme for sterigmatocystin biosynthesis, respectively, than the WT. These results imply that NdxA lowers the levels of cellular NAD+, through which NdxA up-regulates the synthesis of these secondary metabolites. The expression of genes involved in secondary metabolism is regulated by specific transcription regulators and by the posttranslational modification of nucleosomal histones. Acetylation is the type of modification that correlates with conformational changes in chromatin. Two groups of histone deacetylases (HDAC) deacetylate acetylated histones. One is classical HDAC and the other is sirtuin that deacetylates lysine residues of histones H3 and/or H4 using NAD+ as a cosubstrate [Citation63,66,67]. Yeast Sir2p is a prototype sirtuin that silences genes at mating type, ribosomal DNA and sub-telomeric loci [Citation68,69]. Its mammalian counterparts control aging, stress responses and circadian rhythms [Citation70,71]. Sirtuin activity is controlled by cellular NAD+ production [Citation64,72] that links cellular metabolic status and gene regulation, since NAD+ is a crucial coenzyme for biological redox reactions and energy conservation. The NAD+-independent HDAC of A. nidulans HdaA regulates histone H3 acetylation and the expression of secondary metabolite cluster genes [Citation73], whereas the sirtuin isozyme HstA has predicted obscure enzymatic and physiological functions [Citation74]. The fungal genome encodes a gene encoding a sirtuin isozyme (SirA) with nicotinamide-sensitive NAD+-dependent HDAC activity. Chromatin immunoprecipitation analyses have shown that SirA removes H4K16 acetylation and represses the expression of these secondary metabolite genes. These findings indicate that SirA regulates the amounts of H4K16 acetylation and secondary metabolite production through deacetylating H4K16. The increased cellular NAD+ and the decreased secondary metabolite production induced by adding nicotinamide riboside accompanies decreased amounts of H4K16 acetylation at the promoters of secondary metabolite genes; this is not evident in ΔSirA. These indicate that cellular NAD+ modulates the HDAC reaction by SirA, and fungal NdxA decreases NAD+, represses the HDAC activity of SirA, increases H4K16 acetylation and de-represses secondary metabolite gene expression (Figure ). These also indicate a hitherto undiscovered mechanism of NAD(H) regulation that is governed by the novel NAD(H) regulator NdxA (Figure ); NdxA controls total levels of NAD+/NADH, and negatively regulates sirtuin function and chromatin structure. Hypoxia induces biosynthesis of the secondary metabolite pseurotin A in the airborne fungal pathogen A. fumigatus [Citation75]. SirE is a recently discovered NAD+-dependent deacetylase that represses A. nidulans events during the stationary growth phase (that mammals and yeasts lack), autolysis, conidia development, secondary metabolites and extracellular hydrolase production [Citation76].
Concluding remarks
Filamentous fungi balance their cellular NAD+:NADH ratios to adapt to environmental redox stimuli. The cellular NAD(H) state triggers changes in metabolic pathways such as glycolysis and fermentation, as well as the production of organic acids, amino acids and secondary metabolites. Hypoxia imposes various obstacles upon eukaryotes, such as reduced ATP availability due to a lack of O2 respiration and a high NADH ratio caused by a lack of terminal electron acceptors. Under such conditions, filamentous Aspergillus nidulans uses nitrate as an alternative electron acceptor to re-oxidize NADH to NAD+, and these mechanisms are defined as denitrification and ammonia fermentation. The mechanism of BCAA fermentation is also significant in providing an alternative electron acceptor (pyruvate) to re-oxidize NAD(P)H to NAD(P)+, and supporting the growth of A. nidulans under O2-limited conditions. Furthermore, NdxA hydrolyzes cellular NADH, derepresses GAPDH activity, and hence increases glycolytic flow in hypoxic A. nidulans cells. Eukaryotic microbes thrive under various O2 concentrations in several types of environments. Transient flooding often depletes O2 in the soil where fungi reside. Pathogenic fungi proliferate in animal tissues or cells where O2 concentrations are below atmospheric levels. These imply that filamentous fungi utilize multi-adaptive systems to survive under O2 limitation.
