Abstract
Serum starvation induces binucleation in HeLa cells, but the effects of serum starvation on mitosis and the significance of binucleation remain unknown. We investigated the effect of serum starvation on mitosis and analyzed the growth of binucleated cells. The frequency of binucleation caused by cytokinesis failure in DMEM without FBS (0% medium) was higher than that in DMEM with FBS (10% medium). In 0% medium, the metaphase spindle location was off-center, and RhoA localization significantly lacked symmetry. The frequency of the extension of intercellular bridge with the midbody in 0% medium was significantly higher than that in 10% medium. Moreover, all mononucleated mitotic cells caused bipolar mitosis and produced only mononucleated daughter cells, but binucleated cells produced various nucleated cells by multipolar mitosis in 0% medium. These results suggest that serum starvation may have various effects on mitosis, and binucleated cells may be related to formation of aneuploidy.
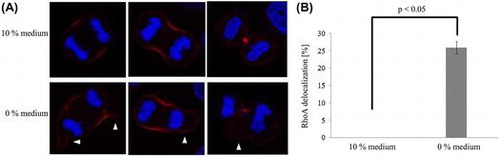
RhoA localization in two different media.
Serum includes hormones and proteins that are necessary for cell proliferation, survival, and function. Serum starvation induces cell cycle arrest [Citation1] and has been the commonly used method to arrest and synchronize cultured cells for experiments. However, some studies have reported that serum or glucose starvation induces apoptosis and mitotic chromosomal instability [Citation2–4]. We also reported that serum starvation induces cytokinesis failure and binucleation in HeLa cells [Citation5]. These studies suggest that serum starvation may have various effects on mitosis.
A study has shown that a chemical can induce mitotic abnormalities. Nakayama et al. reported that genistein induces cytokinesis failure via an anaphase chromosome bridge and RhoA delocalization [Citation6]. It is known that cytokinesis is regulated by various factors such as RhoA and Ect2 [Citation7]. RhoA also plays an important role in spindle formation and mitotic rounding [Citation8]. Thus, chemicals may have various effects on mitosis. However, the effects of serum starvation on mitosis are not fully understood.
Binucleated cells are sometimes observed in malignant lesions, including thyroid carcinoma and malignant mesothelioma [Citation9,10]. However, binucleated cells have also been detected in benign lesions, such as cervical squamous cells and liver cells [Citation11,12]. Therefore, the exact significance of binucleated cells in lesions is unclear. Recent studies report new meanings of existence of binucleated cells. Mammary epithelial binucleated cells evolved to maximize milk production and promote the survival of offspring in all mammalian species [Citation13]. In contrast, tetraploids such as binucleated cells formed by cytokinesis failure promote tumorigenesis [Citation14]. Thus, the significance of binucleated cells in lesions may be due to a variety of reasons. We have previously found that some binucleated cells induced by cytokinesis failure maintain proliferation potential [Citation5]. However, the significance of binucleated cells under serum starvation conditions remains unknown.
Investigating the growth of binucleated cells under serum starvation conditions may be helpful in understanding the significance of binucleated cells in lesions. In this study, we aimed to investigate the effects of serum starvation on mitosis and analyze the growth of binucleated cells undergoing serum starvation to gain insight about the formation mechanism and the significance of binucleation caused by serum starvation.
Materials and methods
Cells and culture conditions
HeLa cells (courtesy of Saga Medical School, Saga, Japan) were cultured in two different media: Dulbecco’s modified Eagle’s medium (DMEM; D5796, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; S1820, BioWest, Nuaillé, France) or DMEM without FBS, 10 and 0% medium, respectively. All cells were cultured at 37 °C with 5% CO2.
The frequency of binucleation
HeLa cells (1 × 105 cells/μL) were grown on tissue culture dishes (l-Dish, 35 mm, Ibidi, Martinsried, Germany) for 1 day in 10 or 0% medium. Cultured cells were observed using a time-lapse microscopy system (Biostation IM, Nikon, Japan). The frequency of binucleation per mitotic cell was observed in both 10 and 0% medium.
