Abstract
The Zn2Cys6-type transcription factor MalR controls the expression of maltose-utilizing (MAL) cluster genes and the production of amylolytic enzymes in Aspergillus oryzae. In the present study, we demonstrated that MalR formed a complex with Hsp70 and Hsp90 chaperones under non-inducing conditions similar to the yeast counterpart Mal63 and that the complex was released from the chaperone complex after the addition of the inducer maltose. The MalR protein was constitutively localized in the nucleus and mutation in both the putative nuclear localization signals (NLSs) located in the zinc finger motif and the C-terminal region resulted in the loss of nuclear localization. This result indicated the involvement of NSLs in the MalR nuclear localization. However, mutation in both NLSs did not affect the dissociation mode of the MalR-Hsp70/Hsp90 complex, suggesting that MalR activation induced by maltose can occur regardless of its intracellular localization.
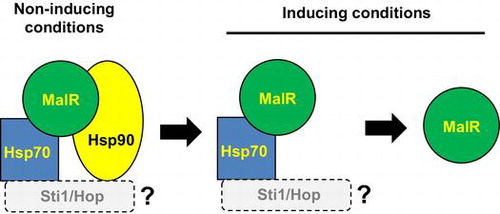
Model for the regulation of A. oryzae MalR by molecular chaperones. The figure is depicted based on the model of the yeast Mal63 (Ran et al., 2008).
Aspergillus oryzae, a filamentous fungus, possesses the ability to produce a large amount of diverse hydrolytic enzymes, including amylolytic, proteolytic, and lipolytic enzymes. Therefore, A. oryzae has been used in Japanese traditional fermentation industries such as in the production of sake, soy sauce, and miso (soybean paste) since over a thousand years [Citation1]. The most important enzymes in sake fermentation are amylolytic enzymes, the production of which is induced in the presence of starch or malto-oligosaccharides [Citation2,3]. This inducible production of amylolytic enzymes requires at least two Zn2Cys6-type transcription activators, AmyR and MalR, which were identified in past studies [Citation4–7]. MalR is also essential for maltose utilization in A. oryzae. MalR regulates the expression of malP and malT that encode maltose transporter (MalP) and intracellular α-glucosidase (maltase, MalT), respectively, both of which constitute the maltose-utilizing cluster (MAL cluster) together with malR. The expression of malP and malT was induced by maltose, but not by glucose or isomaltose, while malR was constitutively expressed, albeit at a low level [Citation6,7]. This malR feature suggested that maltose acts as the inducing sugar for the activation of the transcription factor MalR, which in turn induces the expression of malP and malT in A. oryzae.
The gene organization of the A. oryzae MAL cluster resembles that of the MAL cluster of the yeast Saccharomyces cerevisiae. The transcriptional activation of the transcription factor Mal63 [Citation8] – which is the counterpart of A. oryzae MalR in the yeast MAL cluster – occurs through sequential complex formation with the chaperones Hsp70 and Hsp90 and the co-chaperone Hop/Sti1 in the presence of the inducing sugar maltose [Citation9,10]. The orthologous genes of these chaperones also exist in the genome of A. oryzae. Because Mal63 and MalR share a high homology of zinc finger motifs and also maltose as the common inducing sugar, the transcription activation of MalR is also assumed to be caused through the formation of a complex with such chaperone proteins. In our previous study, we found that MalR protein was constitutively localized in the nucleus, irrespective of the sugar species [Citation7]; thus, if MalR is activated by some modification in the presence of maltose, the corresponding complex formation with chaperones would occur in the nucleus.
In the present study, to examine the protein–protein interaction between MalR and the chaperone proteins orthologous to the yeast Hsp70 and Hsp90, co-immunoprecipitation (Co-IP) analyses were conducted using FLAG-tagged MalR-expressing strains that also harbored the expression constructs of c-Myc-tagged Hsp70 or Hsp90. Furthermore, to examine the influence of subcellular localization of MalR on the complex formation with chaperones, MalR mutants, in which the amino acid residues of the putative nuclear localization signals (NLSs) were substituted by alanine, were constructed and Co-IP analyses were performed.
