Abstract
Pteris multifida (PM) and Cortex phellodendri (CP) are medicinal foods used for gastrointestinal protection. Lactic-acid bacteria are probiotics. Salmonella Typhimurium strain ST21-infected mice were used to examine the alleviative effects of two lactic-acid bacteria (LAB) as well as aqueous extracts of PM and CP for a 4-day treatment. CP and LAB decreased fecal ST counts. CP and PM reduced the ST21 count in the blood, intestine, and liver. LAB lowered the ST21 count in the intestine and spleen. CP and LAB decreased the IFN-gamma level; PM lowered the TNF-alpha level; and both LAB and PM reduced the IL-1beta level in serum. PM and CP lowered the IgG level in serum. The data in a macrophage infection model indicate that TNF-alpha was partial involved in this alleviative effects, other mechanisms might be involved. In sum, these novel findings suggest that PM, CP, and LAB probiotics are potential anti-Salmonellae agents.
Pteris multifida (PM), Cortex phellodendri (CP) and probiotics [Lactic-acid bacteria (LAB)] are potential to exhibit anti-Salmonellae effect on infected mice.
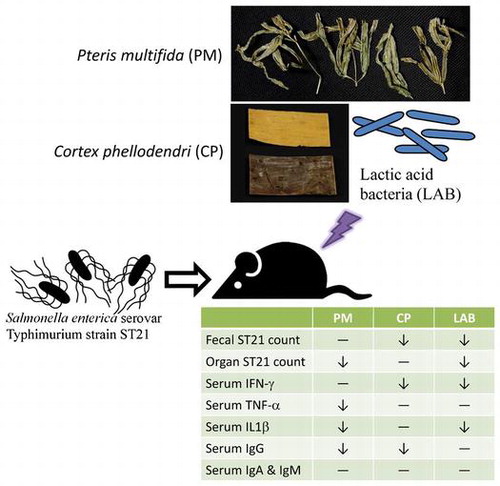
Salmonellosis, an infectious disease resulting from Salmonella infection, commonly occurs in humans and as well as livestock animals. Among many Salmonella serotypes, Salmonella enterica serovar Typhimurium (ST) is an important one as it is responsible for the transmission of a food-borne pathogen disease from animals to animals and from animals to humans. It is for this reason that ST is a well-known zoonotic pathogen [Citation1]. ST infections, due mostly to the consumption of contaminated water or foods, cause gastroenteritis, fever, diarrhea, and even death. Although antibiotics can be used for salmonellosis therapy, the overuse has led to the occurrence of antibiotic resistant bacterial strains [Citation2]. Therefore, in order to reduce the development of antibiotic resistant strains caused by treatment of ST infection, the investigation and application of potential appropriate supplements with anti-ST activity is necessary and of an ever-emergent need.
ST is able to invade host cells, including intestinal epithelial cells and macrophages, where it evokes inflammatory stress, disturbs the host’s immune response, and even induces cell apoptosis [Citation3,4]. It has been reported that ST infection increases the production of inflammatory cytokines including interferon (IFN)-gamma, interleukin (IL)-6, and tumor necrosis factor (TNF)-alpha, all of which enhance inflammatory injury for the host [Citation5]. In addition, ST infection also promotes the host’s immune responses through stimulating a massive generation of immunoglobulins (Igs) such as IgA, IgG, and IgM [Citation3,6]. Also, these studies’ authors have indicated that these Igs contribute to eliminating ST. Thus, if an agent or supplement alleviates ST infection, a decreased release of inflammatory cytokines and/or Igs in ST might be observed.
Pteris multifida (PM) and Cortex phellodendri (CP) are two herbs commonly used in traditional Chinese medicine for anti-hyperlipidemic and diarrhea therapy [Citation7,8]. PM contains pterosins and diterpenoids, both of which are components that are able to regulate immune function and exert anti-tumor activities [Citation9]. CP is rich in phytochemicals such as alkaloids and berberine, and both of these agents are considered to be major ingredients responsible for the anti-diarrheal effects of CP [Citation7,10]. The anti-microbial effects of CP and/or PM against Helicobacter pylori, Mycoplasma hominis, and some viruses have already been reported [Citation11–13]. Up to this point, little information is available regarding the anti-ST effects of these two herbs. On the other hand, the healthy benefits of lactic acid bacteria have been widely reported [Citation14,15]. Those studies indicated that lactic acid bacteria offers probiotic properties such as acting as immune-modulators and modifying the enzymatic activity of intestinal microbes. One of our previous studies found that a supplement that was a mixture of two lactic acid bacteria strains, Lactobacillus acidophilus strain LAP5 and Lactobacillus reuteri strain Pg4, in feed markedly prevented S. Choleraesuis infection in swine via enhancing host defenses against subsequent infection [Citation16]. Another of our studies revealed that pre-treatments of this mixture effectively prevented ST infection in chickens through modulating their inflammatory response [Citation17]. Besides these preventive effects, it is unknown whether these lactic acid bacteria can provide therapeutic effects to overcome ST in infected subjects.