Immunofluorescence analysis and microscopy
HeLa cells (1 × 105 cells/μL) were grown on chamber slides (FALCON354114, Becton Dickinson, Franklin Lakes, NJ, USA) for 12 h in the two media, and the centrality of metaphase spindle formation, RhoA localization, and the frequency of the extension of intercellular bridge with the midbody were analyzed (details explained below). For RhoA, cells were fixed with 10% TCA for 15 min. For α-tubulin, cells were fixed with cold methanol for 10 min. Cells were rinsed twice with PBS and then treated with 0.2% Triton X-100 to permeabilize cells. Afterward, cells were incubated at 4 °C overnight with the RhoA (26C4, Alexa Fluor 647 conjugated monoclonal antibody, Santa Cruz Biotechnology, Dallas, TX, USA) and α-tubulin antibodies (DM1A, FITC-conjugated monoclonal antibody, Sigma-Aldrich). DNA was stained with DAPI for 15 min. Images were obtained using a laser scanning microscope (LSM700, Zeiss, Germany).
The centrality of metaphase spindle formation
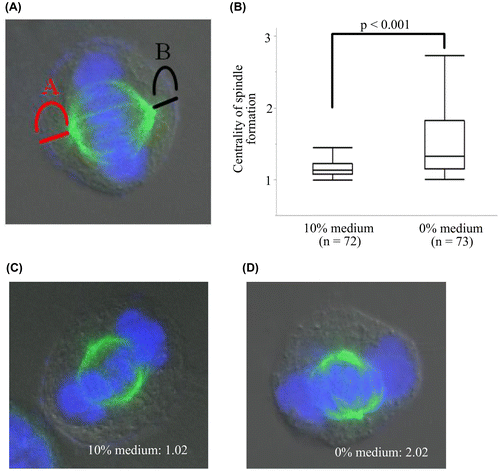
The centrality of metaphase spindle formation in 10 and 0% medium was analyzed as previously described [Citation6]. Briefly, the distances of the poles to their respective polar cortex A and B (Figure (A)) were measured, and the ratio of A to B in the two media was analyzed. The ratio was always at least 1. When the spindle formation was centered, the ratio was 1.
Figure 1. The centrality of spindle formation in two different media. (A) A and B are distances of the poles to their respective polar cortex. We measured and analyzed the ratio of A to B and evaluated the centrality of metaphase spindle formation. The ratio is always at least 1. (B) The ratio in 0% medium was significantly higher than that in 10% medium indicating that the spindle location in 0% medium was a little off-center. (C) Spindle formation in 10% medium. (D) Spindle formation in 0% medium.
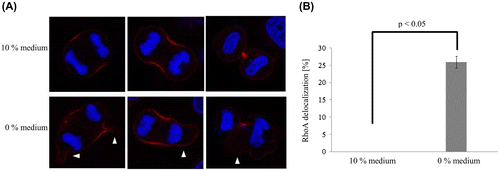
RhoA localization
RhoA localization during mitosis in 10 and 0% medium was analyzed. RhoA delocalization was defined as when RhoA was not present at or localized symmetrically to the equatorial cell cortex.
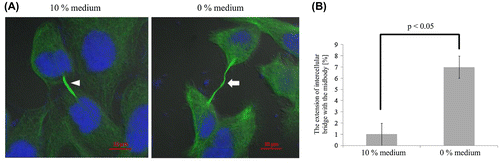
The frequency of the extension of intercellular bridge with the midbody
The frequency of the extension of intercellular bridge with the midbody during cytokinesis was observed and analyzed. The extension of intercellular bridge with the midbody was defined as when the length of intercellular bridge was more than 10 μm.
The spindle formation and the products of binucleated and mononucleated cells
HeLa cells (1 × 105 cells/μL) were grown on tissue culture dishes as described above for 1 day in 0% medium. The spindle formation and the products of binucleated and mononucleated cells for 2 more days in 0% medium were analyzed using the time-lapse microscopy system.
