Abstract
Prenylquinones are isoprenoid compounds with a characteristic quinone structure and isoprenyl tail that are ubiquitous in almost all living organisms. There are four major prenylquinone classes: ubiquinone (UQ), menaquinone (MK), plastoquinone (PQ), and rhodoquinone (RQ). The quinone structure and isoprenyl tail length differ among organisms. UQ, PQ, and RQ contain benzoquinone, while MK contains naphthoquinone. UQ, MK, and RQ are involved in oxidative phosphorylation, while PQ functions in photosynthetic electron transfer. Some organisms possess two types of prenylquinones; Escherichia coli has UQ8 and MK8, and Caenorhabditis elegans has UQ9 and RQ9. Crystal structures of most of the enzymes involved in MK synthesis have been solved. Studies on the biosynthesis and functions of quinones have advanced recently, including for phylloquinone (PhQ), which has a phytyl moiety instead of an isoprenyl tail. Herein, the synthesis and applications of prenylquinones are reviewed.
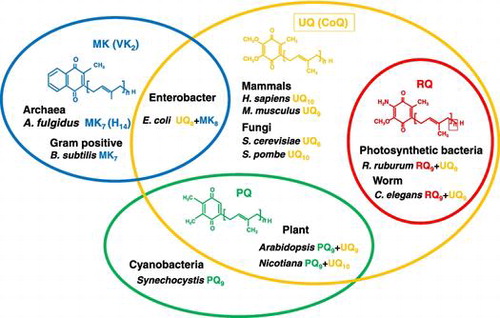
Distribution of prenylquinones in living organisms
Abbreviations:
- DHFL: dehypoxanthinylfutalosine
- DHNA: 1,4-dihydroxy-2-naphthoate
- DMK: demethylmenaquinone
- DMAPP: dimethylallyl pyrophosphate
- DXP: 1-deoxy-D-xylulose-5-phosphate
- FPP: farnesyl pyrophosphate
- GPP: geranyl pyrophosphate
- GGPP: geranylgeranyl pyrophosphate
- HGA: homogentisate
- IPP: isopentenyl pyrophosphate
- MEP: 2C-methyl-D-erythritol-4-phosphate
- MK: menaquinone
- MVA: mevalonate
- PDS: prenyl diphosphate synthase
- PHB: p-hydroxybenzoate
- PhQ: phylloquinone
- PQ: plastoquinone
- RQ: rhodoquinone
- UQ: ubiquinone
Isoprenoids are compounds built from two common precursors, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). More than 50,000 isoprenoid compounds are found in nature [Citation1]. Among them, isoprenylated quinones, in which the length of the isoprenoid side chain or tail varies, are widely distributed in almost all living organisms, and they function in electron transfer. Living organisms must acquire energy through oxidative phosphorylation or photosynthetic phosphorylation, and these processes require lipid molecules to transfer electrons and protons between protein complexes. Typically, ubiquinone (UQ) transfers electrons from Complex I or II to Complex III in oxidative phosphorylation, while plastoquinone (PQ) transfers electrons from photosystem II to the cytochrome b6f complex in photosynthesis [Citation2].
The isoprenoid side chain is responsible for the lipid-soluble nature of quinones, and anchors them in membrane lipid bilayers, while the electron transfer capacity is derived from the quinone head. The quinone ring undergoes a two-step reversible oxidation/reduction between reduced and oxidized forms. This common property allows electrons and protons to shuttle between different protein complexes in biological membranes, allowing it to function as both a cofactor in enzyme reactions, and as an antioxidant.
Widely distributed (major) and more restricted (minor) quinones are present in living organisms. UQ, menaquinone (MK), PQ, and rhodoquinone (RQ) are major quinones. UQ and RQ are distributed in prokaryotes and eukaryotes, while MK is found in bacteria and archaea, and PQ is restricted to cyanobacteria and plants. Minor quinones include thermoplasmaquinone, methionaquinone, chlorobiumquinone, sulfolobusquinone, and caldariellaquinone, and are found in bacteria and archaea [Citation3].
The length of the isoprenoid side chain and the type of quinone are variable (side chain length is annotated in subscript in this review). For example, bacteria such as Bacillus subtilis produce MK7, Escherichia coli synthesize UQ8 and MK8, and Synechocystis spp. generate PQ9. Yeasts such as Saccharomyces cerevisiae and Schizosaccharomyces pombe produce UQ6 and UQ10, respectively. Plants such as Arabidopsis thaliana produce UQ9 and PQ9, while Nicotiana tabacum synthesize UQ10 and PQ9. Nematodes such as Caenorhabditis elegans produce UQ9 and RQ9, and higher animals such as Mus musculus and Homo sapiens make UQ9 and UQ10, respectively (Figure ). The types of prenylquinones in organisms are highly variable; hence they have been used for classification of microbes [Citation4,5].
Figure 1. Distribution of four major prenylquinones in different organisms. Ubiquinone (UQ) is present in almost all living organisms from bacteria to higher eukaryotes. Menaquinone (MK) is distributed in bacteria and archaea, and therefore considered the oldest type of prenylquinone, first synthesized in primitive living organisms. Plastoquinone (PQ) occurs in cyanobacteria, and was presumably subsequently transferred to plants. Rhodoquinone (RQ) is the most recently evolved quinone, and is synthesized from UQ.
The biosynthesis of prenylquinones has been extensively studied, and despite significant knowledge accumulated, some biosynthetic reactions remain poorly understood. In this review, the biosynthesis of four major prenylquinones and phylloquinone (PhQ) is summarized in detail.
Isoprenoid side chains are synthesized via 2C-methyl-D-erythritol-4-phosphate (MEP) and mevalonate (MVA) pathways
The isoprenoid side chains of prenylquinones are synthesized by prenyl diphosphate synthase (PDS) from DMAPP, geranyl pyrophosphate (GPP), geranylgeranyl pyrophosphate (GGPP), or farnesyl pyrophosphate (FPP) by condensation of IPP. IPP and DMAPP are synthesized from either the 2C-methyl-D-erythritol-4-phosphate (MEP) pathway [Citation6–8] or the mevalonate (MVA) pathway [Citation9].The MEP pathway is present in most prokaryotes, and the MVA pathway occurs in archaea and eukaryotes [Citation10]. Plants and Streptomycetes possess both pathways.
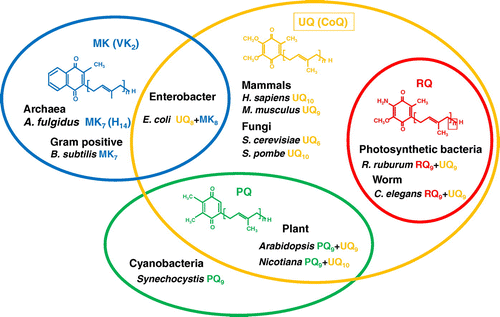
The MVA pathway was discovered in the 1960s and consists of seven enzyme-catalyzed reactions. It performs several key functions within cells, and is an important central metabolic pathway in all higher eukaryotes. The MVA pathway of S. cerevisiae is shown in Figure as representative of eukaryotes. Formation of acetoacetyl-CoA from two acetyl-CoA molecules by acetyl-CoA acetyltransferase (Erg10) is followed by the synthesis of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by Erg13. In the third step, MVA is generated by reduction of HMG-CoA by HMG-CoA reductase (Hmg), the target of the famous ‘statin’ drugs [Citation11]. MVA is phosphorylated by Erg12 to generate phosphomevalonate, and further phosphorylated by Erg8. Finally, diphosphomevalonate is used by Erg19/Mvd1 to generate IPP or DMAPP. Idi isomerizes between IPP and DMAPP, and DMAPP and IPP are further utilized in condensation reactions for the biosynthesis of isoprenoids. The reactions of all enzymes in the MVA pathway and their three-dimensional structures have been summarized previously [Citation9].
Figure 2. The mevalonate (MVA) pathway in Saccharomyces cerevisiae. The MVA pathway consists of seven enzyme-catalyzed reactions. The first step is the formation of acetoacetyl-CoA from two acetyl-CoA molecules by Erg10 (acetyl-CoA acetyl transferase). Subsequently, Erg13 (HMG-CoA synthase), Hmg1/Hmg2 (HMG-CoA reductase), Erg12 (mevalonate kinase), Erg8 (phosphomevalonate kinase), and Erg19 (diphosphomevalonate decarboxylase) lead to the production of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Idi1 isomerizes between IPP and DMAPP.
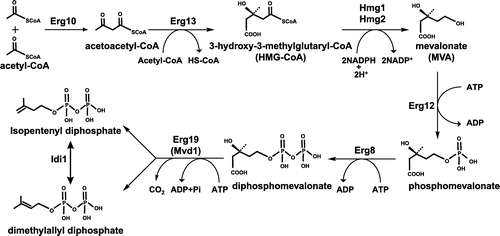
The MEP pathway for the biosynthesis of IPP and DMAPP was discovered in the 1990s and consists of eight enzyme-catalyzed reactions. The MEP pathway of E. coli is shown in Figure . Condensation of pyruvate and D-glyceraldehyde-3-phosphate to form 1-deoxy-D-xylulose-5-phosphate (DXP) is catalyzed by Dxs (Figure ). The second step is the conversion of DXP to MEP by IspC, and 4-diphosphocytidyl-2C-methyl-D-erythritol (CDP-ME) is then generated from MEP and CTP by IspD. The fourth step is the phosphorylation of CDP-ME to generate 4-diphosphocytidyl-2C-methyl-D-erythritol-2-phosphate (CDP-ME2P) by IspE, and IspF subsequently removes CMP from CDP-ME2P to generate 2C-methyl-D-erythritol-2,4-cyclodiphosphate (MEcPP). In the sixth step, IspG catalyzes the ring opening of the cyclic pyrophosphate and C3-reductive dehydration of MEcPP to generate 4-hydroxy-3-methylbut-2-enyl diphosphate (HMB-PP). Finally, IspH generates IPP or DMAPP from HMB-PP by reduction. Idi catalyzes isomerization between IPP and DMAPP in the eighth step, and some organisms lack this enzyme. Enzymes of this MEP pathway are attractive targets for the development of drugs against infectious diseases because this pathway occurs in pathogenic prokaryotes but is absent in humans. The antimalarial drug fosmidomycin, which inhibits Dxr, is one of the best-known examples of a drug that targets the MEP pathway. Three-dimensional structures of E. coli Dxs, IspC, IspD, IspE, IspF, IspG, and IspH have been solved, and their precise structure-based catalytic mechanisms have been described [Citation6]. Further details about these biosynthetic pathways synthesizing IPP can be found in previous reviews [Citation6–10].
