Abstract
The aim of this study was to evaluate the effect of consumption of coffee polyphenols (CPPs) on the autonomic nervous system activity and decreased skin barrier function caused by sodium dodecyl sulfate (SDS) treatment. In this single-blind, placebo-controlled study, ten healthy male subjects consumed either a beverage containing CPPs or a placebo beverage for four weeks. CPPs significantly suppressed the deterioration in skin barrier function and skin moisture content induced by SDS treatment after the third week. Furthermore, in the heart rate variability analysis, CPPs significantly produced an increase in parasympathetic nervous activity, and a decrease in sympathetic nervous activity after the four weeks of beverage consumption. These results suggest that CPPs might influence the regulation of the autonomic nervous system and contribute to the suppressive effect on deterioration of skin barrier function.
Coffee polyphenols suppress the deterioration in skin barrier function, concomitant with the modulation of autonomic nervous system activity
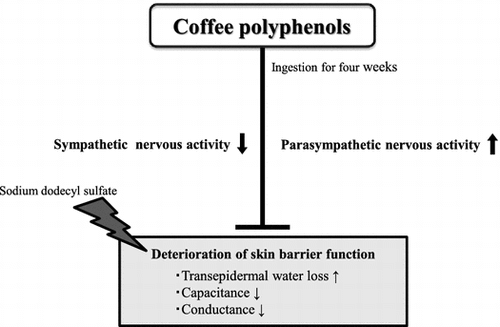
The skin is the largest organ in humans, and one of its most important functions is acting as a barrier to protect the body from environmental fluctuations [Citation1]. Failure of the skin barrier is a phenomenon associated with numerous skin disorders, including atopic dermatitis [Citation2]. The skin barrier function is known to be affected not only by external environmental factors, such as temperature, humidity, ultraviolet (UV) rays, and chemicals, but also by a variety of other factors, including psychological stress and physiological conditions of the body, such as nutritional status [Citation3,4]. We recently investigated the relationship between autonomic nervous system function and skin barrier function in healthy individuals, and reported that in heart rate variability (HRV) analysis, there was a negative correlation between the high-frequency (HF) component (which reflects parasympathetic tone) and transepidermal water loss (TEWL) (as an indicator for skin barrier function), and there is a positive correlation between low-frequency/high-frequency (LF/HF) (which reflects sympathovagal balance) and TEWL [Citation5]. These findings suggested the possibility that autonomic nervous system function could affect skin barrier function.
Coffee is one of the most widely consumed beverages in the world. Coffee is rich in coffee polyphenols (CPPs), chief among which is chlorogenic acid, and coffee is one of the primary sources of polyphenols consumed in Europe and Japan [Citation6,7]. Consumption of CPPs, including chlorogenic acid, has been reported to benefit a variety of tissue functions throughout the body. CPPs reportedly ameliorate lifestyle-related diseases such as obesity, diabetes, and hypertension. CPP consumption promotes energy metabolism in humans, suppressing the accumulation of fat [Citation8,9]. CPPs have also been reported to result in an improvement with respect to glucose tolerance and insulin resistance, demonstrated by an oral glucose tolerance test in obese individuals [Citation10]. In addition, there are reports that CPPs have the effect of ameliorating reduced vascular endothelial function following meals and glucose loading [Citation11,12], as well as improving vascular endothelial function and vasoreactivity and reducing hypertension [Citation13,14].
In this way, CPPs have a variety of physiological functions, but their effect upon the skin is unclear. Although a cohort study has reported a negative correlation between the amount of coffee consumption and skin wrinkling due to UV rays [Citation15], CPPs have not been reported to affect skin barrier function. In addition, despite a number of reports on the effect of coffee on the autonomic nervous system, their results have been inconsistent [Citation16,17], Monda et al. reported that espresso coffee increases parasympathetic tone while supine, but does not have an effect while seated [Citation16]. Conversely, Zimmermann-Viehoff et al. reported that in healthy individuals, espresso coffee containing caffeine does not affect parasympathetic tone, but decaffeinated espresso coffee does decrease parasympathetic tone [Citation17]. In addition, there have not been any reports on the effect of CPPs on autonomic nervous system activity.
In this study, we investigated the effects of CPP consumption on autonomic nervous system activity and the decrease in skin barrier function induced by sodium dodecyl sulfate (SDS) treatment in healthy individuals.