Statistical analysis
Statistical analyses were carried out using JMP pro 12 (SAS Institute, Cary, NC, USA). Student’s t test, the Mann–Whitney U test, and the Fisher’s exact test were used as appropriate. A p value of < 0.05 was considered statistically significant.
Results
Serum starvation induced binucleation by cytokinesis failure
The frequency of binucleation is shown in Table . In 10% medium, the frequency of binucleation was 0.4%. In contrast, the frequency of binucleation in 0% medium was 9.4%. All binucleated cells were formed due to cytokinesis failure rather than cell fusion.
Table 1. The frequency of binucleation in the two media.
Serum starvation induced abnormal spindle location
The analysis of the centrality of metaphase spindle formation is shown in Figure . The average of the ratio was 1.17 and 1.54 in 10 and 0% medium, respectively. The ratio in 0% medium was significantly higher than that in 10% medium (p < 0.001), indicating that the metaphase spindle location in 0% medium was a little off-center.
Serum starvation induced RhoA delocalization
In cells cultured in 10% medium, RhoA was present at the equatorial cell cortex during anaphase and accumulated at the cleavage furrow (Figure (A)). In contrast, in cells cultured in the 0% medium, RhoA was not significantly present at or localized symmetrically to the equatorial cell cortex (p < 0.05; Figure (A) and (B)).
Figure 2. RhoA localization in two different media. (A) In 10% medium, RhoA was present at the equatorial cell cortex in anaphase and accumulated at the cleavage furrow. In contrast, in 0% medium, RhoA lacked symmetry (arrowhead). (B) The frequency of RhoA delocalization in 0% medium was significantly higher than that in 10% medium. Each value is the mean ± SD of three independent experiments.
Serum starvation induced the extension of intercellular bridge with the midbody
The extension of intercellular bridge with the midbody is shown in Figure (A). In 10% medium, the maximum length of the intercellular bridge with the midbody was about 12 μm. In contrast, the maximum length of the intercellular bridge with the midbody in 0% medium was about 20 μm. The frequency of the extension of intercellular bridge with the midbody was approximately 1% in the 10% medium and significantly higher at 7% in the 0% medium (p < 0.05; Figure (B)).
Figure 3. The extension of intercellular bridge with the midbody in two different media. (A) In 10% medium, the length of the extension of intercellular bridge with the midbody (arrowhead) was about 12 μm. In contrast, the maximum length of the extension of intercellular bridge with the midbody (arrow) in 0% medium was about 20 μm. (B) In 10% medium, the frequency of the extension of intercellular bridge with the midbody was about 1%. In contrast, the frequency of the extension of intercellular bridge with the midbody in 0% medium was about 7%, which was significantly higher than that in 10% medium. Each value is the mean ± SD of three independent experiments.
Binucleated cells caused multipolar mitosis and produced variably nucleated daughter cells
The analysis of the spindle formation in the 0% medium is depicted in Table . All mononucleated mitotic cells caused bipolar mitosis and produced only mononucleated daughter cells. In contrast, binucleated cells caused various multipolar mitoses such as bipolar, tripolar, and tetrapolar mitoses (p < 0.001). Moreover, binucleated cells produced various nucleated daughter cells such as mono-, bi-, tri-, and tetranucleated cells (p < 0.001).
Table 2. The spindle formation and the products of mononucleated and binucleated cells.
Discussion
In this study, we investigated the effects of serum starvation on mitosis and analyzed the growth of binucleated cells under serum starvation conditions. First, we investigated the mechanism and the frequency of binucleation in two different media. Binucleated cells may arise due to a variety of causes including cytokinesis failure [Citation5,15], cell–cell fusion [Citation16,17], and multipolar spindles [Citation18]. In this study, all binucleated cells were formed by cytokinesis failure, which is in agreement with our previous study [Citation5]. Furthermore, the frequency of binucleation in 0% medium was 9.4%, which was higher than in the 10% medium. Previous studies have shown that serum or glucose starvation induces chromosomal damage [Citation2,4]. Chromosomal damage has also been shown to produce binucleated cells [Citation19]. Chromosomal mis-segregation occurs at a high frequency in binucleated cells that arise spontaneously [Citation20]. These findings indicate that serum starvation may affect chromosomal stability, which may be related to binucleation. Tumor cells are continuously exposed to hypoxia and glucose starvation [Citation21], which suggests that the presence of binucleated cells in lesions may indicate environmental deterioration affecting cell proliferation. However, we analyzed only HeLa cells, and the frequency of binucleation caused by serum starvation was negligible. Therefore, we should conduct further studies to analyze other cell lines for clarifying the effect of serum starvation on binucleation.