Figure 3. The 2C-methyl-D-erythritol-4-phosphate (MEP) pathway in Escherichia coli. The MEP pathway consists of eight enzyme-catalyzed reactions. The first step is the condensation of pyruvate and glyceraldehyde-3-phosphate to form 1-deoxy-D-xylulose-5-phosphate by Dxs (DXP synthase). Subsequent steps catalyzed by IspC (DXP reductoisomerase), IspD (MEP cytidyltransferase), IspE (CDP-ME kinase), IspF (MECDP synthase), IspG (4-hydroxy-3-methylbut-2-enyl diphosphate synthase), and IspH (4-hydroxy-3-methylbut-2-enyl diphosphate reductase) lead to the production of IPP and DMAPP.
Prenyl diphosphate synthase
The side chain of prenylquinone is supplied by polyprenyl diphosphate synthase (PDS) and determines the side chain length of prenylquinones (Figure ) [Citation12,13]. Numerous PDS enzymes have been analyzed [Citation5,14–17], and all consist of seven conserved regions including two DDXXD motifs involved in binding substrates such as FPP (GPP or GGPP) and IPP. PDS occurs in both homomeric and heteromeric forms. PDSs in Gram-negative bacteria are mostly heteromeric, while those in Gram-positive bacteria are mostly heteromeric [Citation15,18]. Eukaryotes have both homomeric (e.g. Coq1 and SPS1) and heteromeric types (e.g. human PDSS1 and PDSS2) [Citation19–21]. The distribution of these PDSs differs among organisms, and among different components in organisms. For example, E. coli contains only one polyprenyl diphosphate synthase (IspB) and shares the side chain of UQ and MK [Citation22,23]. Meanwhile, plants such as Arabidopsis possess three different PDSs, in this case three solanesyl diphosphate synthases (SPS1, SPS2, and SPS3), which are localized to different subcellular organelles (the ER, chloroplasts, and mitochondria, respectively) [Citation24,25]. Humans contain one PDS comprising two subunits, PDSS1 and PDSS2 [Citation21] similar to S. pombe PDS, which also consists of a heteromeric complex and served as the basis for analysis of the human enzyme [Citation20,21]. A heteromeric form of PDS was probably evolved in S. pombe or earlier organisms and succeeded to humans. The result that artificial heteromeric PDSs between Coq1 and Dps1 or IspB and Dps1 is functional [Citation19,26] supported an idea that heteromeric form was evolved from homomeric form.
Figure 4. Biosynthetic pathway of the isoprenoid tail of prenylquinones. Polyprenyl diphosphate synthase synthesizes trans-polyprenyl diphosphate of a certain length. S. cerevisiae Coq1 (hexaprenyl diphosphate synthase) forms products from six isoprene units, E. coli IspB (octaprenyl diphosphate synthase) synthesizes products with eight isoprene units, Arabidopsis SPS1, SPS2, and SPS3 (solanesyl diphosphate synthase) generate products with nine isoprene units, and human and Schizosaccharomyces pombe decaprenyl diphosphate synthase (DPS; a heteromer of PDSS1 and PDSS2 or Dps1 and Dlp1, respectively) catalyzes the formation of products with ten isoprene units. S. cerevisiae Coq2 (PHB-hexaprenyl diphosphate transferase), E. coli UbiA (PHB-octaprenyl diphosphate transferase), and human COQ2 (PHB-decaprenyl diphosphate transferase) or S. pombe Ppt1 (Coq2; PHB-decaprenyl diphosphate transferase) condense p-hydroxybenzoate (PHB) with trans-polyprenyl diphosphate to form UQ6, UQ8, and UQ10, respectively. MenA prenylates DHNA, and homogentisate solanesyl transferase (HST) prenylates homogentisate (HGA). DPP, decaprenyl diphosphate; HexPP, hexaprenyl diphosphate; NPP, nonaprenyl diphosphate; OPP, octaprenyl diphosphate.
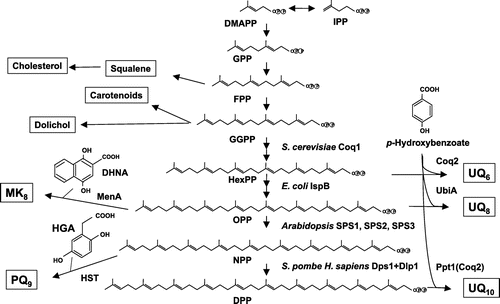
The three-dimensional structure of PDS has been solved, and octaprenyl diphosphate synthase (IspB) from E. coli consists of 14 α-helices [Citation27]. Recent co-crystallization of IspB with its substrates (FPP and IPP) revealed aspartate-rich motifs surrounding the binding regions of substrates, and indicated a product pocket that determines the chain length. The three-dimensional structure of the heteromeric heptaprenyl diphosphate synthase from Staphylococcus aureus was solved [Citation28], revealing a regulatory subunit that does not resemble the catalytic subunit.
E. coli ispB is essential for growth, while ispA encoding FPS is not [Citation22], presumably because ispB replaces the function of ispA [Citation29]. Since ispB is required for the synthesis of the side chain of both UQ and MK, E. coli cannot survive without both quinones. Coq1 in S. cerevisiae [Citation19] and Dps1 (or Dlp1) in S. pombe are not essential for growth [Citation20,30], while C. elegans coq1 and PDSS1 (or PDSS2) in mouse are essential for development [Citation31,32].
Prenylquinones
Different groups of prenylquinones such as UQ, MK, PQ, and RQ (Figure ) are present in different taxonomic groups, and prenylquinone profiling is a useful taxonomic tool [Citation4]. Exactly why such a wide variety of quinones are found in nature is an interesting question. The isoprenoid side chain gives these molecule their lipid-soluble character, the quinone ring defines the redox mid-potential, and organisms have evolved the optimal quinone types for survival. The redox potential (E0) of MK is −74 mV, compared with −63 mV for RQ and + 100 mV for UQ. The lower redox potential of MK and RQ explains why they are used in electron transfer systems under anaerobic conditions, while UQ is employed in aerobic conditions. Differences in the natural environment of living organisms probably affect selection of the preferred quinone type. Evolutionally, MK probably arose in archaea, while PQ and UQ evolved later in bacteria and became distributed in eukaryotes, and RQ forms evolved most recently. However, some researchers believe that PQ may have evolved first in cyanobacteria, and was then distributed to other organisms, because the pathway for UQ biosynthesis is similar to that of PQ, and PQ has the simplest structure among prenylquniones [Citation33].
Some organisms possess two types of quinones, such as E. coli that synthesizes UQ8 and MK8, and C. elegans that has UQ9 and RQ9. Possessing different types of quinones may be beneficial for adapting to changing environmental conditions. In E. coli, the level of UQ8 is 4–5 times higher than that of MK8 and demethyl menaquinone (DMK)8 when growing under aerobic conditions, whereas UQ is three times less abundant than MKs under anaerobic conditions. In Euglena gracilis, RQ9 is present at a similar concentration to UQ9 under aerobic conditions, but is more abundant under anaerobic culture conditions. In C. elegans, UQ9 is 3.56-fold more abundant than RQ9, indicating a preference for aerobic growth, although anaerobic growth also occurs [Citation34].
The significance of the length of the side chain of prenylquinones remains contentious, and only UQ has been thoroughly investigated. The side chain of UQ is determined by the supplied prenyl diphosphate synthesized by PDS [Citation12]. Genetically engineered S. cerevisiae produce UQ5 to UQ10 and grow well, but the native form (UQ6 in this case) is preferred for better growth [Citation13]. E. coli producing UQ6 to UQ10 also grow well, but a longer side chain is preferable for better growth [Citation22]. C. elegans clk-1 mutant, which lacks the penultimate enzyme (Coq7) in UQ synthesis, lives longer than wild type. When engineering E. coli producing UQ6 to UQ10 were used as diet, they reverse the longevity of this mutant, but the effect is different [Citation35]. C. elegans prefers longer UQs such as UQ8 to UQ10, and the preference for a certain length may reflect the affinity for binding proteins or the membrane lipid composition. Why plants prefer the isoprene unit 9 form in PQs, while animals prefer the isoprene unit 4 form in MKs (or PQs) is also interesting.
A variety of scarcer prenylquinones other than those widely distributed in nature have been identified. Thermoplasmaquinone and methionaquinone are found in Thermoplasma spp. and Hydrogenobacter thermophilus, respectively [Citation36,37]. Chlorobiumquinone, containing oxygenized isoprenoid in MK, is found in the photosynthetic bacterium Chlorobium limicola and in Leishmania parasitic protozoans [Citation38]. Sulfolobusquinone, caldariellaquinone, and benzodithiophenoquinone, containing sulfur in an additional heterocyclic ring, are found in Sulfolobales, an order of thermophilic and aerobic archaebacteria [Citation39]. Sulfomenaquinone, containing sulfur in the end of the side chain, is found in Mycobacterium tuberculosis [Citation40], and a saturated isoprenoid in UQ is found in Fungi [Citation41]. There are a few known organisms, such as obligatory fermentative bacteria, that lack prenylquinones [Citation4].