The Ascomycetes genus Aspergillus includes numerous strains that produce secondary metabolites. Classical HDACs are regulators of secondary metabolite synthesis whereas no known sirtuin regulates such synthesis in response to cellular NAD(H) levels. Nudix hydrolase A combined with a novel fungal sirtuin constitutes a novel epigenetic mechanism that degrades cellular NAD(H) and negatively regulates sirtuin function and chromatin structure. The NdxA-mediated down-regulation mechanisms of total NAD(H) is considered to counteract biosynthetic mechanisms and fine-tune NAD(H), which in turn regulates levels of histone acetylation. Nudix hydrolases are ubiquitous among eukaryotes. The distribution of Nudix hydrolase orthologs in most analyzed fungal genomes and of histone modifications on the secondary metabolite genes of mycotoxigenic fungi suggests that Nudix hydrolase inhibitors could help to diminish cereal contamination by mycotoxins.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research [grant number 25850057 to MS].
Acknowledgments
I am very grateful to Dr. Naoki Takaya (University of Tsukuba) and Dr. Masashi Kato (Meijo University) for helpful advice and support. I thank Norma Foster for helpful discussion and critical reading of the manuscript. I also thank Kiyota Sakai and Shunsuke Murata for help with manuscript preparation.
Notes
* This review was written in response to the author’s receipt of the JSBBA Award for Young Scientists in 2016.
References
- Rytioja J, Hildén K, Yuzon J, et al. Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol Mol Biol Rev. 2014;78:614–649.10.1128/MMBR.00035-14
- Segato F, Damásio AR, de Lucas RC, et al. Genomics review of holocellulose deconstruction by aspergilli. Microbiol Mol Biol Rev. 2014;78:588–613.10.1128/MMBR.00019-14
- Shimizu M, Kaneko Y, Ishihara S, et al. Novel β-1,4-mannanase belonging to a new glycoside hydrolase family in Aspergillus nidulans. J Biol Chem. 2015;290:27914–27927.10.1074/jbc.M115.661645
- Muraguchi H, Umezawa K, Niikura M, et al. Strand-specific RNA-seq analyses of fruiting body development in Coprinopsis cinerea. PLoS One. 2015;10:e0141586.10.1371/journal.pone.0141586
- Shimizu M, Yamamoto T, Okabe N, et al. Novel 4-methyl-2-oxopentanoate reductase involved in synthesis of the Japanese sake flavor, ethyl leucate. Appl Microbiol Biotechnol. 2016;100:3137–3145.10.1007/s00253-015-7182-0
- Sakai K, Mochizuki M, Yamada M, et al. Biochemical characterization of thermostable β-1,4-mannanase belonging to the glycoside hydrolase family 134 from Aspergillus oryzae. Appl Microbiol Biotechnol. 2017;101:3237–3245.10.1007/s00253-017-8107-x
- Abe K, Gomi K, Hasegawa F, et al. Impact of Aspergillus oryzae genomics on industrial production of metabolites. Mycopathologia. 2006;162:143–153.10.1007/s11046-006-0049-2
- Kobayashi T, Abe K, Asai K, et al. Genomics of Aspergillus oryzae. Biosci Biotechnol Biochem. 2007;71:646–670.10.1271/bbb.60550
- Mullbacher A, Eichner RD. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc Natl Acad Sci USA. 1984;81:3835–3837.10.1073/pnas.81.12.3835
- Brown DW, Yu JH, Kelkar HS, et al. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc Natl Acad Sci USA. 1996;93:1418–1422.10.1073/pnas.93.4.1418
- MacCabe AP, Riach MB, Unkles SE, et al. The Aspergillus nidulans npeA locus consists of three contiguous genes required for penicillin biosynthesis. EMBO J. 1990;9:279–287.