Materials and methods
Strains and media
Aspergillus oryzae ΔligD::ptrA strain (ΔligD::ptrA, niaD−, sC−) [Citation11] was used as the recipient strain for tagging malR, hsp70, and hsp90 with 3 × FLAG or c-Myc at the genomic locus for Co-IP analysis. The ΔmalR strain [Citation7] was used as the recipient strain for the expression of GFP-fused MalR and FLAG-tagged MalR. Escherichia coli DH5α (Promega, Madison, WI, USA) was used for the construction and propagation of plasmid DNAs. Czapek–Dox (CD) was used as the minimal medium (MM) for A. oryzae culture, and it contained 0.6% NaNO3; 0.05% KCl; 0.2% KH2PO4; 0.05% MgSO4; a trace amount of FeSO4, ZnSO4, CuSO4, MnSO4, Na2B4O7, and (NH4)6Mo7O24; and 1% sugar. For cultivation of the niaD-deficient and sC-deficient strains, 0.6% NaNO3 was replaced with 0.5% (NH4)2SO4 as the nitrogen source and 0.0003% methionine as the sulfur source, respectively, in MM.
Construction of plasmids for Co-IP analyses
The plasmids for introducing the c-Myc-tag at the C-terminus of Hsp70 and Hsp90 were constructed as follows: the c-Myc-fused hsp70 fragment was amplified by PCR with the primers Hsp70senNotI and Hsp70Pma-mycRVanti (Supplementary Table S1) on A. oryzae genomic DNA. The amplified PCR fragment was digested with NotI and EcoRV and then inserted into the plasmid pPTRI-PenoA-TagdA to yield pPTRI-PenoA-hsp70myc. The plasmid pPTRI-PenoA-TagdA is a modified pPTRI (Takara Bio Inc., Shiga, Japan) containing the enoA promoter and agdA terminator, which were obtained from PstI/SmaI-digested pNE [Citation7]. The hsp90 fragment was amplified by PCR with the primers Hsp90senNotI and Hsp90antiPmaCI (Supplementary Table S1). The amplified fragment was digested with NotI and PmaCI, and replaced with hsp70 of pPTRI-PenoA-hsp70myc to yield pPTRI-PenoA-hsp90myc. The partial c-Myc-fused hsp70 and hsp90 fragments attached with the agdA terminator were obtained from KpnI-digested pPTRI-PenoA-hsp70myc and pPTRI-PenoA-hsp90myc and then inserted into KpnI-digested pUSC [Citation12]. Because the resulting plasmids harbored the N-terminally truncated hsp70 or hsp90, the plasmids were digested with the restriction enzyme whose unique site was located within the coding region and then introduced into the recipient ΔligD::ptrA strain, so that the hsp70-myc and hsp90-myc constructs were expressed by their own promoters (Supplementary Figure S1).
The plasmid for expressing 3 × FLAG-tagged MalR was constructed as follows: the fragment of malR was amplified by PCR with the primers malRsenPmaCI and malRantiXbaI (Supplementary Table S1) on A. oryzae genomic DNA. The amplified fragment was digested with PmaCI and XbaI and inserted into pNE. The resultant plasmid was digested with PstI and PmaCI, and the enoA promoter was replaced with the thiA promoter attached to the 3 × FLAG-encoding sequence obtained from PstI/PmaCI-digested pNT-3FLAG (Tanaka et al. unpublished). The resulting plasmid was introduced into the expression strains of c-Myc tagged Hsp70 or Hsp90 used for Co-IP analyses.