Ubiquinone (coenzyme Q)
Ubiquinone (UQ; 2,3-dimethoxy-5-methyl-6-polyprenyl-1,4-benzoquinone) is an essential cofactor in oxidative phosphorylation, present in all eukaryotes and alpha-, beta-, and gamma-proteobacteria [Citation4]. UQ was discovered by F. Crane in 1957, and the structure was determined by K. Folkers the following year [Citation42]. UQ functions in many physiological processes including sulfide oxidation [Citation43,44], first discovered in fission yeast and later in humans, as well as regulation of the mitochondrial permeability transition pore, and the translocation of protons and Ca2+ across biological membranes in eukaryotes [Citation45]. UQ is the only lipid-soluble antioxidant produced in humans, and it is present in almost all membranes, ranging from mitochondrial membranes, Golgi, ER, and plasma membranes, to very low density lipoproteins. UQ10 production decreases with aging in humans, as does the antioxidant capability of cells [Citation46]. In humans, the heart, liver, and kidney have higher UQ10 levels than other organs [Citation47].
In model organisms such as E. coli, S. cerevisiae, and S. pombe, UQ deficiency is not lethal, but causes growth defects on minimum medium, and a heightened sensitivity to oxidative stress [Citation17]. In C. elegans [Citation31], UQ deficiency leads to gamma-aminobutyric acid (GABA) neuron degeneration, and in Drosophila melanogaster [Citation48], it can cause mitochondrial stress and neuronal apoptosis. In Arabidopsis, UQ is necessary for seed development [Citation49]. In humans, UQ10 deficiency has been implicated in various diseases involving muscle and neural development, with the severity of the disease correlated with the acuteness of the UQ10 shortfall [Citation50].
The biosynthetic pathway of UQ has been reviewed previously [Citation17,51–55], but important progress has been made in recent years. Biosynthesis of UQ has received greatest attention in E. coli and S. cerevisiae, serving as representative prokaryotes and eukaryotes, respectively (Figure ). Some variation in UQ biosynthetic enzymes is observed in prokaryotes and eukaryotes; in particular, decarboxylation and C1 hydroxylation enzymes are not defined in eukaryotes, and likely to be different from prokaryotic enzymes [Citation51].
Figure 5. Overview of the proposed UQ biosynthetic pathway. The UQ biosynthetic pathways of E. coli and S. cerevisiae are shown. In E. coli, PHB is first condensed with trans-polyprenyl diphosphate, and the ring structure is then modified. Decarboxylation catalyzed by UbiD (3-octaprenyl-4-hydroxybenzoate decarboxylase) and UbiX (flavin prenyl transferase) follows. The ring is further hydroxylated by UbiI (2-octaprenylphenol hydroxylase), O-methylated by UbiG (2-octaprenyl-6-hydroxy phenol methylase), hydroxylated by UbiH (2-octaprenyl-6-methoxyphenol hydroxylase), C-methylated by UbiE (2-octaprenyl-6-methoxy-1,4-benzoquinone methylase), hydroxylated by UbiF (2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone oxygenase), and O-methylated by UbiG (3-demethylubiquinone 3-methyltransferase). In S. cerevisiae, para-amino benzoic acid (pABA) and PHB are used for UQ synthesis. The first ring is modified via hydroxylation by Coq6 (PHB-2-hexaprenyl hydroxylase), followed by O-methylation by Coq3 (2-hexaprenyl-6-hydroxy phenol methyltransferase). After decarboxylation and hydroxylation steps, the ring is further modified via C-methylation by Coq5 (2-hexaprenyl-6-methoxy-1,4-benzoquinone methyltransferase), a final hydroxylation by Coq7 (2-hexaprenyl-3-methyl-6-methoxy-1,4-benzoquinone oxygenase), and O-methylation by Coq3. H. sapiens contains similar enzymes with S. cerevisiae except PDSS1 and PDSS2 [Citation70].
![Figure 5. Overview of the proposed UQ biosynthetic pathway. The UQ biosynthetic pathways of E. coli and S. cerevisiae are shown. In E. coli, PHB is first condensed with trans-polyprenyl diphosphate, and the ring structure is then modified. Decarboxylation catalyzed by UbiD (3-octaprenyl-4-hydroxybenzoate decarboxylase) and UbiX (flavin prenyl transferase) follows. The ring is further hydroxylated by UbiI (2-octaprenylphenol hydroxylase), O-methylated by UbiG (2-octaprenyl-6-hydroxy phenol methylase), hydroxylated by UbiH (2-octaprenyl-6-methoxyphenol hydroxylase), C-methylated by UbiE (2-octaprenyl-6-methoxy-1,4-benzoquinone methylase), hydroxylated by UbiF (2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone oxygenase), and O-methylated by UbiG (3-demethylubiquinone 3-methyltransferase). In S. cerevisiae, para-amino benzoic acid (pABA) and PHB are used for UQ synthesis. The first ring is modified via hydroxylation by Coq6 (PHB-2-hexaprenyl hydroxylase), followed by O-methylation by Coq3 (2-hexaprenyl-6-hydroxy phenol methyltransferase). After decarboxylation and hydroxylation steps, the ring is further modified via C-methylation by Coq5 (2-hexaprenyl-6-methoxy-1,4-benzoquinone methyltransferase), a final hydroxylation by Coq7 (2-hexaprenyl-3-methyl-6-methoxy-1,4-benzoquinone oxygenase), and O-methylation by Coq3. H. sapiens contains similar enzymes with S. cerevisiae except PDSS1 and PDSS2 [Citation70].](/cms/asset/ad26797c-6ee4-4674-87eb-bf2e0e39bcf2/tbbb_a_1433020_f0005_oc.gif)
In E. coli, PHB is first condensed with trans-polyprenyl diphosphate by UbiA [Citation56], and the ring structure is then modified. The decarboxylation step is catalyzed by UbiD with the assistance of UbiX, which generates the prenylated FMN cofactor for UbiD [Citation57]. UbiX functions as a flavin prenyltransferase. The ring is further hydroxylated by UbiI [Citation58], O-methylated by UbiG [Citation59], hydroxylated by UbiH, C-methylated by UbiE [Citation60], hydroxylated by UbiF [Citation61], then O-methylated by UbiG. It is reported that ubiK and ubiJ are required for efficient biosynthesis of UQ in E. coli [Citation62]. Hydroxylation and ring formation are reportedly catalyzed by enzymes encoded by ubiM and ubiL in Rhodospirillum [Citation33]. The ubiZ gene product is predicted to be involved in UQ synthesis in Acinetobacter junii, based on genomic analysis of 254 human gut microbes [Citation63]. However, verification of these genes in the biosynthesis of UQ awaits further evidence. An attempt to produce a higher amount of UQ by genetic engineering was first succeeded in E. coli by expressing ubiA, ubiB, ubiC, ubiG, ubiH and ispB [Citation64].
In S. cerevisiae, PHB and para-amino benzoic acid (pABA) are used for UQ synthesis. PHB is synthesized from 4-hydroxybenzaldehyde by Hfd1 in S. cerevisiae [Citation65]. A conserved homolog of Hfd1 is found in humans, but it is still not clear how many steps are required to form 4-hydroxybenzaldehyde from tyrosine [Citation65]. pABA was originally identified as a precursor of ring formation in S. cerevisiae, and we observed that it is also used in S. pombe (unpublished). The first ring is prenylated by Coq2 [Citation66] modified via hydroxylation by Coq6 [Citation67] followed by O-methylation by Coq3 [Citation59]. The enzymes responsible for decarboxylation and hydroxylation remain unclear. The ring is then modified further via C-methylation by Coq5 [Citation68] a final hydroxylation by Coq7 [Citation69] and O-methylation by Coq3. The genes involved in biosynthesis in eukaryotes are well conserved among yeasts, plants, and humans [Citation70], although there is some variation among species. Even between the two model yeasts S. cerevisiae and S. pombe, components of PDS are different [Citation19]. There are at least four genes (COQ4, COQ8, COQ9, and COQ11) responsible for the synthesis of UQ, but their functions are not known. The function of Coq4 is clearly conserved in humans and plants [Citation70]. Conservation of Coq9 in higher eukaryotes is not so obvious, but interestingly, a homolog is also found in some prokaryotes [Citation33]. Coq11 is associated with the UQ synthetic enzyme complex named CoQ synthome, and is required for UQ synthesis in S. cerevisiae [Citation71]. Coq11 is also required for efficient UQ synthesis in S. pombe (unpublished). A deamination step is required for the synthesis of UQ from pABA, and the involvement of Coq9 or Coq6 has been proposed [Citation72,73]. The UbiD and UbiX homologs Pad1 and Fdc1 found in yeasts are not involved in UQ synthesis, but are required for ferulic acid synthesis [Citation74]. How decarboxylation takes place during ring formation in eukaryotes is a long-standing question in UQ synthesis.
In addition to the three-dimensional structure described previously [Citation51], the structure of Coq3 was recently solved [Citation75]. Coq3 forms a typical Class I S-adenosyl methionine methyltransferase (SAM-MTase) fold. Coq3 is a membrane-binding protein specifically binding to liposomes containing phosphatidylglycerol (PG), cardiolipin (CL), or diphosphatidylglycerol (DPPG). The three-dimensional structures of Coq7 and Coq11 are yet to be reported.
How UQ is transported has been a long-standing question. By searching for the binding protein using UQ, three UQ-binding proteins, Coq10, saposin, and voltage-dependent anion channel 1 (VDAC1), were identified [Citation76,77]. Coq10 is localized to mitochondria in eukaryotes, and homologs are found in prokaryotes. Lack of Coq10 results in respiration deficiency in yeasts [Citation76,78]. Coq10 itself is not required for the biosynthesis of UQ, but it is thought to be required for efficient operation of electron transfer systems. The binding site of Coq10 for UQ10 was determined by affinity-purified Coq10 using a UQ analog in S. pombe [Citation77]. Saposin is another protein that binds UQ, but it is only found in mammals. Among different types, saposin B was shown to bind UQ as well as tocopherol [Citation79]. VDAC1, located in the mitochondrial outer membrane of S. cerevisiae, is another UQ-binding protein [Citation80]. The role of VDAC1 in Ca2+-induced mitochondrial permeability is affected by binding to UQ, but whether this function is conserved in other organisms is not known.