We also investigated the effect of serum starvation on the centrality of metaphase spindle formation. In 10% medium, spindle formation was centered. In contrast, the spindle formation in 0% medium was off-center. The spindle position determines the location of contractile ring assembly [Citation22], and accurate spindle location in the center of the cell is essential for normal cell division. Genistein induces abnormal spindle position, which results in cytokinesis failure [Citation6]. RhoA regulates spindle formation and cytokinesis [Citation8]; therefore, we next investigated the effect of serum starvation on RhoA localization during mitosis. In this study, RhoA was present at the equatorial cell cortex in anaphase and symmetrically accumulated at the cleavage furrow in 10% medium. In contrast, in 0% medium, RhoA significantly lacked symmetry. Accurate RhoA localization at the equatorial cell cortex is critical for the execution of mitosis and inhibition of Rho GTPases by C3 toxin leads to spindle mis-orientation [Citation23]. Cells depleted of RhoA by siRNA exhibit defective cytokinesis [Citation24]. These findings indicate that RhoA delocalization is directly related to binucleation caused by cytokinesis failure under serum starvation conditions. However, the frequency of binucleation per round of mitosis in 0% medium was lower than the frequency of RhoA delocalization, indicating that binucleation caused by serum starvation may be adjusted not only by RhoA delocalization but also other factors. Therefore, further investigation of cytokinesis-related factors that regulate RhoA, such as Ect2 and Mklp1, is warranted.
In addition, we investigated the intercellular bridge during cytokinesis. The frequency of the extension of intercellular bridge with the midbody in 0% medium was higher than that in 10% medium. The mechanism of cell separation during cytokinesis consists of three processes: constricting midbody, severance of microtubules in the midbody, and abscission of a thin membrane stalk [Citation25]. RhoA also plays an important role in contractile activity during cytokinesis. The intermediate filament bridge-like formation connects the two daughter cells during cytokinesis and tetraploid cells are formed by cytokinesis failure [Citation26]. The bridge formed by vimentin is mediated by activation of RhoA [Citation27]. Therefore, we suggest that RhoA delocalization may also be related to the extension of intercellular bridge with the midbody under serum starvation conditions. Interestingly, multiple studies have reported that RhoA is related to cell motility, invasion, and metastasis in malignant tumors [Citation28–30]. Cancer cells adjust activation and suppression of RhoA in response to changes in their environment [Citation31]. Microenvironmental stimulation, such as malnutrition and hypoxia, promote Angptl2 transcription, which promotes cell motility and metastasis [Citation32]. These findings suggest that external stimulation, such as serum starvation, may exhibit effects on RhoA localization and cell motility in lesions. Therefore, we plan to investigate the mechanism of RhoA delocalization caused by serum starvation in a further study.
Additionally, we investigated division dynamics of binucleated cells. Our data indicate that spindle formation and the products of binucleated cells differed from mononucleated cells. All mononucleated mitotic cells underwent bipolar mitosis, but binucleated cells underwent bipolar, tripolar, or tetrapolar mitoses. Binucleated cells also produced various nucleated daughter cells such as mono-, bi-, tri-, and tetranucleated cells. Cytokinesis failure and multipolar mitoses have been demonstrated to drive aneuploidy [Citation33]. Moreover, binucleated cells induced by cytokinesis failure promote tumorigenesis [Citation14]. Binucleated cells produce aneuploid cells due to high rates of chromosomal mis-segregation resulting from multipolar mitosis [Citation20]. Based on these findings, our results suggest that binucleated cells induced by serum starvation may also be related to various ploidy or aneuploidy formation via multipolar mitosis. However, we did not analyze aneuploidy in binucleated cells in this study. Therefore, future studies will focus on chromosomal aneuploidy in binucleated cells using fluorescent in situ hybridization. Serum starvation is a commonly used method to arrest and synchronize cells in culture for experiments. Our studies suggest that serum starvation should not be used to arrest and synchronize cells due to abnormal mitosis such as cytokinesis failure and binucleation.