- Shimizu M, Takaya N. Nudix hydrolase controls nucleotides and glycolytic mechanisms in hypoxic Aspergillus nidulans. Biosci Biotechnol Biochem. 2013;77:1888–1893.10.1271/bbb.130334
- Sato I, Shimizu M, Hoshino T, et al. The glutathione system of Aspergillus nidulans involves a fungus-specific glutathione S -transferase. J Biol Chem. 2009;284:8042–8053.10.1074/jbc.M807771200
- Zhou S, Narukami T, Masuo S, et al. NO-inducible nitrosothionein mediates NO removal in tandem with thioredoxin. Nat Chem Biol. 2013;9:657–663.10.1038/nchembio.1316
- Rocha S. Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci. 2007;32:389–397.10.1016/j.tibs.2007.06.005
- Masuo S, Terabayashi Y, Shimizu M, et al. Global gene expression analysis of Aspergillus nidulans reveals metabolic shift and transcription suppression under hypoxia. Mol Genet Genomics. 2010;284:15–24.
- Terabayashi Y, Shimizu M, Kitazume T, et al. Conserved and specific responses to hypoxia in Aspergillus oryzae and Aspergillus nidulans determined by comparative transcriptomics. Appl Microbiol Biotechnol. 2012;93:305–317.10.1007/s00253-011-3767-4
- Hughes AL, Todd B, Espenshade PJ. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell. 2005;120:831–841.
- Ter Linde JJ, Steensma HY. A microarray-assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae. Yeast. 2002;19:825–840.10.1002/(ISSN)1097-0061
- Znaidi S, Weber S, AI-Abdin OZ, et al. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot Cell. 2008;7:836–847.10.1128/EC.00070-08
- ter Linde JJM, Liang H, Davis RW, et al. Genome-wide transcriptional analysis of aerobic and anaerobic chemostat cultures of Saccharomyces cerevisiae. J Bacteriol. 1999;181:7409–7413.
- Piper MDW, Daran-Lapujade P, Bro C, et al. Reproducibility of oligonucleotide microarray transcriptome analyses. An interlaboratory comparison using chemostat cultures of Saccharomyces cerevisiae. J Biol Chem. 2002;277:37001–37008.10.1074/jbc.M204490200
- Kwast KE, Lai L, Menda N, et al. Genomic analyses of anaerobically induced genes in Saccharomyces cerevisiae: functional roles of Rox1 and other factors in mediating the anoxic response. J Bacteriol. 2002;184:250–265.10.1128/JB.184.1.250-265.2002
- Kobi D, Zugmeyer S, Potier S, et al. Two-dimensional protein map of an “ale”-brewing yeast strain: proteome dynamics during fermentation. FEMS Yeast Res. 2004;5:213–230.10.1016/j.femsyr.2004.07.004
- de Groot MJL, Daran-Lapujade P, van Breuklen B, et al. Quantitative proteomics and transcriptomics of anaerobic and aerobic yeast cultures reveals post-transcriptional regulation of key cellular processes. Microbiology. 2007;153:3864–3878.10.1099/mic.0.2007/009969-0
- Keng T. HAP1 and ROX1 form a regulatory pathway in the repression of HEM13 transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2616–2623.10.1128/MCB.12.6.2616
- Zitomer RS, Lowry CV. Regulation of gene expression by oxygen in Saccharomyces cerevisiae. Microbiol Rev. 1992;56:1–11.
- Todd BL, Stewart Burg JS, Hughes AL, et al. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol Cell Biol. 2006;26:2817–2831.10.1128/MCB.26.7.2817-2831.2006
- Takasaki K, Shoun H, Yamaguchi M, et al. Fungal ammonia fermentation, a novel metabolic mechanism that couples the dissimilatory and assimilatory pathways of both nitrate and ethanol. Role of acetyl CoA synthetase in anaerobic ATP synthesis. J Biol Chem. 2004;279:12414–12420.10.1074/jbc.M313761200