The unique fission yeast Schizosaccharomyces japonicus produces 200-fold less UQ10 than S. pombe [Citation81]. This fission yeast acquires energy through fermentation and has abandoned respiration. How this yeast survives in its natural environment with such a small amount of UQ10 is interesting and worthy of study.
Menaquinones
Menaquinone (MK; 2-methyl-3-polyprenyl-1,4-naphthoquinone) is found in bacteria, and is the sole quinone in anaerobically growing bacteria [Citation82,83]. MK was discovered in 1939 by E. A. Doisy [Citation84]. MKs are found in archaea and bacteria such as γ-, δ-, and ε-proteobacteria, Gram-positive bacteria, green sulfur bacteria, green filamentous bacteria, and flavobacteria. As MKs have a low midpoint redox potential, they are believed to have appeared early in evolution before UQ, since they function in a reducing atmosphere as was present before the increase in oxygen concentration following the arrival of photosynthetic organisms.
MKs occur in different forms, with the number of isoprene units varying between 4 and 13. Some bacteria such as E. coli possess both UQ8 and MK8, and the relative amounts of each depend on oxygen levels; while UQ levels are higher under aerobic conditions, MK8 is more abundant under anaerobic conditions. Neither MK nor UQ is essential for survival in E. coli, but at least one of these prenylquinones is needed [Citation22]. The side chain of MK is usually fully unsaturated, but it can be also be partially or fully saturated in some organisms [Citation85].
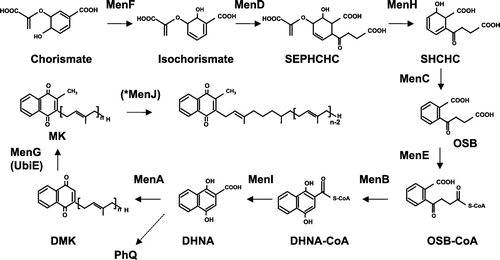
Two pathways are known for the synthesis of MK. The classical pathway involves nine steps catalyzed by MenF, MenD, MenH, MenC, MenE, MenB, MenI, MenA, and MenG (Figure ) [Citation83,86]. The biosynthesis of MK in E. coli starts from the conversion of chorismate to isochorismate by MenF, and succination is then catalyzed by MenD. In the third step, 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate (SHCHC) is synthesized by MenH, and O-succinylbenzoate (OSB) is then synthesized from SHCHC by MenC. In the fifth step, CoA is adducted to OSB by MenE, and MenB then cyclizes OSB-CoA to form DHNA-CoA. In the seventh step, MenI synthesizes naphthoate [Citation87], which is prenylated by MenA, and the product is methylated by MenG (UbiE) in the final step. Prenylation takes place during the later stages via MenA, in contrast with the synthesis of UQ in which it occurs earlier via UbiA (Figure ). The methylation enzyme (MenG) for MK is homologous to UbiE functioning in UQ synthesis in E. coli. MenJ works as a reductase of the side chain in Mycobacterium tuberculosis [Citation88]. Further methylation of MK is observed in some bacterium such as Shewanella oneidensis [Citation89].
Figure 6. Menaquinone biosynthesis in E. coli. The biosynthesis of menaquinone in E. coli starts from the conversion of chorismate to isochorismate by MenF. Subsequently, MK is synthesized by MenD (succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexadiene-1-carboxylate synthase), MenH (2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase), MenC (O-succinylbenzoate synthase), MenE (O-succinylbenzoic acid-CoA ligase), MenB (naphthoate synthase), MenI (DHNA-CoA thioesterase), MenA (1,4-dihydroxy-2-naphthoate octaprenyltransferase), and MenG (UbiE). *MenJ functions as a reductase of the side chain in Mycobacterium tuberculosis.
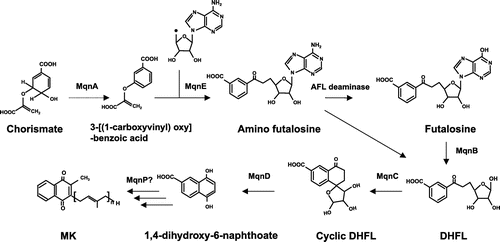
The novel pathway for MK synthesis was first discovered in Streptomyces coelicolor and subsequently in Helicobacter pylori and Thermus thermophilus [Citation90,91]. Six enzymes are engaged in 1,4-dihydroxy-6-naphthoate biosynthesis. MqnA converts chorismate to 3-[(1-carboxyvinyl)oxy]benzoic acid, which is condensed with SAM by MqnE, leading to aminofutalosine (AFL). MqnE is a radical SAM enzyme that catalyzes the addition of the adenosyl radical to the double bond of 3-[(1-carboxyvinyl)oxy]benzoic acid. Deamination of AFL is catalyzed by a specific deaminase for which no common gene name has been assigned [Citation92]. MqnB (futalosine hydrolase) then removes hypoxanthine, forming dehypoxanthinylfutalosine (DHFL) [Citation93]. MqnC cyclizes DHFL, and MqnD cleaves the cyclic 1,4-dihydroxy-6-naphthoate to release 1,4-dihydroxy-6-naphthoate (Figure ). In H. pylori, MqnB directly converts aminodeoxyfutalosine into DHFL [Citation94], indicating an alternative way in the futalosine pathway.
Figure 7. Novel pathway of menaquinone synthesis via futalosine. A novel menaquinone biosynthesis pathway was originally discovered in Streptomyces coelicolor, in which 1,4-dihydroxy-6-naphthoate is synthesized by MqnA (chorismate dehydratase), MqnD (1,4-dihydroxy-6-naphthoate synthase), MqnE (aminofutalosine synthase), AFL deaminase, MqnB (futalosine hydrolase), and MqnC (dehypoxanthine futalosine cyclase). MK synthesis from 1,4-dihydroxy-6-naphthoate is still not clearly understood, but a prenylation step catalyzed by MqnP has been proposed.
The novel genes mqnP, mqnL, and mqnN were predicted to encode enzymes involved in MK synthesis in Helicobacter cinaedi following genomic analysis of 254 human gut microbes. [Citation63]. MqnP is predicted to be involved in prenylation of 1,4-dihydroxy-6-naphthoate, and MqnL and MqnN are likely involved in decarboxylation. Comparing gene clusters can be useful for predicting biosynthetic genes such as those orchestrating the synthesis of MK. However, biochemical analysis is essential for confirming any predictions, and analysis of this futalosine pathway leading to MK is still under investigation.
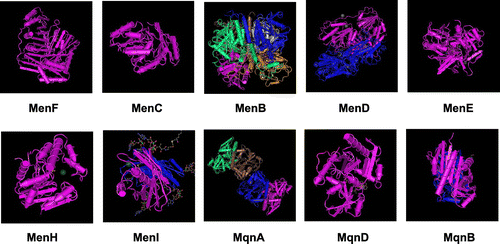
The three-dimensional structures of MK biosynthetic enzymes MenC from E. coli [Citation95] and Thermosynechococcus elongatus [Citation96], and MenB [Citation97], MenD [Citation98], MenE [Citation99], MenF [Citation100], MenH [Citation101], and MenI [Citation102] from E. coli, have been determined (Figure ). The structure of MenC is similar to that of other members of the enolase superfamily [Citation95]. The structure of MenB, a crotonase superfamily member, was solved in complex with a substrate analog, revealing an intramolecular Claisen condensation reaction mechanism [Citation97]. MenD is highly dependent on thiamine diphosphate for its structural stability [Citation98]. MenE requires a conserved arginine for binding the OSB carboxylate, and catalyzes CoA ligation via an acyl-adenylate intermediate [Citation99]. Structural and biochemical analyses of MenF revealed Lys190 as the base that activates a water molecule for nucleophilic attack at the chorismate C2 carbon [Citation100]. MenH has an α/β-hydrolase fold with a catalytic triad comprising Ser86, His232, and Asp210 [Citation101]. MenI (YdiI) belongs to the hotdog fold enzyme superfamily [Citation102]. The three-dimensional structures of MqnA [Citation103] and MqnD, and AFL deaminase (Nis0429) functioning in the futalosine-mediated MK pathway, have been solved [Citation92]. The structure of MqnA (DUF178) from Deinococcus radiodurans was originally solved as a domain of unknown function before being identified as MqnA [Citation103]. The structure of MqnD from Thermus thermophilus HB8 comprises two alpha/beta domains, a large domain, and a small domain [Citation104]. The three-dimensional structure of MqnB from H. pylori has a Rossmann fold [Citation93]. The structures of AFL deaminase (Nis0429) from Nitratiruptor sp. and Dr0824 from D. radiodurans reveal that Ser145 interacts with the carboxylate moiety of the substrate [Citation92].
Figure 8. Crystal structures of menaquinone biosynthetic enzymes. (A) MenF from E. coli (PDB ID: 2EUA). (B) MenC from E. coli (PDB ID: 1FHU). (C) MenB from E. coli (PDB ID: 3T89). (D) MenD from E. coli (PDB ID: 3HWX). (F) MenE from E. coli (PDB ID: 5C5H). (G) MenH from E. coli (PDB ID: 4GDM). (H) MenI from E. coli (PDB ID: 4K4B). (I) MqnA from Deinococcus radiodurans (PDB ID: 216E). (J) MqnD from Thermus thermophilus HBB (PDB ID: 3A3U). (K) MqnB from Helicobacter pylori (PDB ID: 4BMX).