Materials and methods
CPP beverages and placebo beverages
CPPs were prepared from raw coffee beans (Robusta, Coffea canephora) using a hot water extraction method, and the caffeine that is contained in CPP extracts was eliminated through absorption by activated carbon. CPPs primarily consist of nine compounds: 5-caffeoylquinic acid (5-CQA), 3-caffeoylquinic acid (3-CQA), 4-caffeoylquinic acid (4-CQA), 3,4-dicaffeoylquinic acid (3,4-diCQA), 3,5-dicaffeoylquinic acid (3,5-diCQA), 4,5-dicaffeoylquinic acid (4,5-CQA), 3-feruloylquinic acid (3-FQA), 4-feruloylquinic acid (4-FQA), and 5-feruloylquinic acid (5-FQA) (the nomenclature is based on the International Union of Pure and Applied Chemistry numbering system). The CPP constituents were analyzed via high-performance liquid chromatography using a Cadenza CD C18 column (4.6 mm i.d. × 150 mm; Intact, Kyoto, Japan) and a Prominence Inert LC system (Shimadzu, Kyoto, Japan). In our study, 270 mg of CPPs (CQAs + FQAs) were consumed daily as 100 mL of a beverage containing a sweetener, an acidulant, and flavorings. The placebo beverage was prepared using the same quantities of sweetener, acidulant, and flavorings but did not contain CPPs.
Subjects
Ten healthy male participants (27–49 years old, average age: 35.6 ± 8.3 years) were recruited for this study. Those with a disease affecting the skin or vascular function, those with allergies, and smokers were excluded. The experiments were conducted with the approval of the Human Research Ehics Committee of the Kao Corporation in accordance with ethical guidelines for clinical research as well as ethical principles based on the Declaration of Helsinki. The subjects received an explanation of the experiment conditions and methods, and we obtained informed consent.
Study design
Our study was conducted with a single-blind, placebo-controlled, randomized, crossover trial design. The study was a crossover trial in which the same subjects continuously consumed the CPP-containing beverage or the placebo beverage once per day for two four-week periods from November through March, including a six-week ingestion cessation in between those periods. The ingestion cessation period was provided to avoid any impact caused by the order of CPP consumption and placebo consumption. The subjects consumed the provided test beverages daily for four weeks during the period between dinner and bedtime. An evaluation of autonomic nervous system function, sampling of the stratum corneum, and sampling of the saliva were performed at week zero of ingestion (prior to ingestion) and after the fourth week. In addition, chapped skin was induced by SDS treatment after the third week of ingestion, and the properties of the skin were measured over time until after the fourth week of ingestion. All the subjects were forbidden from consuming coffee beverages during the test period, and did not alter their lifestyle (diet, supplements, use of cosmetics, etc.). The consumption of alcohol and caffeinated beverages (tea, colas, etc.) was prohibited from the day prior until the completion of the autonomic nervous system function evaluation, the sampling of the stratum corneum, and the sampling of the saliva.
SDS treatment and skin barrier evaluation
The subjects’ right medial forearms were cleaned using a cleaning agent, and then allowed to rest for 20 min at a temperature of 20 ± 1 °C and a humidity of 40 ± 5%. First, the TEWL at the medial forearm was measured using a Tewameter TM300 (Courage + Khazaka, Köln, Germany) and the moisture content of the stratum corneum (capacitance, conductance) was measured using a Skicon-200EX (Yayoi Co., Ltd., Tokyo, Japan). Next, the skin barrier function was reduced through SDS treatment of the medial forearm. Specifically, a glass cup with a diameter of 3.5 cm containing 10 mL of SDS 10 wt.% solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was applied and agitated for 30 min. Immediately after the SDS treatment, and after one and seven days, the TEWL, capacitance, and conductance were measured.