In our present study, ST-infected mice were studied to examine the alleviative effects of two lactic acid bacteria strains (LAB), as well as CP and PM aqueous extracts. Streptomycin was the antibiotic used for comparison. The alteration of ST count, cytokines, and Igs was measured in all the separate groups. The effects of LAB, and CP and PM aqueous extracts were also tested on macrophages in vitro to investigate possible mechanisms for treating ST21 infection. If these aqueous extracts and LAB can ameliorate ST infection with their natural and less expensive properties, then they are potential anti-Salmonellae agents that could replace antibiotics as alternatives to treat ST infection.
Materials and methods
Material
Dry PM and CP were obtained from Kaiser Pharmaceutical Co. Ltd. (Tainan City, Taiwan). Fifty gram of each herb was chopped and mixed with 500 mL of sterile distilled water by a Waring blender, and this was followed by 30 min of boiling. An aqueous extract of each herb was collected and freeze-dried as a fine powder. Same as our previous study [Citation17], two lactic acid bacteria strains, Lactobacillus acidophilus strain LAP5 (LAP5) [Citation18], and L. reuteri strain Pg4 (Pg4) [Citation19], isolated from the gastrointestinal tract of swine and broilers, respectively, were used as described previously [Citation16]. LAP5 and Pg4 were combined at a 1:1 ratio for the experiments in this study and defined as LAB. LAB was further lyophilized and freeze-dried to form a powder. Lyophilized cells were prepared as follows: LAP5 and Pg4 were incubated in De Man, Rogosa, and Sharpe (MRS) broth (Difco, Detroit, MI, USA) at 37 °C for 18 h. The cells were collected by centrifugation at 2000 × g for 10 min at 4 °C and washed twice with sterilized phosphate buffered saline (PBS). The culture supernatants were combined at a 1:1 ratio and adjusted to pH 7.0 (LAB supernatant) for further assays. The cells were suspended in 10 % reconstituted skim milk. Samples were first frozen at −20 °C and then desiccated under vacuum (50 mTorr) for 48 h at −20 °C (Freeze dry system and stoppering tray dryer; Labconco, Kansas, USA). The lyophilized cells were stored at −20 °C [Citation20]. This powder contained 109 colony-forming unit (CFU) of LAB per gram. Streptomycin was purchased from Bio Basic Inc. (Canada SB0494). S. Typhimurium strain ST21 (ST21) was supplied by Dr. Chao-chin Chang from National Chung Hsing University, Taiwan. The agar, broth, and plate were bought from Difco Co. (NJ, USA).
Animals and diet
Eight-week old male Balb/c mice were obtained from the National Laboratory Animal Center (Taipei City, Taiwan). The mice were kept under a specific pathogen-free condition with a 12-h dark and 12-h light cycle. All mice consumed water and a standard diet ad libitum. This animal study was approved by the Animal Care and Use Committee of China Medical University (permission number: 103-64-N).
Animal experiment
On day 0 of the experiment, each mouse was randomly placed into one of the nine groups, a control group without infection, an infection group that received ST21 challenge but not treatment, and seven infection groups that received different treatments. For infection, each mouse was given 1010 CFU of ST21 via a stomach tube. On day 1, PM and CP at a dose of 2.5 or 5 mg/day, LAB at a dose of 5 × 105 or 106 CFU/day, or 20 mg/day of streptomycin was given orally for 4 days continuously via a stomach tube. The dosage of LAB and streptomycin used was based on previous studies [Citation16,17]. Body weight was monitored and feces was collected starting on day 0. On day 4, after fasting for 12 h, the mice were asphyxiated with carbon dioxide. Blood, intestine, spleen, and liver were also collected. Blood was obtained from the heart via microsyringe, and the serum was immediately separated. Organ or tissue was homogenized for further analysis.
ST21 count determination
We collected daily fecal samples from day 0 to day 4, with the samples on day 0 being taken pre-infection and the samples on days 1–4 always being taken prior to treatment each day. Two gram of fecal sample was mixed with 20 mL PBS, and then vigorously shaken for 30 min. After being diluted from 10−2 × to 10−9 ×, 100 μL fecal suspension, serum, or organ homogenate was loaded onto a Salmonella-Shigella (SS) agar plate for ST21 enumeration. After overnight incubation at 37 °C, CFU were counted. ST21 was confirmed by PCR assay according to the method enumerated before by Alvarez et al. [Citation21].
Fecal coliform and lactic acid bacteria count
We collected daily fecal samples from day 0 to day 4, with the samples on day 0 being taken pre-infection and the samples on days 1–4 always being taken prior to treatment each day. Both coliform and lactic acid bacteria enumerations in the fecal samples were processed within 2 h after sampling. After the feces were diluted by PBS, coliforms and lactic acid bacteria counts were respectively determined by using Eosin Methylene Blue (EMB) agar and MRS agar. EMB plates were put into a regular incubator for 24 h incubation at 37 °C, and MRS plates were stored in a BD GasPak™ EZ Anaerobe Container System Sachets for 48 h incubation at 37 °C.