Phylloquinones (PhQs, vitamin K1)
Phylloquinone (VK1; 2-methyl-3-phytyl-1,4-naphthoquinone) functions as an essential photosynthetic electron transporter in photosystem I, and was discovered by H. C. P. Dam in 1934 as a vitamin [Citation105]. Higher amounts are found in green leafy vegetables because it is directly involved in photosynthesis. Humans rely on PhQ uptake from vegetables as a precursor for the synthesis of MK4. PhQ is thought to be converted to MK4 by UBIAD in humans. Experiments performed on rodents showed that at least some of their tissues are able to convert PhQ to MK4. UBIAD mediates the conversion of PhQ into MK4, probably by cleaving the side chain of PhQ to generate 2-methyl-1,4-naphthoquinone (menadione; VK3), then prenylating it with GGPP to form MK4 [Citation106].
The biosynthetic pathway of PhQ in cyanobacteria and plants is thought to resemble the MK pathway [Citation2,107]. Four genes, menF, menD, menC, and menH, involved in PhQ biosynthesis in Arabidopsis, are fused at a single locus named PHYLLO [Citation108]. The structure of the MenI ortholog AtDHNAT1 (DHNA-CoA thioesterase) has been solved [Citation109]. O-succinylbenzoyl-coenzyme A (OSB-CoA) ligase (a MenE ortholog) encoded by aae14 is essential for PhQ synthesis [Citation110]. A MenG ortholog was identified as the methyltransferase catalyzing the last step of PhQ synthesis in Arabidopsis [Citation111], and a MenB homolog has been identified in the Arabidopsis genome sequence. MenB, MenI, and MenG orthologs localize to the peroxisome [Citation112], while PHYLLO, comprising MenF, MenD, MenC, and MenH orthologs, is localized to chloroplasts [Citation108]. Carboxy-1,4-naphthoquinone phytyltransferase (a MenA ortholog) is involved in PhQ synthesis chloroplasts [Citation113]. The phytyl moiety of PhQ is synthesized either by reduction of GGPP in de novo synthesis, or via the salvage pathway. Recent analysis of Arabidopsis vte6 encoding phytyl phosphate kinase revealed that it performs an essential role in PhQ synthesis [Citation114]. The entire biosynthetic pathway of PhQ in plants is still under investigation.
Plastoquinones
Plastoquinone (PQ; 2,3-dimethyl-1,4-benzoquinone), discovered in 1946 [Citation115] functions in the electron transport chain of oxygenic photosynthesis, and plays an indispensable role in plant growth and development. PQ is found in cyanobacteria and plants. PQ9 is distributed widely among organisms, while PQ8 is found in maize. In the biosynthesis of PQ in plants, tyrosine is converted to p-hydroxyphenylpyruvate (PHPP) by tyrosine aminotransferase (TAT) [Citation2]. Homogentisate (HGA) is then synthesized from HPP by p-hydroxyphenylpyruvate dioxygenase (Figure ). The prenyl tail is synthesized independently from the head group by SPS1, and IPP for prenyl tail synthesis is supplied by the MEP pathway in chloroplasts. Condensation of HGA with the prenyl tail is catalyzed by homogentisate solanesyl transferase (HST) [Citation116]. Finally, a methylation reaction is catalyzed by methyl transferase (Vte3) [Citation117]. The vte genes are required for vitamin E synthesis and perform some functions in PQ synthesis. The lipid-associated protein Fibrillin5 (FBN5), required for PQ synthesis, interacts with SPS1 and SPS2 [Citation118]. Overexpression of SPS1 in Arabidopsis resulted in enhanced phototolerance [Citation119].
Figure 9. The biosynthesis of plastoquinone. In plants, tyrosine is converted to p-hydroxyphenylpyruvate (PHPP) by TAT (tyrosine amino transferase), and the product is oxidized to HGA by HPPD (p-hydroxyphenylpyruvate dioxygenase). HGA is prenylated by HST (homogentisate prenyltransferase), and methylated by VTE3 (MSBQ methyltransferase) to yield PQ. In Synechocystis, PHB is used as a quinone backbone, and after prenylation of PHB by Slr0926, decarboxylation, oxidation, and methylation take place.
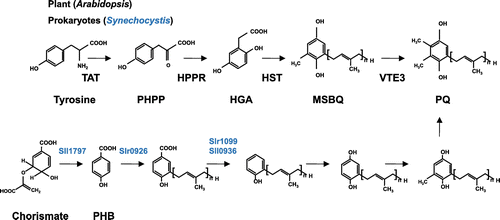
Similar to UQ, the biosynthesis of PQ differs in eukaryotes and prokaryotes. Chorismate lyase generates PHB in the synthesis of PQ in the cyanobacterium Synechocystis [Citation120]. PHB is prenylated by Slr0926, and decarboxylated by Slr1099 and Sll0936, then oxygenized and methylated to make PQ (Figure ).
There are other forms of PQ with shorter side chains such as PQ3 and PQ4, as well as analogs such as PQ-B, and PQ-C, which differ in the modification pattern of their side chains [Citation115]. PQ-C contains hydroxyl group in the prenyl chain and PQ-B is a fatty acid ester form of PQ-C [Citation115].
Rhodoquinones
Rhodoquinone (RQ; 2‐methoxy‐3‐amino-5‐methyl-6-polyprenyl-1,4-benzoquinone) was discovered in the bacterium Rhodospirillum rubrum in 1965, and subsequently in other organisms such as Rhodoferax fermentansi [Citation121], E. gracilis [Citation122], C. elegans [Citation34], planaria, parasitic helminths, snails, mussels, lungworms, and oysters. Anaerobically and aerobically grown E. gracilis cells contain similar total amounts of RQ and UQ, but RQ constitutes 43 and 28% of the pool under anaerobic and aerobic conditions, respectively [Citation122]. Helminth parasites can use fumarate as a terminal electron acceptor in the respiratory chain since they possess RQ-fumarate oxidoreductase. C. elegans produce both UQ9 and RQ9, and the relative amounts are thought to be of relevance to lifespan [Citation123]. Rhodoplanes serenus produces UQ10 and RQ10 [Citation124]. As these examples demonstrate, organisms possessing RQ also have UQ, and UQ was shown to be required for the biosynthesis of RQ in R. rubrum. A novel gene named rquA was found in R. rubrum that is required only for RQ synthesis but not UQ synthesis [Citation125]. The biosynthesis of RQ is still not fully understood.
Applications of prenylquinones
UQ10 (coenzyme Q10) is popular as a food supplement and sold worldwide in both reduced and oxidized forms. The demand for skin care cosmetics and public awareness of the importance of antioxidants such as UQ10 has increased, and UQ10 is also used therapeutically in Alzheimer’s, Huntington’s, Parkinson’s, and cardiovascular diseases [Citation47]. As UQ10 is naturally produced in humans, and available from foods such as meat and fish, side effects are very rare. Taking statins to reduce the amount of cholesterol also lowers UQ10 levels, and so taking both simultaneously is recommended [Citation47]. The UQ10 commercial market is large, and UQ10 is purified from yeast or photosynthetic bacteria. Several native producers of UQ10 have been investigated to optimize UQ10 production. S. pombe, Sporidiobolus johnsonii, Rhodobacter sphaeroides, and Agrobacterium tumefaciens reportedly produce 1.0, 10.5, 8.7, and 4.5 mg/g dry cell weight (DCW), respectively [Citation126]. However, because these amounts were measured by different groups using different methods, direct comparison is necessary to assess the efficiency of UQ10 production by these microorganisms. Attempts to produce UQ10 in rice and tobacco have proven successful [Citation127,128], and regulation of genetically modified organisms (GMOs) hampers the commercial production of UQ10 in rice.
MK is also sold as a food supplement in the form of MK4 or MK7. MK7 is from B. subtilis, and MK4 is produced in animals. The Japanese food Natto, fermented by B. subtilis, contains MK7, and increases bone mineral density and reduces bone fractures. Humans do not biosynthesize MK, but it is utilized for blood coagulation, bone metabolism, and cell-cycle regulation. The major sources of MK in humans are the diet and gut flora. As MK is only synthesized in bacteria, inhibitors of MK synthesis are useful for inhibiting the growth of harmful bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). Using this concept, analogs of each reaction step were synthesized and shown to be efficient inhibitors such as 7-methoxy naphthalene derivatives, methyl 4-oxo-4-phenylbut-2-enoate, and lysocin E of MenA [Citation129], MenB [Citation130], and MenE, respectively [Citation131].
PhQ (VK1) is used as a vitamin supplement since mammals are not able to synthesize it, and must obtain it from their diet. PhQ is as a cofactor for coagulation factors II, VII, IX, and X, required for the formation of anticoagulant factors protein C and S, and for bone protein formation. PhQ is commonly used to treat warfarin toxicity.
PQ itself is not a commercially useful product, but derivatives such as plastoquinonyl-decyl-triphenylphophonium (SkQ1) and its methylated derivative SkQ3 are under consideration for usage as antioxidants. Mitochondrial-targeted SkQ1 is currently under clinical trial for glaucoma treatment and prevention of dry eye [Citation132].
RQ is only found in a limited number of organisms that are not used for food, and applications for RQ have not been reported.
Concluding remarks
There are a wide variety of prenylquinones in nature, but their synthesis is not fully understood. In this review, the biosynthesis of prenylquinones was summarized, with emphasis on UQ, MK, PhQ, PQ, and RQ. Extensive studies have uncovered in detail MEP and MVA biosynthetic pathways that lead to the synthesis of IPP and DMAPP. Enzymes condensing IPP with DMAPP to produce polyprenyl diphosphate have been particularly well studied. While the synthesis of the isoprenoid side chain is relatively well characterized, many aspects of the modification of prenylquinones remain obscure. UQ synthesis in bacteria is quite well defined, but our knowledge of UQ synthesis in eukaryotes is incomplete. Classical MK synthesis is mostly understood, but the novel MK pathway mediated by futalosine is vague in comparison. Many three-dimensional structures related to UQ and MK synthesis have been solved, but some reactions in PhQ synthesis remain undefined. Furthermore, exactly how RQ is synthesized after UQ remains to be solved. Bioinformatics and genomics approaches are proving useful for predicting the biosynthetic pathways of these prenylquinones, but details of such work fall beyond the scope of this review. Nevertheless, this summary of recent progress on the biosynthesis of prenylquinones should prove useful, and will likely accelerate the characterization of unknown reactions and enzymes.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan [grant number 17H03806].