We previously reported that the frequency of binucleated HeLa cells increases in serum starvation, and some binucleated cells have the potential of proliferation [Citation5]. In the present study, we sought to address this phenomenon in further detail. This is the first report demonstrating that serum starvation induces abnormal spindle location, RhoA delocalization, and extension of intercellular bridge with the midbody. These abnormalities are known to be related to cytokinesis failure, and we considered these abnormalities in relation to binucleation. However, only in this study, it remains unclear whether the three abnormalities cause binucleation. In order to address this question, we should investigate the connection between these abnormalities and binucleation. Moreover, we showed that binucleated cells produce various nucleated cells by multipolar mitosis under serum starvation conditions. These findings further contribute to the understanding and underscore the significance of binucleated cells in tumors. Further investigation into the mechanisms of binucleation induced by serum starvation is warranted to understand binucleation dynamics in tumor cells.
Disclosure statement
The authors declare no conflicts of interest.
Author contributions
K. Nishimura performed the experiments, and drafted the manuscript. S. Watanabe contributed reagents/materials/analysis tools. S. Watanabe, T. Kaku and S. Sugishima contributed to the implementation of the research, and provided valuable advices.
References
- Shin JS, Hong SW, Lee SL, et al. Serum starvation induces G1 arrest through suppression of Skp2-CDK2 and CDK4 in SK-OV-3 cells. Int J Oncol. 2008;32(2):435–439.
- Dai C, Sun F, Zhu C, et al. Tumor environmental factors glucose deprivation and lactic acidosis induce mitotic chromosomal instability – an implication in aneuploid human tumors. PLoS ONE. 2013;8(5):e63054.10.1371/journal.pone.0063054
- Kulkarni GV, McCulloch CA. Serum deprivation induces apoptotic cell death in a subset of Balb/c 3T3 fibroblasts. J Cell Sci. 1994;107(5):1169–1179.
- Li CY, Little JB, Hu K, et al. Persistent genetic instability in cancer cells induced by non-DNA-damaging stress exposures. Cancer Res. 2001;61(2):428–432.
- Nishimura K, Watanabe S, Hayashida R, et al. Binucleated HeLa cells are formed by cytokinesis failure in starvation and keep the potential of proliferation. Cytotechnol. 2016;68:1123–1130.10.1007/s10616-015-9869-6
- Nakayama Y, Saito Y, Soeda S, et al. Genistein induces cytokinesis failure through RhoA delocalization and anaphase chromosome bridging. J Cell Biochem. 2014;115(4):763–771.10.1002/jcb.24720
- Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119(1):104–114.10.1242/jcs.02737
- Heng YW, Koh CG. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell B. 2010;42(10):1622–1633.10.1016/j.biocel.2010.04.007
- Kane SV, Sharma TP. Cytologic diagnostic approach to poorly differentiated thyroid carcinoma: a single-institution study. Cancer Cytopathol. 2015;123(2):82–91.10.1002/cncy.21500
- Kimura N, Dota K, Araya Y, et al. Scoring system for differential diagnosis of malignant mesothelioma and reactive mesothelial cells on cytology specimens. Diagn Cytopathol. 2009;37:885–890.10.1002/dc.v37:12
- Washiya K, Motoi M, Kobayashi T, et al. Significance of binucleated cells with compression in atypical squamous cells of undetermined significance. Acta Cytologica. 2013;57(6):599–603.10.1159/000353802
- Morizur SC, Merlen G, Couton D, et al. Polyploidy and liver proliferation: central role of insulin signaling. Cell Cycle. 2010;9:460–466.10.4161/cc.9.3.10542
- Rios AC, Fu NY, Jamieson PR, et al. Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat Commun. 2016;7:11400.10.1038/ncomms11400
- Fujiwara T, Bandi M, Nitta M, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047.10.1038/nature04217
- Vinciguerra P, Godinho SA, Parmar K, et al. Cytokinesis failure occurs in Fanconi anemia pathway-deficient murine and human bone marrow hematopoietic cells. J Clin Invest. 2010;120(11):3834–3842.10.1172/JCI43391
- Hu L, Plafkera K, Vorozhko V, et al. Human papillomavirus 16 E5 induces bi-nucleated cell formation by cell–cell fusion. Virology. 2009;384:125–134.10.1016/j.virol.2008.10.011
- Ma G-F, Miettinen S, Poroka P, et al. Human parainfluenza virus type 2 (HPIV2) induced host ADAM8 expression in human salivary adenocarcinoma cell line (HSY) during cell fusion. BMC Microbiol. 2009;9:1–7.