Assay of inflammatory factors
IFN-gamma, IL-1beta, and TNF-alpha levels in the serum and culture supernatant were analyzed by using cytoscreen immunoassay kits (BioSource International, Camarillo, CA, USA). Samples were run in duplicates according to the manufacturer’s instructions.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC and MBC values of herbal aqueous extract or LAB supernatant against ST21 were determined. Briefly, ST21 cultured in Mueller Hinton broth was diluted to 106 CFU/mL. ST21 suspension at 100 μL was loaded into a 96-well plate, and series dilutions of herbal aqueous extracts or LAB supernatant was added. After incubation at 37 °C for 24 h, the MIC was the lowest concentration of herbal aqueous extracts or the highest dilution of LAB supernatant without visible turbidity. The mixtures of ST21 suspension and either herbal aqueous extracts or LAB supernatant without visible turbidity were used for MBC determination by adding a 10 μL mixture onto a Luria Bertani agar plate and incubating at 37 °C for 16 h. The MBC was the lowest concentration of herbal aqueous extracts or the highest dilution of LAB supernatant that displayed no growth.
Invasion assay
The murine monocyte/macrophage cell line RAW 264.7 (ATCC TIB-71) was obtained from American Type Culture Collection (ATCC). RAW264.7 cells were seeded into 96-well plates at a density of 1 × 105 cells/well and grown at 37 °C in CO2 in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) for 18 h. The culture supernatant was removed and then 100 μL of antibiotic-free DMEM was added in each well. RAW264.7 cells were then infected by 100 μL of ST21 at a multiples of infection (MOI) of 10. Hundred microliter of herbal aqueous extracts or LAB supernatant was then added to the cells to match their indicated concentrations or dilution (coincubation group). For the pretreatment groups, before infection, RAW264.7 cells (cell-pretreatment group) or ST21 (ST21-pretreatment group) were pretreated with the indicated amount of herbal aqueous extracts or LAB supernatant for 1 h and then removed. For the TNF-alpha depletion, anti-mouse TNF-alpha capture antibody (BioSource International, Camarillo, CA, USA) at a 100-fold dilution was added along with the treatment. Cell-associated bacteria were then quantified at 1 h after infection, then treated with 100 mg/L of gentamicin for 1.5 h. Before antibiotic treatment, the supernatant was collected for cytokine analysis. The cell cultures were then gently separated from the supernatant, washed and resuspended with PBS, and then bacterial numbers were derived by plating serial dilutions on SS agar plates (Difco, NJ, USA) at 37 °C for 18 h. Invasive activity was then calculated from a triplicate mean. The results were then expressed as a percent relative to the invasion of RAW264.7 cells, which was then compared to the infection without treatment group.
Statistical analysis
Each group had ten mice (n = 10). The differences between the mean values of the treatment and ST21-infected groups were evaluated using the one-way analysis of variance (ANOVA) followed by Dunnett’s test (SAS version 9.1 software). Results were then presented as the mean ± the standard deviation (SD). p < 0.05 was considered to indicate a statistically significant difference compared with the infection without treatment group. The differences between the mean values of the coincubation group with the same treatment groups and the control group with TNF-alpha depletion group with the same treatment groups were evaluated by Student’s t-test using SPSS ver. 12.0. software (SPSS, Inc., Chicago, Ill., USA). p < 0.05 was considered to indicate a statistically significant difference.
Results
PM, CP, and LAB affected body weight
Until day 3, the body weight of all groups did not show a significant difference. Only the control group maintained body weight for 4 days. The rest of the groups slightly decreased their body weight at day 4 (Figure ).
Figure 1. Body weight (relative to day 0) of mice without infection (Control), ST21-infected mice with no treatment (Infection), and treatments of 5 mg or 2.5 mg of PM (PM-A or PM-B), 5 mg or 2.5 mg of CP (CP-A or CP-B), 106 or 5 × 105 CFU of LAB (LAB-A or LAB-B), or 20 mg streptomycin for 4 days. Values are mean ± SD, n = 10. *Means significantly different from infection without treatment group, p < 0.05.
PM, CP, and LAB decreased ST21 count
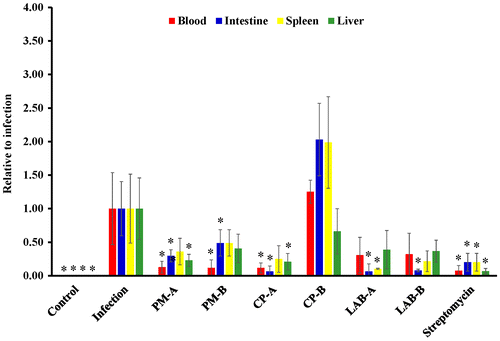
ST21 infection increased fecal ST21 count at days 3–4. The supplementation of PM, CP, LAB, and streptomycin at test dosages decreased fecal ST21 count at day 3 when compared to the infection without treatment group (Figure (a)). The fecal ST21 counts of the LAB groups already had decreased at day 2. At day 4, there was a rebound of fecal ST21 counts in the PM-treated groups. Streptomycin treatment lowered fecal ST21 count starting at day 3. As shown in Figure (b), ST21 infection did not alter fecal coliform count; however, the treatments of CP at 5 mg (CP-A group) and streptomycin increased fecal coliform counts when compared with the infection without treatment group at day 1 and day 2, respectively. The control and PM at 5 mg (PM-A) groups showed a slight decrease in fecal lactic acid bacteria count at day 2 (Figure c) The rest of the treatment did not change lactic acid bacteria count for 4 days. As shown in Figure , both PM and CP at 5 mg (PM-A and CP-A groups) reduced ST21 count in the blood, intestine, and liver when compared with the infection without treatment group. PM at 2.5 mg (CP-B group) reduced ST21 count only in blood and intestine, LAB at 106 CFU (LAB-A group) decreased ST21 count in the intestine and spleen, and at 5 × 105 CFU (LAB-B group) only lowered ST21 count in the intestine. CP at 2.5 mg (CP-B group) did not decrease ST21 count in the blood and all the organs that we collected samples from. However, streptomycin treatment reduced ST21 count in all the samples.