Acknowledgments
I thank Dr. T. Kaino for checking this manuscript.
References
- Shrader J, Bohkmann J, editors. Biotechnology of isoprenoids. Springer; 2015.
- Liu M, Lu S. Plastoquinone and ubiquinone in plants: biosynthesis, physiological function and metabolic engineering. Front Plant Sci. 2016;7:1898.
- Nowicka B, Kruk J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim Biophys Acta. 2010;1797:1587–1605.10.1016/j.bbabio.2010.06.007
- Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981;45:316–354.
- Kawamukai M. Biosynthesis, bioproduction and novel roles of ubiquinone. J Biosci Bioeng. 2002;94:511–517.10.1016/S1389-1723(02)80188-8
- Frank A, Groll M. The methylerythritol phosphate pathway to isoprenoids. Chem Rev. 2017;117:5675–5703.10.1021/acs.chemrev.6b00537
- Kuzuyama T. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem. 2002;66:1619–1627.10.1271/bbb.66.1619
- Zhao L, Chang WC, Xiao Y, et al. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu Rev Biochem. 2013;82:497–530.10.1146/annurev-biochem-052010-100934
- Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505:131–143.10.1016/j.abb.2010.09.028
- Vranova E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol. 2013;64:665–700.10.1146/annurev-arplant-050312-120116
- Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:484–493.10.2183/pjab.86.484
- Okada K, Suzuki K, Kamiya Y, et al. Polyprenyl diphosphate synthase essentially defines the length of the side chain of ubiquinone. Biochim Biophys Acta. 1996;1302:217–223.10.1016/0005-2760(96)00064-1
- Okada K, Kainou T, Matsuda H, et al. Biological significance of the side chain length of ubiquinone in Saccharomyces cerevisiae. FEBS Lett. 1998;431:241–244.10.1016/S0014-5793(98)00753-4
- Koyama T. Molecular analysis of prenyl chain elongating enzymes. Biosci Biotechnol Biochem. 1999;63:1671–1676.10.1271/bbb.63.1671
- Moriyama D, Kaino T, Yajima K, et al. Cloning and characterization of decaprenyl diphosphate synthase from three different fungi. Appl Microbiol Biotechnol. 2017;101:1559–1571.10.1007/s00253-016-7963-0
- Ferella M, Montalvetti A, Rohloff P, et al. A solanesyl-diphosphate synthase localizes in glycosomes of Trypanosoma cruzi. J Biol Chem. 2006;281:39339–39348.10.1074/jbc.M607451200
- Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem. 2009;53:217–226.10.1042/BA20090035
- Okada K, Kainou T, Tanaka K, et al. Molecular cloning and mutational analysis of the ddsA gene encoding decaprenyl diphosphate synthase from Gluconobacter suboxydans. Eur J Biochem. 1998;255:52–59.10.1046/j.1432-1327.1998.2550052.x
- Zhang M, Luo J, Ogiyama Y, et al. Heteromer formation of a long-chain prenyl diphosphate synthase from fission yeast Dps1 and budding yeast Coq1. FEBS J. 2008;275:3653–3668.10.1111/j.1742-4658.2008.06510.x
- Saiki R, Nagata A, Uchida N, et al. Fission yeast decaprenyl diphosphate synthase consists of Dps1 and the newly characterized Dlp1 protein in a novel heterotetrameric structure. Eur J Biochem. 2003;270:4113–4121.10.1046/j.1432-1033.2003.03804.x
- Saiki R, Nagata A, Kainou T, et al. Characterization of solanesyl and decaprenyl diphosphate synthases in mice and humans. FEBS J. 2005;272:5606–5622.10.1111/ejb.2005.272.issue-21
- Okada K, Minehira M, Zhu X, et al. The ispB gene encoding octaprenyl diphosphate synthase is essential for growth of Escherichia coli. J Bacteriol. 1997;179:3058–3060.10.1128/jb.179.9.3058-3060.1997
- Kainou T, Okada K, Suzuki K, Nakagawa T, Matsuda H, Kawamukai M. Dimer formation of octaprenyl-diphosphate synthase (IspB) is essential for chain length determination of ubiquinone. J Biol Chem. 2001;276:7876–7883.10.1074/jbc.M007472200
- Ducluzeau AL, Wamboldt Y, Elowsky CG, et al. Gene network reconstruction identifies the authentic trans-prenyl diphosphate synthase that makes the solanesyl moiety of ubiquinone-9 in Arabidopsis. Plant J. 2012;69:366–375.10.1111/tpj.2011.69.issue-2
- Jun L, Saiki R, Tatsumi K, et al. Identification and subcellular localization of two solanesyl diphosphate synthases from Arabidopsis thaliana. Plant Cell Physiol. 2004;45:1882–1888.10.1093/pcp/pch211
- Cui TZ, Kaino T, Kawamukai M. A subunit of decaprenyl diphosphate synthase stabilizes octaprenyl diphosphate synthase in Escherichia coli by forming a high-molecular weight complex. FEBS Lett. 2010;584:652–656.10.1016/j.febslet.2009.12.029
- Han X, Chen CC, Kuo CJ, et al. Crystal structures of ligand-bound octaprenyl pyrophosphate synthase from Escherichia coli reveal the catalytic and chain-length determining mechanisms. Proteins. 2015;83:37–45.10.1002/prot.v83.1
- Desai J, Liu YL, Wei H, et al. Structure, function, and inhibition of Staphylococcus aureus heptaprenyl diphosphate synthase. Chem Med Chem. 2016;11:1915–1923.10.1002/cmdc.v11.17
- Takahashi H, Aihara Y, Ogawa Y, et al. (in press). Suppression of phenotype of Escherichia coli mutant defective in farnesyl diphosphate synthase by overexpression of gene for octaprenyl diphosphate synthase. Biosci Biotechnol Biochem. 1–8.
- Suzuki K, Okada K, Kamiya Y, Biosci Biotechnol Biochem. Analysis of the decaprenyl diphosphate synthase (dps) gene in fission yeast suggests a role of ubiquinone as an antioxidant. J Biochem (Tokyo). 1997;121:496–505.10.1093/oxfordjournals.jbchem.a021614
- Gavilán Á, Asencio C, Cabello J, et al. C. elegans knockouts in ubiquinone biosynthesis genes result in different phenotypes during larval development. BioFactors. 2005;25:21–29.10.1002/biof.v25:1/4
- Saiki R, Lunceford AL, Shi Y, et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–1544.10.1152/ajprenal.90445.2008
- Degli Esposti M. A journey across genomes uncovers the origin of ubiquinone in cyanobacteria. Genome Biol Evol. 2017;9:3039–3053.10.1093/gbe/evx225
- Takamiya S, Matsui T, Taka H, et al. Free-living nematodes Caenorhabditis elegans possess in their mitochondria an additional rhodoquinone, an essential component of the eukaryotic fumarate reductase system. Arch Biochem Biophys. 1999;371:284–289.10.1006/abbi.1999.1465
- Yang YY, Gangoiti JA, Sedensky MM, et al. The effect of different ubiquinones on lifespan in Caenorhabditis elegans. Mech Ageing Dev. 2009;130:370–376.10.1016/j.mad.2009.03.003
- Ishii M, Kawasumi T, Igarashi Y, et al. 2-Methylthio-1,4-naphthoquinone, a unique sulfur-containing quinone from a thermophilic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. J Bacteriol. 1987;169:2380–2384.10.1128/jb.169.6.2380-2384.1987
- Nandi N, Bera T, Kumar S, et al. Involvement of thermoplasmaquinone-7 in transplasma membrane electron transport of Entamoeba histolytica trophozoites: a key molecule for future rational chemotherapeutic drug designing. J Bioenerg Biomembr. 2011;43:203–215.10.1007/s10863-011-9347-6
- Biswas S, Haque R, Bhuyan NR, et al. Participation of chlorobiumquinone in the transplasma membrane electron transport system of Leishmania donovani promastigote: effect of near-ultraviolet light on the redox reaction of plasma membrane. Biochim Biophys Acta. 2008;1780:116–127.10.1016/j.bbagen.2007.09.006
- Zhou D, White RH. Biosynthesis of caldariellaquinone in Sulfolobus spp. J Bacteriol. 1989;171:6610–6616.10.1128/jb.171.12.6610-6616.1989
- Sogi KM, Holsclaw CM, Fragiadakis GK, et al. Biosynthesis and regulation of sulfomenaquinone, a metabolite associated with virulence in Mycobacterium tuberculosis. ACS Infect Dis. 2016;2:800–806.10.1021/acsinfecdis.6b00106
- Yamada Y, Kanematsu Y, Ohashi M, Kondo K. On the partly reduced coenzyme Q isolated from Rhodotorula lactose IFO1058 and its relation to the taxonomic position. Agric Biol Chem. 1973;37:621–628.
- Crane FL. Biochemical functions of coenzyme Q. J Am Coll Nutr. 2001;20:591–598.10.1080/07315724.2001.10719063
- Zhang M, Wakitani S, Hayashi K, et al. High production of sulfide in coenzyme Q deficient fission yeast. BioFactors. 2008;32:91–98.10.1002/biof.v32:1/4
- Quinzii CM, Luna-Sanchez M, Ziosi M, et al. The role of sulfide oxidation impairment in the pathogenesis of primary CoQ deficiency. Front Physiol. 2017;8:525.10.3389/fphys.2017.00525
- Bogeski I, Gulaboski R, Kappl R, et al. Calcium binding and transport by coenzyme Q. J Am Chem Soc. 2011;133:9293–9303.10.1021/ja110190t
- Kalen A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579–584.10.1007/BF02535072
- Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition. 2010;26:250–254.10.1016/j.nut.2009.08.008
- Grant J, Saldanha JW, Gould AP. A Drosophila model for primary coenzyme Q deficiency and dietary rescue in the developing nervous system. Dis Model Mech. 2010;3:799–806.10.1242/dmm.005579
- Okada K, Ohara K, Yazaki K, Nozaki K, Uchida N, Kawamukai M, Nojiri H, Yamane H. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol Biol. 2004;55:567–577.