Autonomic nervous system function evaluation
The method used by Nomura et al. [Citation5] was referenced for the autonomic nervous system function evaluation. Continuous electrocardiogram (ECG) monitoring was performed using a small heart rate-measuring device (ActiHR4, CamNtech, Cambridge, United Kingdom) during sleep. HRV analyses were conducted using Actiheart 4 software (CamNtech, Cambridge, United Kingdom) for a frequency domain analysis and a time domain analysis. One-minute epochs from the heart beat series were extracted for short-term HRV analysis. Regarding the frequency domain analysis, the integrated spectral power of the periodic components in the total power (TP, 0–4 Hz), low-frequency (LF power, 0.04–0.15 Hz), and high-frequency (HF power, 0.15–0.4 Hz) ranges were calculated. HF variables were expressed in normalized units: normalized HF (HF nu = HF/TP). The ratio of LF to HF power (LF/HF) was calculated as a marker of the sympathovagal balance. Furthermore, for the time domain analysis, the root mean square of successive differences (RMSSD) between adjacent R-R intervals was calculated [Citation18,19]. We used HF nu and RMSSD as indices for parasympathetic tone and LF/HF as indices for sympathovagal balance. The means of these values during sleep were calculated. The times at which each subject went to bed and woke up were recorded based on each subject’s report. If the quality of ECG data was evaluated as < 0.8 on the Actiheart 4 software, the data were regarded as invalid and were not used for the calculation of each mean according to the manufacturer’s instruction manual.
Salivary cortisol quantification
In the morning (within one hour of awakening) and the evening (within one hour of going to bed), naturally released saliva was sampled using a Salivette tube (SARSTEDT K. K., Nűmbrecht, Germany) by introducing absorbent cotton into the mouth and allowing it to soak for a one minute period after having rinsed the oral cavity with water. The saliva sample was obtained via centrifugal processing (2000 g, 5 min). Cortisol in the saliva was quantified using the IDELISA Rapid Salivary Free Cortisol ELISA Kit (ID Labs Inc., London, Canada).
Statistical analysis
The obtained values are all indicated as the mean ± SD. The paired t-test or two-way repeated measures ANOVA (SPSS statistics version 23, IBM, Armonk, NY, USA) was used to test for a statistically significant difference between the two groups. The values at baseline and immediately, 1 day, and 7 days after SDS treatment were tested by using the one-factor ANOVA, and if a significant difference was found, a multiple comparisons test (post hoc test) was performed according to the Dunnett method (StatView, SAS Institute, Cary, NC, USA).
Results
The ingestion compliance rate for the test beverages consumed once per day for four successive weeks was 98.6% for the CPP-containing beverage and 98.2% for the placebo-containing beverage. In addition, no adverse events stemming from the test beverages were found.
Effect of CPP consumption on chapped skin induced by SDS treatment
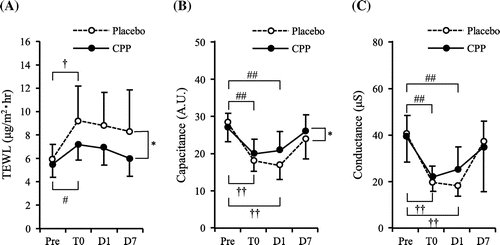
TEWL was measured to evaluate skin barrier function, and capacitance and conductance were measured to evaluate the moisture content of the stratum corneum. The TEWL results are indicated in Figure (A). There was a significant time-by-group interaction between the CPP group and the placebo group (p < 0.05), and a significant effect of the group was detected by ANOVA (p < 0.01). The CPP group and the placebo group exhibited a significant increase in TEWL immediately after SDS treatment (T0) compared to Pre. However, TEWL was 22% lower in the CPP group than the placebo group at T0. In addition, TEWL was 22% and 28% lower in the CPP group than the placebo group at day 1 (D1) and day 7 (D7) after SDS treatment, respectively. Furthermore, there was a significant time-by-group interaction between the CPP group and placebo group in capacitance (p < 0.05) (Figure (B)), but there was no significant difference between the groups in conductance (Figure (C)). Both groups exhibited significant decreases at T0 and D1 compared to the Pre levels. At D1, the capacitance and conductance in the CPP group were respectively 24 and 40% higher than in the placebo group (Figure (B) and (C)). These results indicated that the decreased functionality of the stratum corneum, including the skin barrier function, as a result of SDS treatment, was suppressed by CPP consumption.
Figure 1. Effects of CPP on skin functions. After three weeks of ingestion of a CPP or placebo beverage, the skin of the ventral side of the upper forearm was treated with a 10% SDS solution for 30 min. TEWL (A), capacitance (B), and conductance (C) were measured at baseline (Pre) and immediately (T0), 1 day (D1) and 7 days (D7) after SDS treatment. Data are mean ± SD, n = 10. *: P < 0.05, significant difference in placebo group vs. CPP group (two-way repeated measures ANOVA). †: P < 0.05, ††: P < 0.01, significant differences vs. placebo pre-value in the placebo group (Dunnett test). #: P < 0.05, ##: P < 0.01, significant differences vs. CPP pre-value in the CPP group (Dunnett test).