Figure 2. Bacterial count of ST21 (a), coliform (b) and lactic acid bacteria (c) in the feces of mice without infection (Control), ST21-infected mice with no treatment (Infection), and treatments of 5 mg or 2.5 mg of PM (PM-A or PM-B), 5 mg or 2.5 mg of CP (CP-A or CP-B), 106 or 5 × 105 CFU of LAB (LAB-A or LAB-B), or 20 mg streptomycin for 4 days. Values are mean ± SD, n = 10. *Means significantly different from infection without treatment group, p < 0.05.
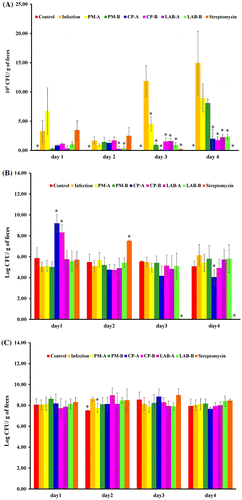
Figure 3. ST21 count in the serum, small intestine, spleen, and liver of mice without infection (Control), ST21-infected mice with no treatment (Infection), and treatments of 5 mg or 2.5 mg of PM (PM-A or PM-B), 5 mg or 2.5 mg of CP (CP-A or CP-B), 106 or 5 × 105 CFU of LAB (LAB-A or LAB-B), or 20 mg streptomycin for 4 days. Values are mean ± SD, n = 10. *Means significantly different from infection without treatment group, p < 0.05.
PM, CP, and LAB attenuated inflammatory and immune response
ST21 infection increased the release of IFN-gamma (Figure (a)). CP and LAB supplementation at both dosages decreased the IFN gamma level in the serum. In Figure (b), it can be seen that ST21 infection raised the serum TNF-alpha level, and PM supplementation at both dosages as well as streptomycin lowered the serum TNF-alpha level. ST21 infection also elevated the serum IL-1beta level (Figure (c)). The supplementation of PM at 5 mg (PM-A group), LAB at both dosages, and streptomycin all reduced the IL-1beta level in serum. ST21 infection increased the levels of IgA, IgM and IgG in the serum (Figure ). PM at 2.5 mg (PM-B group) and CP at 5 mg (CP-A group) lowered the IgG levels, while streptomycin reduced the serum level of IgM and IgG induced by infection.
Figure 4. Level (relative to control) of IFN-gamma (a), TNF-alpha (b) and IL-1beta (c) in the serum of mice without infection (Control), ST21-infected mice without any treatment (Infection), and treated by 5 mg or 2.5 mg of PM (PM-A or PM-B), 5 mg or 2.5 mg of CP (CP-A or CP-B), 106 or 5 × 105 CFU of LAB (LAB-A or LAB-B), or 20 mg streptomycin for 4 days. Values are mean ± SD, n = 10. *Means significantly different from infection without treatment group, p < 0.05.
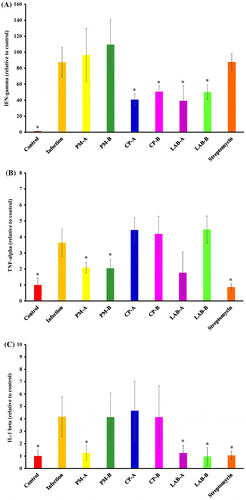
Figure 5. Level (relative to control) of IgA, IgM, and IgG in the serum of mice without infection (Control), ST21-infected mice without any treatment (Infection), and treated by 5 mg or 2.5 mg of PM (PM-A or PM-B), 5 mg or 2.5 mg of CP (CP-A or CP-B), 106 or 5 × 105 CFU of LAB (LAB-A or LAB-B), or 20 mg streptomycin for 4 days. Values are mean ± SD, n = 10. *Means significantly different from infection without treatment group, p < 0.05.
PM, CP, and LAB supernatant showed weak anti-ST21 activity
MIC and MBC values of PM and CP aqueous extract against ST21 were >100 mg/mL. The LAB supernatant did not show anti-ST21 activity even without dilatation.