- Fujii T, Takaya N. Denitrification by the fungus fusarium oxysporum involves NADH-nitrate reductase. Biosci Biotechnol Biochem. 2008;72:412–420.10.1271/bbb.70538
- Zhou Z, Takaya N, Sakairi MAC, et al. Oxygen requirement for the denitrification by the filamentous fungus Fusarium oxysporum. Arch Microbiol. 2001;175:19–25.10.1007/s002030000231
- Zhou Z, Takaya N, Nakamura A, et al. Ammonia fermentation, a novel anoxic metabolism of nitrate by fungi. J Biol Chem. 2002;277:1892–1896.10.1074/jbc.M109096200
- Takasaki K, Shoun H, Nakamura A, et al. Unusual transcription regulation of the niaD gene under anaerobic conditions supporting fungal ammonia fermentation. Biosci Biotechnol Biochem. 2004;68:978–980.10.1271/bbb.68.978
- Shimizu M, Fujii T, Masuo S, et al. Proteomic analysis of Aspergillus nidulans cultured under hypoxic conditions. Proteomics. 2009;9:7–19.10.1002/pmic.v9:1
- Takaya N. Response to hypoxia, reduction of electron acceptors, and subsequent survival by filamentous fungi. Biosci Biotechnol Biochem. 2009;73:1–8.10.1271/bbb.80487
- Zhou Z, Takaya N, Shoun H. Multi-energy metabolic mechanisms of the fungus fusarium oxysporum in low oxygen environments. Biosci Biotechnol Biochem. 2010;74:2431–2437.10.1271/bbb.100482
- Kim SW, Fushinobu S, Zhou S, et al. Eukaryotic nirK genes encoding copper-containing nitrite reductase: originating from the protomitochondrion? Appl Environ Microbiol. 2009;75:2652–2658.10.1128/AEM.02536-08
- Nakanishi Y, Zhou S, Kim SW, et al. A eukaryotic copper-containing nitrite reductase derived from a NirK homolog gene of Aspergillus oryzae. Biosci Biotechnol Biochem. 2010;74:984–991.10.1271/bbb.90844
- Kim SW, Fushinobu S, Zhou S, et al. The possible involvement of copper-containing nitrite reductase (NirK) and flavohemoglobin in denitrification by the fungus Cylindrocarpon tonkinense. Biosci Biotechnol Biochem. 2010;74:1403–1407.10.1271/bbb.100071
- Shiro Y, Fujii M, Iizuka T, et al. Spectroscopic and kinetic studies on reaction of cytochrome P450nor with nitric oxide. Implication for its nitric oxide reduction mechanism. J Biol Chem. 1995;270:1617–1623.10.1074/jbc.270.4.1617
- Shimizu M, Fujii T, Masuo S, et al. Mechanism of de novo branched-chain amino acid synthesis as an alternative electron sink in hypoxic Aspergillus nidulans cells. Appl Environ Microbiol. 2010;76:1507–1515.10.1128/AEM.02135-09
- Cano NJ, Fouque D, Leverve XM. Application of branched-chain amino acids in human pathological states: renal failure. J Nutr. 2006;136:299–307.
- Gurr SJ, Hawkins AR, Drainas C, et al. Isolation and identification of the Aspergillus nidulans gdhA gene encoding NADP-linked glutamate dehydrogenase. Curr Genet. 1986;203:761–766.
- Ward DE, Kengen SW, van der Oost J, et al. Purification and characterization of the alanine aminotransferase from the hyperthermophilic archaeon Pyrococcus furiosus and its role in alanine production. J Bacteriol. 2000;182:2559–2566.10.1128/JB.182.9.2559-2566.2000
- Limami AM, Glevarec G, Ricoult C, et al. Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J Exp Bot. 2008;59:2325–2335.10.1093/jxb/ern102
- Kräutler Bernhard. Vitamins. Angewandte Chemie. 1998;101:1569–1570.