- Gisselsson D, Jin Y, Lindgren D, et al. Generation of trisomies in cancer cells by multipolar mitosis and incomplete cytokinesis. PNAS. 2010;107(47):20489–20493.10.1073/pnas.1006829107
- Huang X, Tran T, Zhang L, et al. DNA damage-induced mitotic catastrophe is mediated by the Chk1-dependent mitotic exit DNA damage checkpoint. PNAS. 2005;102(4):1065–1070.10.1073/pnas.0409130102
- Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042.10.1038/nature03958
- Esumi H, Izuishi K, Kato K, et al. Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 50-AMP-activated protein kinase-dependent manner. J Biol Chem. 2002;277:32791–32798.10.1074/jbc.M112270200
- Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annu Rev Cell Dev Biol. 2012;28:29–58.10.1146/annurev-cellbio-101011-155718
- Heng YW, Lim HH, Mina T, et al. TPPP acts downstream of RhoA-ROCK-LIMK2 to regulate astral microtubule organization and spindle orientation. J Cell Sci. 2012;125(6):1579–1590.10.1242/jcs.096818
- Yüce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170(4):571–582.10.1083/jcb.200501097
- Sagona AP, Stenmark H. Cytokinesis and cancer. FEBS Lett. 2010;584:2652–2661.10.1016/j.febslet.2010.03.044
- Tanaka H, Goto H, Inoko A, et al. Cytokinetic failure-induced tetraploidy develops into aneuploidy, triggering skin aging in phosphovimentin-deficient mice. J Biol Chem. 2015;290(21):12984–12998.10.1074/jbc.M114.633891
- Jin Y, Peranen J, Schaible N, et al. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J Cell Sci. 2017;130(5):892–902.
- Vega FM, Fruhwirth G, Ng T, et al. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193(4):655–665.10.1083/jcb.201011038
- Jeong D, Park S, Kim H, et al. RhoA is associated with invasion and poor prognosis in colorectal cancer. Int J Oncol. 2016;48(2):714–722.10.3892/ijo.2015.3281
- Liu X, Chen D, Liu G. Overexpression of RhoA promotes the proliferation and migration of cervical cancer cells. Biosci Biotechnol Biochem. 2014;78:1895–1901.10.1080/09168451.2014.943650
- Aoki K, Maeda F, Nagasako T, et al. A RhoA and Rnd3 cycle regulates actin reassembly during membrane blebbing. Proc Natl Acad Sci USA. 2016;113(13):E1863–E1871.10.1073/pnas.1600968113
- Endo M, Kadomatsu T, Oike Y. The roles of angiopoietin-like protein ANGPTL2 in inflammatory carcinogenesis and tumor metastasis. Inflamm Regen. 2012;32:158–164.10.2492/inflammregen.32.158
- Telentschak S, Soliwoda M, Nohroudi K, et al. Cytokinesis failure and successful multipolar mitoses drive aneuploidy in glioblastoma cells. Oncol Rep. 2015;33(4):2001–2008.10.3892/or.2015.3751