PM, CP, and LAB supernatant affected ST21 invasion, IL-1 beta, and TNF-alpha expression in infected RAW264.7 cells
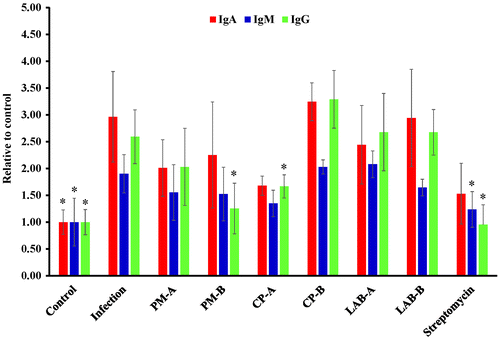
ST21 were able to invade RAW 264.7 cells (Figure (a)) and then induce the expression of IL-1β (Figure (b)) at MOI 10 after 1 h of coincubation. Both PM and CP aqueous extracts at concentrations of 2.5 and 5 mg/mL, as well as 2-fold dilution of the LAB supernatant significantly suppressed both the number of invading ST21 (Figure (a)) and the IL-1 beta levels (Figure (b)) in infected cells. ST21 infection also induced the expression of TNF-alpha (Figure (c)). However, both herbal aqueous extracts and the LAB supernatant induced TNF-alpha expression in RAW264.7 cells with and without infection. In order to clarify if TNF-alpha was involved in ST21 clearance in RAW264.7 cells, the TNF-alpha was depleted by adding antibodies against murine TNF-alpha along with 5 mg/mL of herbal aqueous extracts or 2-fold dilution of the LAB supernatant, and this increased the number of invading ST21 (Figure (a)) as compared with those groups without TNF-alpha depletion (coincubation group). TNF-alpha levels indeed dramatically decreased by antibody depletion (Figure (c)) without changing IL-beta expression as compared with the coincubation group (Figure (b)).
Figure 6. ST21 invasion ability in RAW267.7 cells (a) and level (relative to control) of IL-1beta (b) and TNF-alpha (c) in infected RAW264.7 cells. ST21-infected cells without any treatment (Infection), and treated by 5, 2.5, 1.25, 0.625 mg/ml of PM or CP, or 2-fold (1/2×), 20-fold diluted (1/20×), 200-fold diluted (1/200×), 400-fold diluted (1/400×), or 800-fold diluted (1/800×) LAB supernatant for 1 h. Values are mean ± SD, n = 3. *Means significantly different from infection without treatment group, p < 0.05.
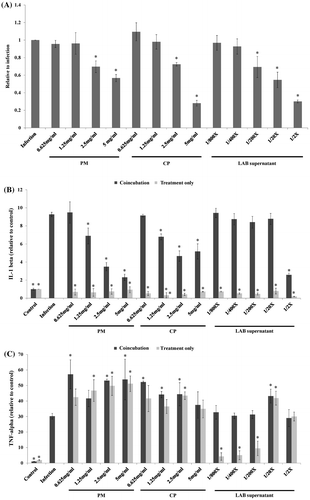
Figure 7. ST21 invasion ability in RAW267.7 cells (a) and level (relative to control) of IL-1beta (b) and TNF-alpha (c) in infected RAW264.7 cells. ST21-infected cells without any treatment (Infection), and treated by 5 mg/ml of PM or CP, or 2-fold (1/2×) diluted LAB supernatant for 1 h. Coincubation groups refer to the cells treated with herbal aqueous extracts or LAB supernatant along with ST21 infection. TNF-alpha depletion groups were the cells treated the same ways as the coincubation groups but with anti-TNF alpha antibody. Cell-pretreatment groups were the cells pretreated by herbal aqueous extracts or LAB supernatant before ST21 infection. ST21-pretreatment groups are ST21 pretreated by herbal aqueous extracts or LAB supernatant before infecting cells. Values are mean ± SD, n = 3. *Means significantly different from infection without treatment group, p < 0.05. **Means significantly different from the coincubation group with the same treatment, p < 0.05. #Means significantly different from the control group with TNF-alpha depletion, p < 0.05.
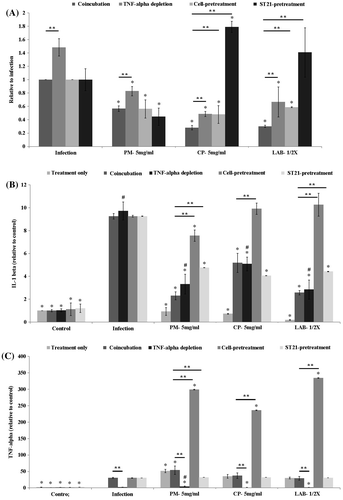
PM, CP, and LAB supernatant acted both on ST21 and RAW264.7 cells
In order to clarify if herbal aqueous extracts or LAB supernatant acted on ST21 or RAW264.7 cells, two pretreatment tests were applied. Pretreating RAW264.7 cells (cell-pretreatment group) with herbal aqueous extracts at a concentration of 5 mg/mL or a 2-fold dilution of LAB supernatant for 1 h still suppressed the number of invading ST21 compared with the infection without treatment group. RAW264.7 cells with or without pretreatment by herbal aqueous extracts did not affect the number of invading ST21 significantly. However, the cells pretreated by LAB supernatant saw an increase in the number of invading ST21 when compared with the coincubation group. The levels of IL-1 beta (Figure (b)) and TNF-alpha (Figure (c)) were both increased by pretreating RAW264.7 cells when compared to the coincubation group. When ST21 was treated by CP at a concentration of 5 mg/mL or a 2-fold dilution of LAB supernatant for 1 h (ST-pretreatment groups), the invasion ability of ST21 was dramatically increased when compared with the coincubation group. However, pretreating ST21 with PM at a concentration of 5 mg/mL did not affect its invasion ability to RAW264.7 cells as compared with the coincubation and cell-pretreatment group (Figure (a)). IL-1 beta levels were increased in ST21 pretreated with PM or LAB supernatant (Figure (b)). TNF-alpha levels were not affected significantly in all ST21-pretreatment groups when compared with the coincubation group, except in the group of PM aqueous extract treatment (Figure (c)).