Effect of CPP consumption on autonomic nervous system activity during sleep
The results are indicated in Table . Each parameter is indicated as the value prior to test beverage consumption (baseline) and after four weeks (4 weeks), as well as the change at 4 weeks compared to the baseline (Δ value). No significant difference in self-reported sleep time was found between the placebo group and the CPP group. RMSSD and HF nu were calculated to evaluate parasympathetic tone. RMSSD is the root mean square of the successive differences between neighboring RR intervals, and reflects parasympathetic tone. HF nu is the result of dividing the power spectrum at the 0.15–0.4 Hz frequency band, which reflects parasympathetic tone, by the power spectrum at the frequencies of 0–0.4 Hz. In the Δ values for HF nu and RMSSD, the CPP group exhibited a significant increase compared to the placebo group (p < 0.05). The LF/HF index is the ratio of the power spectrum at the 0.004–0.15 Hz frequency band (low frequency) to the power spectrum at the 0.15–0.4 Hz frequency band (high frequency), and reflects sympathovagal balance. Specifically, a high value indicates sympathetic dominance while a low value indicates parasympathetic dominance. The LF/HF index Δ values indicated that the CPP group decreased significantly compared to the placebo group (p < 0.05). No significant difference was found between the CPP group and the placebo group in heart rate. These results revealed that CPP consumption shifts the sympathovagal balance towards parasympathetic dominance, and increases parasympathetic tone.
Table 1. HRV indices during sleep at baseline and 4 weeks after ingestion.
Effect of CPP consumption on salivary cortisol
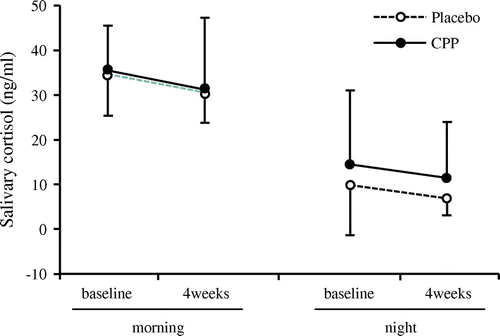
It has been previously known that the elevated cortisol concentration in the body due to stress is associated with decreased skin barrier function [Citation20,21]. Thus, we investigated the relationship between cortisol in the body and skin barrier function by measuring salivary cortisol concentrations. No significant difference was found between the placebo group and the CPP group in salivary cortisol during either the mornings or the evenings (Figure ).
Discussion
The objective of this study was to elucidate the effects of CPPs on skin function and autonomic nervous system function. We showed that in healthy individuals, CPP consumption suppresses the decrease in skin barrier function induced by SDS treatment and simultaneously shifts the sympathovagal balance towards parasympathetic dominance and increases parasympathetic tone.
The skin barrier function is controlled by a variety of factors [Citation3,4], but in recent years, there have also been reports of a correlation with autonomic nervous system function. Skin barrier function anomalies are pronounced in patients with atopic dermatitis, psoriasis, and other similar skin conditions, and it has been reported that sympathetic nervous system activity is elevated in such patients compared to healthy individuals [Citation22,23]. Therefore, it is conceivable that anomalies in autonomic nervous system functionality could be associated with skin barrier function anomalies. In addition, we recently reported that HF as derived from HRV analysis and TEWL as an indicator for skin barrier function, exhibit a negative correlation in healthy individuals, and that LF/HF and TEWL exhibit a positive correlation [Citation5]. These findings suggested a correlation between autonomic nervous system function and skin barrier function in healthy individuals. In the present study, we found that CPP consumption in healthy individuals shifted the sympathovagal balance towards parasympathetic dominance and increased parasympathetic tone, while suppressing the decrease in skin barrier function that resulted from SDS treatment. However, the causal association between the effect of CPP in regulating autonomic nervous system function and suppressing decreased skin barrier function is unclear. Denda et al. reported that when immobilization stress is applied to mice with decreased skin barrier function resulting from tape stripping, the ability for the skin barrier function to recover decreases, but that diazepam, which is a tranquilizer reported to suppress sympathetic nervous system activity [Citation24], improved that ability to recover [Citation25]. This indicates that diazepam’s effect of suppressing the sympathetic nervous system may have contributed to improved skin barrier function. In addition, administering a β2-adrenergic antagonist to the mice subjected to immobilization stress and reduced skin barrier function resulting from tape stripping has been reported to improve skin barrier function, while administering a β2-adrenergic agonist reduced skin barrier function [Citation26]. These results suggested that the increase in noradrenaline due to sympathetic nervous system activation reduces skin barrier function in a manner mediated by β2-adrenergic receptors, and conversely suggests that reduced noradrenaline caused by sympathetic nervous system suppression ameliorates the reduction in skin barrier function. Based on these findings, it is inferred that shifting the sympathovagal balance towards parasympathetic dominance using CPPs could suppress a decrease in skin barrier function via a reduction in noradrenaline.