- Quinzii CM, Emmanuele V, Hirano M. Clinical presentations of coenzyme Q10 deficiency syndrome. Mol Syndromol. 2014;5:141–146.10.1159/000360490
- Kawamukai M. Biosynthesis of coenzyme Q in eukaryotes. Biosci Biotechnol Biochem. 2016;80:23–33.
- Stefely JA, Pagliarini DJ. Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem Sci. 2017;42:824–843.10.1016/j.tibs.2017.06.008
- Lee SQ, Tan TS, Kawamukai M, et al. Cellular factories for coenzyme Q10 production. Microb Cell Fact. 2017;16:39.10.1186/s12934-017-0646-4
- Tran UC, Clarke CF. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion. 2007;7(Suppl):S62–71.10.1016/j.mito.2007.03.007
- Wang Y, Hekimi S. Molecular genetics of ubiquinone biosynthesis in animals. Crit Rev Biochem Mol Biol. 2013;48:69–88.10.3109/10409238.2012.741564
- Suzuki K, Ueda M, Yuasa M, et al. Evidence that Escherichia coli ubiA product is a functional homolog of yeast COQ2, and the regulation of ubiA gene expression. Biosci Biotech Biochem. 1994;58:1814–1819.10.1271/bbb.58.1814
- White MD, Payne KA, Fisher K, et al. UbiX is a flavin prenyltransferase required for bacterial ubiquinone biosynthesis. Nature. 2015;522:502–506.10.1038/nature14559
- Hajj Chehade M, Loiseau L, Lombard M, et al. ubiI, a new gene in escherichia coli coenzyme Q biosynthesis, is involved in aerobic C5-hydroxylation. J Biol Chem. 2013;288:20085–20092.10.1074/jbc.M113.480368
- Poon WW, Barkovich RJ, Hsu AY, et al. Yeast and rat Coq3 and Escherichia coli UbiG polypeptides catalyze both O -methyltransferase steps in coenzyme Q biosynthesis. J Biol Chem. 1999;274:21665–21672.10.1074/jbc.274.31.21665
- Lee PT, Hsu AY, Ha HT, et al. A C-methyltransferase involved in both ubiquinone and menaquinone biosynthesis: isolation snd identification of the Escherichia coli ubiE gene. J Bacteriol. 1997;179:1748–1754.10.1128/jb.179.5.1748-1754.1997
- Kwon O, Kotsakis A, Meganathan R. Ubiquinone (coenzyme Q) biosynthesis in Escherichia coli: identification of the ubiF gene. FEMS Microbiol Lett. 2000;185:157–161.
- Loiseau L, Fyfe C, Aussel L, et al. The UbiK protein is an accessory factor necessary for bacterial ubiquinone (UQ) biosynthesis and forms a complex with the UQ biogenesis factor UbiJ. J Biol Chem. 2017;292:11937–11950.10.1074/jbc.M117.789164
- Ravcheev DA, Thiele I. Genomic analysis of the human gut microbiome suggests novel enzymes involved in quinone biosynthesis. Front Microbiol. 2016;7:128.
- Zhu X, Yuasa M, Okada K, et al. Production of ubiquinone in Escherichia coli by expression of various genes responsible for ubiquinone biosynthesis. J Ferment Bioeng. 1995;79:493–495.10.1016/0922-338X(95)91268-A
- Payet LA, Leroux M, Willison JC, et al. Mechanistic details of early steps in coenzyme Q biosynthesis pathway in yeast. Cell Chem Biol. 2016;23:1241–1250.10.1016/j.chembiol.2016.08.008
- Uchida N, Suzuki K, Saiki R, et al. Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-Hydroxybenzoate polyprenyl diphosphate transferase. J Bacteriol. 2000;182:6933–6939.10.1128/JB.182.24.6933-6939.2000
- Ozeir M, Mühlenhoff U, Webert H, et al. Coenzyme Q biosynthesis: Coq6 is required for the C5-hydroxylation reaction and substrate analogs rescue Coq6 deficiency. Chem Biol. 2011;18:1134–1142.10.1016/j.chembiol.2011.07.008
- Baba SW, Belogrudov GI, Lee JC, et al. Yeast Coq5 C -methyltransferase is required for stability of other polypeptides involved in coenzyme Q biosynthesis. J Biol Chem. 2004;279:10052–10059.10.1074/jbc.M313712200
- Miki R, Saiki R, Ozoe Y, et al. Comparison of a coq7 deletion mutant with other respiration-defective mutants in fission yeast. FEBS J. 2008;275:5309–5324.10.1111/j.1742-4658.2008.06661.x
- Hayashi K, Ogiyama Y, Yokomi K, et al. Functional conservation of coenzyme Q biosynthetic genes among yeasts, plants, and humans. PLoS ONE. 2014;9:e99038.10.1371/journal.pone.0099038
- Allan CM, Awad AM, Johnson JS, et al. Identification of Coq11, a new coenzyme Q biosynthetic protein in the CoQ-synthome in Saccharomyces cerevisiae. J Biol Chem. 2015;290:7517–7534.10.1074/jbc.M114.633131
- He CH, Black DS, Nguyen TP, et al. Yeast Coq9 controls deamination of coenzyme Q intermediates that derive from para-aminobenzoic acid. Biochim Biophys Acta. 2015;1851:1227–1239.10.1016/j.bbalip.2015.05.003
- Ozeir M, Pelosi L, Ismail A, et al. Coq6 is responsible for the C4-deamination reaction in coenzyme Q biosynthesis in Saccharomyces cerevisiae. J Biol Chem. 2015;290:24140–24151.10.1074/jbc.M115.675744
- Mukai N, Masaki K, Fujii T, et al. PAD1 and FDC1 are essential for the decarboxylation of phenylacrylic acids in Saccharomyces cerevisiae. J Biosci Bioeng. 2010;109:564–569.10.1016/j.jbiosc.2009.11.011
- Zhu Y, Wu B, Zhang X, et al. Structural and biochemical studies reveal UbiG/Coq3 as a class of novel membrane-binding proteins. Biochem J. 2015;470:105–114.10.1042/BJ20150329
- Cui T-Z, Kawamukai M. Coq10, a mitochondrial coenzyme Q binding protein, is required for proper respiration in Schizosaccharomyces pombe. FEBS J. 2009;276:748–759.10.1111/j.1742-4658.2008.06821.x
- Murai M, Matsunobu K, Kudo S, et al. Identification of the binding site of the quinone-head group in mitochondrial Coq10 by photoaffinity labeling. Biochemistry. 2014;53:3995–4003.10.1021/bi500347s
- Barros MH, Johnson A, Gin P, et al. The Saccharomyces cerevisiae COQ10 gene encodes a START domain protein required for function of coenzyme Q in respiration. J Biol Chem. 2005;280:42627–42635.10.1074/jbc.M510768200
- Jin G, Kubo H, Kashiba M, et al. Saposin B Is a human coenzyme Q10-binding/transfer protein. J Clin Biochem Nutr. 2008;42:167–174.10.3164/jcbn.2008024
- Murai M, Okuda A, Yamamoto T, et al. Synthetic ubiquinones specifically bind to mitochondrial voltage-dependent anion channel 1 (VDAC1) in Saccharomyces cerevisiae mitochondria. Biochemistry. 2017;56:570–581.10.1021/acs.biochem.6b01011
- Kaino T, Tonoko K, Mochizuki S, et al. (in press). Schizosaccharomyces japonicus has low levels of CoQ10 synthesis, respiration deficiency, and efficient ethanol production at high temperature. Biosci Biotechnol Biochem. 1–12.
- Paudel A, Hamamoto H, Panthee S, et al. Menaquinone as a potential target of antibacterial agents. Drug Discov Ther. 2016;10:123–128.10.5582/ddt.2016.01041
- Meganathan R, Kwon O. Biosynthesis of menaquinone (Vitamin K2) and ubiquinone (coenzyme Q), EcoSal Plus, 3; 2009.
- McKee RW, Binkley SB, Thayer SA, et al. The isolation of vitamin K2. J Biol Chem. 1939;1312:327–344.
- Hemmi H, Takahashi Y, Shibuya K, et al. Menaquinone-specific prenyl reductase from the hyperthermophilic archaeon Archaeoglobus fulgidus. J Bacteriol. 2005;187:1937–1944.10.1128/JB.187.6.1937-1944.2005
- Meganathan R. Ubiquinone biosynthesis in microorganisms. FEMS Microbiol Lett. 2001;203:131–139.