Discussion
PM, CP, and their ingredients are used in traditional Chinese medicine for diarrhea therapy [Citation22–24], and diarrhea is a major symptom of salmonellosis. In our present study, in its mice infection model, after a 4-day treatment, herbal aqueous extracts and LAB supplementation slightly decreased body weight, and ST21 shedding in the mice feces decreased in the CP, LAB, and antibiotic treatment group. All the treatments did not significantly affect the amount of coliform and lactic acid bacteria in the fecal samples, except in the group that received the treatment of CP at 5 mg and the group that received the antibiotic treatment. PM, CP, and LAB treatment decreased ST21 in the intestines. PM and CP at 5 mg also lowered ST21 counts in the blood and liver. ST21 clearance in blood circulation and organs was seen in the antibiotic treatment group. CP and LAB supplementation suppressed INF-gamma expression. PM and antibiotic treatment decreased the TNF-alpha level. PM at 5 mg, LAB supplementation, and the antibiotic treatment lowered the IL-1 beta expression. Antibiotic treatment also decreased the IgM and IgG levels. The bactericidal activities of PM, CP, and LAB supernatant were weak in vitro, and all of them did not affect the Igs levels significantly. Therefore, they might ameliorate ST21 infection through modulating inflammatory responses.
After the infection, ST21 needs to adapt to the host environment. A notable bacteria amplification was seen on day 3 and day 4. CP at 5 mg significantly suppressed ST21 counts at day 4 and also inhibited the presence of ST21 in the blood, intestines, and livers. These data indicate that CP may inhibit ST21 replicating in the intestines as well as decrease ST21 invasion. In contrast, PM treatment suppressed the fecal shedding of ST21 at day 3 but dramatically increased the fecal ST21 count at day 4. The presence of ST21 in the blood, intestines, and liver was all decreased. These data suggest that PM increased the shedding of ST21 and eliminated the pathogen from the internal organs and from circulation.
It has been reported that ST infection results in the proliferation of ST in organs and stimulates systemic inflammatory responses [Citation25,26]. In our present study, ST21 infection promoted systemic inflammatory reactions, which was evidenced by the increased production of three inflammatory indicators: IFN-gamma, TNF-alpha, and IL-1beta in serum. However, the treatments of herbal aqueous extract and LAB reduced the level of these cytokines in circulation, although they accomplished by different mechanisms. In the early stage of infection, IFN-gamma activates macrophages to clear Salmonella [Citation27]. In this study, the presence of ST21 in the feces and intestines was all reduced in the group that received CP at 5 mg and LAB treatment. These data are consistent with the fact that CP and LAB at test dosages revealed the ability to decrease the serum level of IFN-gamma at day 4. It can thus be inferred that CP and LAB treatment may have suppressed ST21 growth by activating macrophages.
In addition, IL-1 beta and TNF-alpha also facilitate inflammatory responses and cause tissue damage [Citation28]. These harmful systemic symptoms caused by infection were diminished by treating the mice with either PM at 5 mg or streptomycin. In our animal model, the infection already caused a system infection. Macrophages play an important role in the establishment of a successful systemic Salmonella infection [Citation29]. It has been shown that ST-infected macrophages undergo NLRC4/NAIP-mediated pyroptosis and increase IL-1 beta secretion in order to recruit and activate neutrophils to uptake bacteria [Citation30]. In our cell infection model, both herbal aqueous extracts and the LAB supernatant significantly reduced the number of intracellular ST21 and consequently reduced IL-1 beta secretion. In addition, macrophages express TNF-alpha to eliminate infections [Citation3]. The herbal aqueous extracts at concentrations higher than 0.625 mg/mL and the LAB supernatant at 2-fold and 20-fold dilution dramatically increased the levels of TNF-alpha in macrophages with and without infection. When TNF-alpha was depleted in the infected cells, the amount of invading ST21 increased. By comparing with the coincubation groups, the number of invading ST21 was decreased by pretreating macrophages with the herbal aqueous extracts or the LAB supernatant. These data indicate that the treatment might prepare the macrophages by increasing their TNF-alpha against ST21 infection. When the TNF-alpha levels were decreased by antibody depletion, the IL-1 beta levels of infected RAW264.7 cells were increased in response to ST21 invasion.