In addition, a number of reports have shown that glucocorticoids, such as cortisol, impact skin barrier function. Altemus et al. reported that interview stress in healthy individuals elevates serum levels of inflammatory cytokines, including TNFα and IL-1β, upregulates serum cortisol levels, and reduces the recovery from skin barrier impairment caused by tape stripping [Citation20]. In addition, reduced skin barrier function induced by examination stress is suppressed by inhaling rose essential oil, during which time the elevation of salivary cortisol levels is also suppressed, suggesting that the hypothalamo-pituitary-adrenocortical axis (HPA axis) activation is involved in the reduced skin barrier function induced by chronic stress [Citation20]. These findings suggest that elevated cortisol, caused by stress mediated by the HPA axis, impairs skin barrier function. Meanwhile, raw coffee bean extract, which is rich in chlorogenic acid, has been reported to reduce urinary cortisol levels in healthy individuals via a reduction in the enzymatic activity of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which is a cortisol-producing enzyme [Citation27]. However, we were not able to find an effect from CPP consumption on salivary cortisol levels. In the present study, we did not stress the subjects, so at least under the current experimental conditions, which did not include stress, it was shown that CPP consumption does not affect cortisol. Consequently, we believe that in the present study, it is possible that CPPs suppressed a reduction in the skin barrier function, resulting from SDS and regulated autonomic nervous system function independent of effects of cortisol.
A limitation of this study is that blood components such as catecholamines and inflammatory cytokines were not measured. Catecholamines, including noradrenaline, and inflammatory cytokines, are known to affect skin barrier function [Citation28], and healthy individuals with high serum chlorogenic acid concentrations reportedly have lower levels of inflammatory cytokines in the blood [Citation29]. Consequently, CPP consumption could contribute to a suppressed decrease in skin barrier function by affecting inflammatory cytokines. In the future, more details of the mechanism by which CPPs suppress the reduction in skin barrier function will be revealed by analyzing a variety of mediators, including blood components.
In this study, we showed that CPP consumption in healthy individuals suppressed the deterioration in skin barrier function resulting from SDS treatment and simultaneously shifted the sympathovagal balance to parasympathetic dominance and elevated parasympathetic tone. These results suggest that CPPs might influence the regulation of the autonomic nervous system and contribute to the suppressive effect on deterioration of the skin barrier function.
Author contributions
Daiji Kagawa, Akihiko Fujii, Mayumi Ohtsuka and Takatoshi Murase designed the study. Daiji Kagawa analyzed the data and wrote the manuscript. Akihiko Fujii and Takatoshi Murase supervised writing the manuscript. All authors reviewed and approved this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was entirely funded and supported by the Kao Corporation.