- Butterworth RF. Thiamin deficiency and brain disorders. Nutr Res Rev. 2003;16:277–283.10.1079/NRR200367
- Jordan F. Current mechanistic understanding of thiamin diphosphate-dependent enzymatic reactions. Nat Prod Rep. 2003;20:184–201.10.1039/b111348 h
- Settembre E, Begley TP, Ealick SE. Structural biology of enzymes of the thiamin biosynthesis pathway. Curr Opin Struct Biol. 2003;13:739–747.10.1016/j.sbi.2003.10.006
- Begley TP, Downs DM, Ealick SE, et al. Thiamin biosynthesis in prokaryotes. Arch Microbiol. 1999;171:293–300.10.1007/s002030050713
- Spenser ID, White RL. Biosynthesis of vitamin B1 (thiamin): an instance of biochemical diversity. Angew Chem Int Ed Engl. 1997;36:1032–1046.10.1002/(ISSN)1521-3773
- Godoi PHC, Galhardo RS, Luche DD, et al. Structure of the thiazole biosynthetic enzyme THI1 from Arabidopsis thaliana. J Biol Chem. 2006;281:30957–30966.10.1074/jbc.M604469200
- Chatterjee A, Jurgenson CT, Schroeder FC, et al. Thiamin biosynthesis in eukaryotes: characterization of the enzyme-bound product of thiazole synthase from Saccharomyces cerevisiae and its implications in thiazole biosynthesis. J Am Chem Soc. 2006;128:7158–7159.10.1021/ja061413o
- Chatterjee A, Jurgenson CT, Schroeder FC, et al. Biosynthesis of thiamin thiazole in eukaryotes: Conversion of NAD to an advanced intermediate. J Am Chem Soc. 2007;129:2914–2922.10.1021/ja067606t
- Chatterjee A, Abeydeera ND, Bale S, et al. Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature. 2011;478:542–546.10.1038/nature10503
- Shimizu M, Masuo S, Itoh E, et al. Thiamine synthesis regulates the fermentation mechanisms in the fungus Aspergillus nidulans. Biosci Biotechnol Biochem. 2016;80:1768–1775.10.1080/09168451.2016.1158631
- Bessman MJ, Frick DN, O’Handley SF. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem. 1996;271:25059–25062.10.1074/jbc.271.41.25059
- McLennan AG. The Nudix hydrolase superfamily. Cell Mol Life Sci. 2006;63:123–143.10.1007/s00018-005-5386-7
- AbdelRaheim SR, Cartwright JL, Gasmi L, et al. The NADH diphosphatase encoded by the Saccharomyces cerevisiae NPY1 Nudix hydrolase gene is located in peroxisomes. Arch Biochem Biophys. 2001;388:18–24.10.1006/abbi.2000.2268
- Ishikawa K, Ogawa T, Hirosue E, et al. Modulation of the Poly(ADP-ribosyl)ation reaction via the arabidopsis ADP-Ribose/NADH pyrophosphohydrolase, AtNUDX7, is involved in the response to oxidative stress. Plant Physiol. 2009;151:741–754.10.1104/pp.109.140442
- Taddei F, Hayakawa H, Bouton M, et al. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science. 1997;278:128–130.10.1126/science.278.5335.128
- Shimizu M, Masuo S, Fujita T, et al. Hydrolase controls cellular NAD, sirtuin, and secondary metabolites. Mol Cell Biol. 2012;32:3743–3755.10.1128/MCB.00032-12
- Dang W, Steffen KK, Perry R, et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807.10.1038/nature08085
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128.10.1126/science.289.5487.2126
- McDonagh A, Fedorova ND, Crabtree J, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154.10.1371/journal.ppat.1000154
- Imai S, Armstrong CM, Kaeberlein M, et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800.10.1038/35001622
- Zhang T, Kraus WL. SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim Biophys Acta. 2010;1804:1666–1675.10.1016/j.bbapap.2009.10.022
- Guarente L. Diverse and dynamic functions of the Sir silencing complex. Nat Genet. 1999;23:281–285.10.1038/15458
- Smeal T, Claus J, Kennedy B, et al. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell. 1996;84:633–642.10.1016/S0092-8674(00)81038-7
- Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921.10.1101/gad.1467506
- Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340.10.1016/j.cell.2008.07.002
- Belenky P, Racette FG, Bogan KL, et al. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007;129:473–484.10.1016/j.cell.2007.03.024
- Palmer JM, Keller NP. Secondary metabolism in fungi: does chromosomal location matter? Curr Opin Microbiol. 2011;13:431–436.
- Shwab EK, Bok JW, Tribus M, et al. Histone deacetylase activity regulates chemical diversity in aspergillus. Eukaryot Cell. 2007;6:1656–1664.10.1128/EC.00186-07
- Vödisch M, Scherlach K, Winkler R, et al. Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin a biosynthesis gene cluster in response to hypoxia. J Proteome Res. 2011;10:2508–2524.10.1021/pr1012812
- Itoh E, Odakura R, Oinuma KI, et al. Sirtuin E is a fungal global transcriptional regulator that determines the transition from the primary growth to the stationary phase. 2017;292:11043–11054.