In order to clarify if PM, CP, and the LAB supernatant acted on ST21 or RAW264.7 cells, we pretreated ST21 and RAW264.7 cells by herbal aqueous extracts or LAB supernatant, and then monitored the invasion ability of ST21 as well as the IL-1 beta and TNF-alpha levels of infected RAW264.7 cells and compared them to those in the coincubation groups. In the RAW264.7 cells pretreated by PM or CP, the number of invading ST21 was not affected; however, the levels of IL-1 beta and TNF-alpha were both increased. On the other hand, with the RAW264.7 cells pretreated by the LAB supernatant, the number of invading ST21 along with the levels of IL-1 beta and TNF-alpha were all increased. The pretreated cells were more sensitive to ST21-induced IL-1 beta expression. And the TNF-alpha levels were added up by the induction of herbs and also from the infection. These data indicate that herbal aqueous extracts and the LAB supernatant were able to modulate inflammatory response in order to respond to bacterial infection. Pretreating ST21 by PM aqueous extract did not affect the invasive ability of ST21 but increased the IL-1 beta level. Pretreating ST21 by either CP or LAB supernatant was able to increase the number of invading ST21, and the LAB supernatant treatment also increased the IL-1 beta level. Because the RAW264.7 cells were not treated by herbal aqueous extracts or the LAB supernatant in ST21-pretreatment groups, the TNF-alpha levels did not change dramatically among them. Even though the invading ability of ST21 was changed by CP and LAB supernatant, IL-1 beta and TNF-alpha levels did not raise coordinately. Our data indicate the TNF-alpha was directly involved in the clearance of ST21 infection by the treatments of herbal aqueous extracts or the LAB supernatant. But the IL-beta expression as compared with the coincubation group was not changed accordantly. Furthermore, pretreated RAW264.7 cells were sensitized to ST21 infection by expressing IL-1 beta and TNF-alpha. Therefore, the effect of TNF-alpha depletion was partial, so several pathways might be involved in this phenomenon, with the regulation of IL-beta expression would be one of them. Further studies are needed to clarify this issue.
Our previous studies reported that supplementation of a mixture consisting of L. acidophilus LAP5 and L. reuteri Pg4 decreased the bacterial load and inflammatory cytokines in S. enterica serovar Choleraesuis-infected swine [Citation16] and ST315-infected chickens [Citation17]. Our present study further found that this lactic acid bacteria mixture (LAB) at dosages of 5 × 105 CFU or 106 CFU was able to enhance bacterial clearance and suppress the inflammatory response in ST21-infected mice. These findings once again support that LAB is an effective anti-Salmonella agent. In vitro, we examined the ability of the LAB supernatant to affect ST21 invasion and IL-1 beta and TNF-alpha levels induced by infection. The LAB supernatant increased TNF-alpha expression and suppressed the intracellular number of ST21. As well, it sequentially decreased the expression level of IL-1 beta. Therefore, the LAB supernatant also showed an immunomodulating effect on ST infection. The health benefits of probiotics such as this LAB supplement was used in our previous and present studies and found to include the ability to improve the microbial balance of the host’s intestine, or the mediation of the host’s immunity [Citation18,31]. Therefore, this LAB supplement could be considered as a natural agent, and supplementation of this LAB may contribute to the prevention and/or therapy of Salmonella spp.
Conclusions
In summary, a 4-day treatment of Pteris multifidi (PM) or Cortex phellodendri (CP) aqueous extract, or a mixture of Lactobacillus acidophilus LAP5 and Lactobacillus reuteri Pg4 (LAB) was found to ameliorate the effects caused by S. Typhimurium strain ST21 infection through modulating the inflammatory response rather than their direct bactericidal effects. These results suggest that these two herbs and the Lactobacillus species could be considered as potent agents for Salmonellae therapy.
Author contribution
M.-C.Y., C.-H.C., B.Y. and Y.-M.H. conceived and designed the experiments. M.-C.Y. and C.-H. S. performed the experiments. C.-H.C., B.Y. and Y.-M.H. analyzed the data M.-C.Y., C.-H.C. wrote the paper with input from the co-authors, and the other authors reviewed the manuscript.
Disclosure statement
The authors wish to report that there are no potential conflict of interest.
Funding
This research work was supported by China Medical University [grant number CMU104-S-21]; Yen Tjing Ling Medical Foundation [grant number CI-103-21]; and by the Tainan Municipal Hospital in Tainan, Taiwan [grant number 104-6].
References
- Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the united states. Emerg Infect Dis. 1999;5:607–625.10.3201/eid0505.990502
- Nisha AR. Antibiotic residues – a global health hazard. Vet World. 2008;1:375–377.10.5455/vetworld.
- Lalmanach AC, Lantier F. Host cytokine response and resistance to salmonella infection. Microbes Infect. 1999;1:719–726.10.1016/S1286-4579(99)80073-2
- LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13:191–205.10.1038/nrmicro3420
- Keestra-Gounder AM, Tsolis RM, Baumler AJ. Now you see me, now you don’t: the interaction of salmonella with innate immune receptors. Nat Rev Microbiol. 2015;13:206–216.10.1038/nrmicro3428
- Beal RK, Wigley P, Powers C, et al. Cross-reactive cellular and humoral immune responses to salmonella enterica serovars Typhimurium and Enteritidis are associated with protection to heterologous re-challenge. Vet Immunol Immunopathol. 2006;114:84–93.10.1016/j.vetimm.2006.07.011
- Wang L, Wang X, Zhu XM, et al. Gastroprotective effect of alkaloids from cortex phellodendri on gastric ulcers in rats through neurohumoral regulation. Planta Med. 2017;83:277–284.