References
- Benson HAE. Skin structure, function, and permeation. In: Benson HAE, Watkinson AC, editors. Topical and Transdermal Drug Delivery: Principles and Practice. Hoboken (NJ): Wiley; 2011. p. 3–22.10.1002/9781118140505
- Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp. Dermatol. 2008;17:1063–1072.10.1111/exd.2008.17.issue-12
- Boelsma E, van de Vijver LP, Goldbohm RA, et al. Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003;77:348–355.10.1093/ajcn/77.2.348
- Denda M, Tsuchiya T, Elias PM, et al. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367–R372.10.1152/ajpregu.2000.278.2.R367
- Nomura T, Yoshida-Amano Y, Yoshida K, et al. Relationships between transepidermal water loss, cutaneous microcirculatory function and autonomic nervous activity. Int J Cosmet Sci. 2017;39:275–283.10.1111/ics.2017.39.issue-3
- Zamora-Ros R, Knaze V, Rothwell JA, et al. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2016;55:1359–1375.10.1007/s00394-015-0950-x
- Fukushima Y, Ohie T, Yonekawa Y, et al. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J Agric Food Chem. 2009;57:1253–1259.10.1021/jf802418j
- Soga S, Ota N, Shimotoyodome A. Stimulation of postprandial fat utilization in healthy humans by daily consumption of chlorogenic acids. Biosci Biotechnol Biochem. 2013;77:1633–1636.10.1271/bbb.130147
- Ota N, Soga S, Murase T, et al. Consumption of coffee polyphenols increases fat utilization in humans. J Health Sci. 2010;56:745–751.10.1248/jhs.56.745
- van Dijk AE, Olthof MR, Meeuse JC, et al. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care. 2009;32:1023–1025.10.2337/dc09-0207
- Ochiai R, SugiuraY Otsuka K, et al. Coffee bean polyphenols ameliorate postprandial endothelial dysfunction in healthy male adults. Int J Food Sci Nutr. 2015;66:350–354.10.3109/09637486.2015.1007453
- Ochiai R, Sugiura Y, Shioya Y, et al. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr Res. 2014;34:155–159.10.1016/j.nutres.2013.11.001
- Watanabe T, Arai Y, Mitsui Y, et al. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin Exp Hypertens. 2006;28:439–449.10.1080/10641960600798655
- Ochiai R, Jokura H, Suzuki A, et al. Green coffee bean extract improves human vasoreactivity. Hypertens Res. 2004;27:731–737.10.1291/hypres.27.731
- Fukushima Y, Takahashi Y, Hori Y, et al. Skin photoprotection and consumption of coffee and polyphenols in healthy middle-aged Japanese females. Int J Dermatol. 2015;54:410–418.10.1111/ijd.2015.54.issue-4
- Monda M, Viggiano A, Vicidomini C, et al. Espresso coffee increases parasympathetic activity in young, healthy people. Nutr Neurosci. 2009;12:43–48.10.1179/147683009X388841
- Zimmermann-Viehoff F, Thayer J, Koenig J, et al. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers – a randomized crossover study. Nutr Neurosci. 2016;19:169–175.10.1179/1476830515Y.0000000018
- Mazurak N, Gunther A, Grau FS, et al. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur J Clin Nutr. 2013;67:401–406.10.1038/ejcn.2013.32
- Mazurak N, Gunther A, Grau FS, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS One. 2014;9:e105328.
- Altemus M, Rao B, Dhabhar FS, et al. Stress-Induced Changes in Skin Barrier Function in Healthy Women. J Invest Dermatol. 2001;117:309–317.10.1046/j.1523-1747.2001.01373.x
- Fukada M, Kano E, Miyoshi M, et al. Effect of ‘rose essential oil’ inhalation on stress-induced skin-barrier disruption in rats and humans. Chem Senses. 2012;37:347–356.10.1093/chemse/bjr108
- Cicek D, Kandi B, Berilgen MS, et al. Does autonomic dysfunction play a role in atopic dermatitis? Br J Dermatol. 2008;159:834–838.10.1111/bjd.2008.159.issue-4
- Tran BW, Papoiu AD, Russoniello CV, et al. Effect of itch, scratching and mental stress on autonomic nervous system function in atopic dermatitis. Acta Derm Venereol. 2010;90:354–361.
- Ikeda T, Doi M, Morita, K, et al. Effects of midazolam and diazepam as premedication on heart rate variability in surgical patients. Br J Anaesth. 1994;73:479–483.
- Denda M, Tsuchiya T, Hosoi J, et al. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol. 1998;138:780–785.10.1046/j.1365-2133.1998.02213.x
- Denda M, Fuziwara S, Inoue K. Beta2-adrenergic receptor antagonist accelerates skin barrier recovery and reduces epidermal hyperplasia induced by barrier disruption. J Invest Dermatol. 2003;121:142–148.10.1046/j.1523-1747.2003.12310.x
- Revuelta-Iniesta R, AI-Dujaili EA. Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: a pilot crossover study using green and black coffee. Biomed Res Int. 2014;2014:482704.
- Hänel KH, Cornelissen C, Lüscher B, et al. Cytokines and the skin barrier. Int J Mol Sci. 2013;14:6720–6745.10.3390/ijms14046720
- Lee AH, Tan L, Hiramatsu N, et al. Plasma concentrations of coffee polyphenols and plasma biomarkers of diabetes risk in healthy Japanese women. Nutr Diabetes. 2016;6:e212.10.1038/nutd.2016.19