Construction of plasmids for GFP-fused MalR wild-type and mutants
The plasmids for the GFP-fused MalR expressed by the thiA promoter were constructed as previously described [Citation7]. The plasmids harboring the MalR mutants with substitution of alanine residues in the putative NLSs were constructed by QuikChange Site-Directed Mutagenesis (Agilent Technologies, Santa Clara, CA, USA). In addition to the putative NLS (NLS1) located within the zinc finger motif [Citation7], another NLS candidate (NLS2) found in the C-terminal region was mutated by following three sequential steps with the primer sets: MalRNLS2m + MalRNLS2m-r, MalRNLS3m + MalRNLS3m-r, and MalRNLS4m + MalRNL4Sm-r (Supplementary Table S1). Briefly, in the first step, MalRNLS2m and MalRNLS2m-r were used to replace the first arginine residue in NLS2 with alanine. In the second step, MalRNLS3m and MalRNLS3m-r were used to replace the second arginine (AARR) with alanine. In the last step, MalRNLS4m and MalRNL4Sm-r were used to replace the third arginine (AAAR) with alanine, resulting in the replacement of all three arginine residues with the corresponding alanine residues (AAAA). The plasmid obtained by QuickChange mutagenesis was examined by sequencing using the BigDye Terminator v1.1 (Life Technologies Corporation, Carlsbad, CA, USA) and a primer designed in MalR (seqMalR; Supplementary Table S1) to confirm the resulting mutation of interest. Mutation in both NLSs was generated by combining the NLS1 and NLS2 mutants.
Fungal transformation
The transformation of A. oryzae was performed according to the procedure described elsewhere [Citation13].
Fluorescence microscopy
Fluorescence microscope imaging of GFP-MalR was performed by using a confocal laser fluorescence microscope (FV1000-D IX81; Olympus, Tokyo, Japan), as previously described [Citation7]. Briefly, approximately 3 × 105 conidiospores were inoculated onto coverslips dipped in 500 μL of MM containing 1% casamino acid without sugars and incubated at 30 °C for 12 h. Then, the hyphae produced were dipped in fresh MM containing 1% casamino acid and 1% maltose with thiamine at a final concentration of 10 μM.
Intracellular protein extraction for Co-IP analysis
For Co-IP analyses, the co-expression strains were pre-cultured in liquid MM containing 1% casamino acid as the carbon source at 30 °C for 24 h and then transferred to liquid MM containing 0.1% sugar (maltose, glucose, or isomaltose). After incubation for the indicated periods, the mycelium was harvested by filtration through Miracloth (EMD Millipore Corporation, Billerica, MA, USA) and then ground to a fine powder in liquid nitrogen by using a mortar and pestle. The powdered mycelium was suspended in the extraction buffer [25 mM Tris-HCl (pH 8.0), 0.25% TritonX-100, 100 mM NaCl, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 100 μM pepstatin A, and complete EDTA-free protease inhibitor (Roche, Indianapolis, IN, USA)] and incubated on an ice bath for 15 min, followed by centrifugation at 15,000 rpm for 10 min at 4 °C. The protein concentration in the resultant supernatant was determined by the Bradford method [Citation14].
Co-immunoprecipitation assays
Intracellular proteins in the cell lysates prepared as described above were used for Co-IP assays with Anti-c-Myc or Anti-DYKDDDDK (FLAG)-tagged Antibody Beads (Wako Pure Chemical Industries Ltd., Osaka, Japan) washed in advance with phosphate-buffered saline buffer (PBS; 0.8% NaCl, 0.11% Na2HPO4, 0.02% KH2PO4, 0.02% KCl). The intracellular proteins (400 μL) were then added to the washed Anti-c-Myc or Anti- FLAG Antibody Beads, agitated for more than 2 h at 4 °C, and centrifuged at 8,000 rpm for 30 s at 4 °C. Next, the precipitated beads were suspended in 1 mL of ice-cold PBS and centrifuged at 8,000 rpm for 30 s at 4 °C. The beads were then washed four times with 1 mL of PBS and resuspended in 2 × SDS sample buffer [100 mM Tris-HCl (pH 6.8), 20% (v/v) glycerol, 2% SDS, 0.2% BPB]. Finally, the proteins bound to the beads were dissociated by boiling for 3 min. After centrifugation at 15,000 rpm for 30 s, 47.5 μL of the resulting supernatant was mixed with 2.5 μL of 2-mercaptoethanol, boiled for 3 min, and then subjected to SDS-PAGE followed by western blotting.