- Wang TC, Lin CC, Lee HI, et al. Anti-hyperlipidemic activity of spider brake (Pteris multifida) with rats fed a high cholesterol diet. Pharm Biol. 2010;48:221–226.10.3109/13880200903085458
- Ni G, Fu NJ, Zhang D, et al. An unusual dihydrobenzofuroisocoumarin and ent-kaurane diterpenoids from Pteris multifida. J Asian Nat Prod Res. 2015;17:423–429.10.1080/10286020.2015.1040777
- Chen ML, Xian YF, Ip SP, et al. Chemical and biological differentiation of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis. Planta Med. 2010;76:1530–1535.10.1055/s-0030-1249774
- Kim JH, Weeratunga P, Kim MS, et al. Inhibitory effects of an aqueous extract from Cortex Phellodendri on the growth and replication of broad-spectrum of viruses in vitro and in vivo. BMC Complement Altern Med. 2016;16:265.10.1186/s12906-016-1206-x
- Li C, Xie J, Chen X, et al. Comparison of Helicobacter pylori Urease Inhibition by Rhizoma Coptidis, Cortex Phellodendri and Berberine: mechanisms of Interaction with the Sulfhydryl Group. Planta Med. 2016;82:305–311.
- Lu H, Hu J, Zhang LX, et al. Bioactive constituents from Pteris multifida. Planta Med. 1999;65:586–587.10.1055/s-2006-960835
- Janardhana V, Broadway MM, Bruce MP, et al. Prebiotics modulate immune responses in the gut-associated lymphoid tissue of chickens. J Nutr. 2009;139:1404–1409.10.3945/jn.109.105007
- Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr. 1999;39:13–126.10.1080/10408699991279187
- Chang CH, Chen YS, Chiou MT, et al. Application of scutellariae radix, gardeniae fructus, and probiotics to prevent salmonella enterica serovar choleraesuis infection in Swine. Evid Based Complement Alternat Med. 2013;2013:568528.
- Hsu YM, Yu B, Tseng CS, et al. Preventive activities of Scutellariae Radix, Gardeniae Fructus, and probiotics in salmonella enterica serovar Typhimurium infection in chickens. Animal Feed Sci Technol. 2016;214:121–129.10.1016/j.anifeedsci.2016.02.004
- Lin WH, Yu B, Lin CK, et al. Immune effect of heat-killed multistrain of Lactobacillus acidophilus against salmonella typhimurium invasion to mice. J Appl Microbiol. 2007;102:22–31.10.1111/jam.2007.102.issue-1
- Liu JR, Lai SF, Yu B. Evaluation of an intestinal Lactobacillus reuteri strain expressing rumen fungal xylanase as a probiotic for broiler chickens fed on a wheat-based diet. Br Poult Sci. 2007;48:507–514.10.1080/00071660701485034
- Tsuda H, Matsumoto T, Ishimi Y. Biotin, niacin, and pantothenic acid assay using lyophilized Lactobacillus plantarum ATCC 8014. J Nutr Sci Vitaminol. 2011;57:437–440.10.3177/jnsv.57.437
- Alvarez J, Sota M, Vivanco AB, et al. Development of a multiplex PCR technique for detection and epidemiological typing of salmonella in human clinical samples. J Clin Microbiol. 2004;42:1734–1738.10.1128/JCM.42.4.1734-1738.2004
- Kim JW, Kim HP, Sung SH. Cytotoxic pterosins from Pteris multifida roots against HCT116 human colon cancer cells. Bioorg Med Chem Lett. 2017;27:3144–3147.10.1016/j.bmcl.2017.05.034
- Ouyang DW, Ni X, Xu HY, et al. Pterosins from Pteris multifida. Planta Med. 2010;76:1896–1900.10.1055/s-0030-1249934
- Fujii A, Okuyama T, Wakame K, et al. Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. J Nat Med. 2017;71:745–756.
- Patel S, McCormick BA. Mucosal inflammatory response to salmonella typhimurium infection. Front Immunol. 2014;5:311.
- Perkins DJ, Rajaiah R, Tennant SM, et al. Salmonella typhimurium co-opts the host type I IFN system to restrict macrophage innate immune transcriptional responses selectively. J Immunol. 2015;195:2461–2471.10.4049/jimmunol.1500105
- Bao S, Beagley KW, France MP, et al. Interferon-gamma plays a critical role in intestinal immunity against salmonella typhimurium infection. Immunology. 2000;99:464–472.10.1046/j.1365-2567.2000.00955.x
- Voinnet O. Micro-balancing innate immunity to salmonella. EMBO J. 2011;30:1877–1879.10.1038/emboj.2011.134
- Lahiri A, Lahiri A, Iyer N, et al. Visiting the cell biology of salmonella infection. Microbes Infect. 2010;12:809–818.10.1016/j.micinf.2010.05.010
- Bierschenk D, Boucher D, Schroder K. Salmonella-induced inflammasome activation in humans. Mol Immunol. 2017;86:38–43.10.1016/j.molimm.2016.11.009
- Castillo NA, Perdigon G, de LeBlanc ADM. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against salmonella enterica serovar typhimurium infection in mice. BMC Microbiol. 2011;11:177.10.1186/1471-2180-11-177