- Chen M, Ma X, Chen X, et al. Identification of a hotdog fold thioesterase involved in the biosynthesis of menaquinone in Escherichia coli. J Bacteriol. 2013;195:2768–2775.10.1128/JB.00141-13
- Upadhyay A, Fontes FL, Gonzalez-Juarrero M, et al. Partial saturation of menaquinone in Mycobacterium tuberculosis: function and essentiality of a novel reductase, MenJ. ACS Cent Sci. 2015;1:292–302.10.1021/acscentsci.5b00212
- Hein S, Klimmek O, Polly M, et al. A class C radical S-adenosylmethionine methyltransferase synthesizes 8-methylmenaquinone. Mol Microbiol. 2017;104:449–462.10.1111/mmi.2017.104.issue-3
- Hiratsuka T, Furihata K, Ishikawa J, et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science. 2008;321:1670–1673.10.1126/science.1160446
- Dairi T. Menaquinone biosyntheses in microorganisms. Methods Enzymol. 2012;515:107–122.10.1016/B978-0-12-394290-6.00006-9
- Goble AM, Toro R, Li X, et al. Deamination of 6-aminodeoxyfutalosine in menaquinone biosynthesis by distantly related enzymes. Biochemistry. 2013;52:6525–6536.10.1021/bi400750a
- Kim RQ, Offen WA, Davies GJ, et al. Structural enzymology of Helicobacter pylori methylthioadenosine nucleosidase in the futalosine pathway. Acta Crystallogr D Biol Crystallogr. 2014;70:177–185.10.1107/S1399004713026655
- Arakawa C, Kuratsu M, Furihata K, et al. Diversity of the early step of the futalosine pathway. Antimicrob Agents Chemother. 2011;55:913–916.10.1128/AAC.01362-10
- Thompson TB, Garrett JB, Taylor EA, et al. Evolution of enzymatic activity in the enolase superfamily: structure of o-succinylbenzoate synthase from Escherichia coli in complex with Mg2+ and o-succinylbenzoate. Biochemistry. 2000;39:10662–10676.10.1021/bi000855o
- Odokonyero D, Sakai A, Patskovsky Y, et al. Loss of quaternary structure is associated with rapid sequence divergence in the OSBS family. Proc Natl Acad Sci U S A. 2014;111:8535–8540.10.1073/pnas.1318703111
- Li HJ, Li X, Liu N, et al. Mechanism of the intramolecular claisen condensation reaction catalyzed by MenB, a crotonase superfamily member. Biochemistry. 2011;50:9532–9544.10.1021/bi200877x
- Priyadarshi A, Kim EE, Hwang KY. Structural and functional analysis of Vitamin K2 synthesis protein MenD. Biochem Biophys Res Commun. 2009;388:748–751.10.1016/j.bbrc.2009.08.093
- Matarlo JS, Evans CE, Sharma I, et al. Mechanism of MenE inhibition by acyl-adenylate analogues and discovery of novel antibacterial agents. Biochemistry. 2015;54:6514–6524.10.1021/acs.biochem.5b00966
- Kolappan S, Zwahlen J, Zhou R, et al. Lysine 190 is the catalytic base in MenF, the menaquinone-specific isochorismate synthase from Escherichia coli: implications for an enzyme family. Biochemistry. 2007;46:946–953.10.1021/bi0608515
- Johnston JM, Jiang M, Guo Z, et al. Crystal structures of E. coli native MenH and two active site mutants. PLoS ONE. 2013;8:e61325.10.1371/journal.pone.0061325
- Latham JA, Chen D, Allen KN, et al. Divergence of substrate specificity and function in the Escherichia coli hotdog-fold thioesterase paralogs YdiI and YbdB. Biochemistry. 2014;53:4775–4787.10.1021/bi500333 m
- Tyagi R, Burley SK, Swaminathan S. X-ray structures of two proteins belonging to Pfam DUF178 revealed unexpected structural similarity to the DUF191 Pfam family. BMC Struct Biol. 2007;7:62.10.1186/1472-6807-7-62
- Arai R, Murayama K, Uchikubo-Kamo T, et al. Crystal structure of MqnD (TTHA1568), a menaquinone biosynthetic enzyme from Thermus thermophilus HB8. J Struct Biol. 2009;168:575–581.10.1016/j.jsb.2009.07.007
- Dam H. The antihaemorrhagic vitamin of the chick. Biochem J. 1935;29:1273–1285.10.1042/bj0291273
- Nakagawa K, Hirota Y, Sawada N, et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–121.10.1038/nature09464
- Basset GJ, Latimer S, Fatihi A, et al. Phylloquinone (Vitamin K1): Occurrence, Biosynthesis and Functions. Mini Rev Med Chem. 2017;17:1028–1038.
- Gross J, Cho WK, Lezhneva L, et al. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. J Biol Chem. 2006;281:17189–17196.10.1074/jbc.M601754200
- Furt F, Allen WJ, Widhalm JR, et al. Functional convergence of structurally distinct thioesterases from cyanobacteria and plants involved in phylloquinone biosynthesis. Acta Crystallogr D Biol Crystallogr. 2013;69:1876–1888.10.1107/S0907444913015771
- Kim HU, van Oostende C, Basset GJ, et al. The AAE14 gene encodes the Arabidopsis o-succinylbenzoyl-CoA ligase that is essential for phylloquinone synthesis and photosystem-I function. Plant J. 2008;54:272–283.10.1111/j.1365-313X.2008.03416.x
- Lohmann A, Schottler MA, Brehelin C, et al. Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J Biol Chem. 2006;281:40461–40472.10.1074/jbc.M609412200
- Reumann S. Biosynthesis of vitamin K1 (phylloquinone) by plant peroxisomes and its integration into signaling molecule synthesis pathways. Subcell Biochem. 2013;69:213–229.10.1007/978-94-007-6889-5
- Shimada H, Ohno R, Shibata M, et al. Inactivation and deficiency of core proteins of photosystems I and II caused by genetical phylloquinone and plastoquinone deficiency but retained lamellar structure in a T-DNA mutant of Arabidopsis. Plant J. 2005;41:627–637.10.1111/tpj.2005.41.issue-4
- Wang L, Li Q, Zhang A, et al. The phytol phosphorylation pathway is essential for the biosynthesis of phylloquinone, which is required for photosystem i stability in Arabidopsis. Mol Plant. 2017;10:183–196.10.1016/j.molp.2016.12.006
- Barr R, Crane FL. Comparative studies on plastoquinones. III. Distribution of plastoquinones in higher plants. Plant Physiol. 1967;42:1255–1263.
- Sadre R, Frentzen M, Saeed M, et al. Catalytic reactions of the homogentisate prenyl transferase involved in plastoquinone-9 biosynthesis. J Biol Chem. 2010;285:18191–18198.10.1074/jbc.M110.117929
- Liu FZ, Guo AQ, Wan YS. Cloning and polymorphism analysis of the 2-methyl-6-phytyl-1,4-benzoquinol methyltransferase gene (VTE3) in Arachis hypogaea, A. duranensis, and A. ipaensis. Genet Mol Res. 2013;12:1859–1871.10.4238/2013.January.4.5
- Kim EH, Lee DW, Lee KR, et al. Conserved function of Fibrillin5 in the plastoquinone-9 biosynthetic pathway in arabidopsis and rice. Front Plant Sci. 2017;8:1197.10.3389/fpls.2017.01197
- Ksas B, Becuwe N, Chevalier A, et al. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci Rep. 2015;5:10919.10.1038/srep10919
- Pfaff C, Glindemann N, Gruber J, et al. Chorismate pyruvate-lyase and 4-hydroxy-3-solanesylbenzoate decarboxylase are required for plastoquinone biosynthesis in the Cyanobacterium Synechocystis sp. PCC6803. J Biol Chem. 2014;289:2675–2686.10.1074/jbc.M113.511709
- Miyadera H, Hiraishi A, Miyoshi H, et al. Complex II from phototrophic purple bacterium Rhodoferax fermentans displays rhodoquinol-fumarate reductase activity. Eur J Biochem. 2003;270:1863–1874.10.1046/j.1432-1033.2003.03553.x
- Hoffmeister M, van der Klei A, Rotte C, et al. Euglena gracilis rhodoquinone:ubiquinone ratio and mitochondrial proteome differ under aerobic and anaerobic conditions. J Biol Chem. 2004;279:22422–22429.10.1074/jbc.M400913200
- Larsen PL, Clarke CF. Extension of life-span in caenorhabditis elegans by a diet lacking coenzyme Q. Science. 2002;295:120–123.10.1126/science.1064653
- Okamura K, Kanbe T, Hiraishi A. Rhodoplanes serenus sp. nov., a purple non-sulfur bacterium isolated from pond water. Int J Syst Evol Microbiol. 2009;59:531–535.10.1099/ijs.0.000174-0
- Lonjers ZT, Dickson EL, Chu TP, et al. Identification of a new gene required for the biosynthesis of rhodoquinone in Rhodospirillum rubrum. J Bacteriol. 2012;194:965–971.10.1128/JB.06319-11
- Zahiri HS, Noghabi KA, Shin YC. Biochemical characterization of the decaprenyl diphosphate synthase of Rhodobacter sphaeroides for coenzyme Q10 production. Appl Microbiol Biotechnol. 2006;73:796–806.10.1007/s00253-006-0524-1
- Takahashi S, Ogiyama Y, Kusano H, et al. Metabolic engineering of coenzyme Q by modification of isoprenoid side chain in plant. FEBS Lett. 2006;580:955–959.10.1016/j.febslet.2006.01.023
- Ohara K, Kokado Y, Yamamoto H, et al. Engineering of ubiquinone biosynthesis using the yeast coq2 gene confers oxidative stress tolerance in transgenic tobacco. Plant J. 2004;40:734–743.10.1111/tpj.2004.40.issue-5
- Choi SR, Frandsen J, Narayanasamy P. Novel long-chain compounds with both immunomodulatory and MenA inhibitory activities against Staphylococcus aureus and its biofilm. Sci Rep. 2017;7:40077.10.1038/srep40077
- Matarlo JS, Lu Y, Daryaee F, et al. A methyl 4-oxo-4-phenylbut-2-enoate with in vivo activity against MRSA that inhibits MenB in the bacterial menaquinone biosynthesis pathway. ACS Infect Dis. 2016;2:329–340.10.1021/acsinfecdis.6b00023
- Hamamoto H, Urai M, Ishii K, et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat Chem Biol. 2015;11:127–133.10.1038/nchembio.1710
- Jezek J, Engstova H, Jezek P. Antioxidant mechanism of mitochondria-targeted plastoquinone SkQ1 is suppressed in aglycemic HepG2 cells dependent on oxidative phosphorylation. Biochim Biophys Acta. 2017;1858:750–762.10.1016/j.bbabio.2017.05.005