Western blotting
The Co-IP supernatants (10 μL) were subjected to SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon P; EMD Millipore Corporation, Billerica, MA, USA) with Towbin buffer (40 mM Tris-HCl, 38 mM glycine, 10% methanol, 0.025% SDS). Similarly, the cell lysates (10 μg of intracellular proteins) were simultaneously subjected to SDS-PAGE and immunoblotted (IB) for references. The PVDF membranes were then blocked in 0.3% skim milk in PBS containing 0.1% Tween 20 (PBST) and probed with the antibodies against DYKDDDDK and c-Myc (Wako Pure Chemical Industries Ltd.). TrueBlot® Anti-IgG HRP (Rockland Immunochemicals Inc., Limerick, PA, USA) was used for the detection of immunoblotted target protein bands. The chemiluminescence signal was detected by using the Chemi-Lumi One L Kit (NacalaiTesque, Kyoto, Japan) along with the ImageQuant LAS-4000 image analyzer (GE Healthcare, Piscataway, NJ, USA).
Results
Interaction analysis of MalR with chaperone proteins Hsp70 and Hsp90
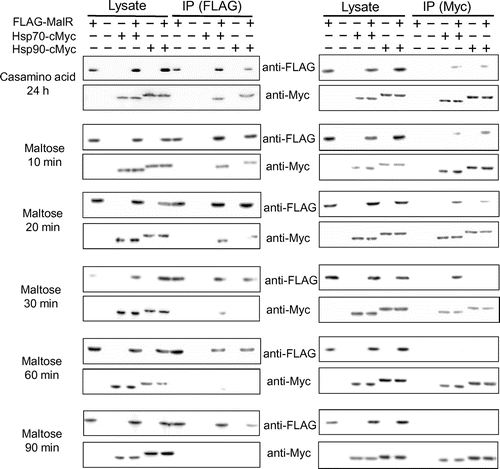
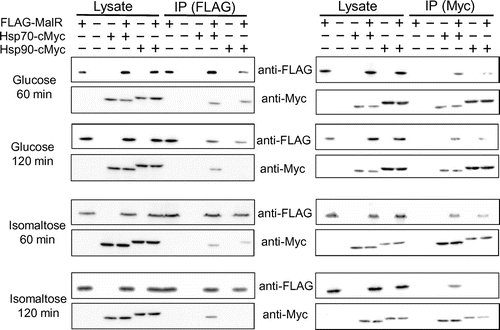
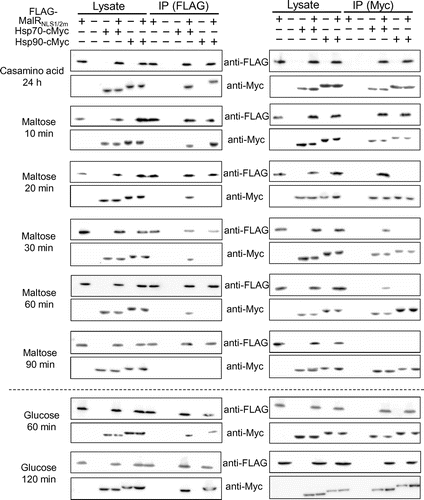
Based on the reports that the transcription activator Mal63 in the yeast MAL cluster is activated in the presence of the inducing sugar maltose through the sequential complex formation with the chaperones and co-chaperone Ssa1/Hsp70, Hsp82/Hsp90, and Sti1 [Citation9,10], we assumed that similar chaperone complex formation event can occur in A. oryzae MalR. We searched for the orthologous genes of these chaperones in the Aspergillus genome database AspGD (http://www.aspgd.org/), based on the genome sequencing analysis [Citation15]. There are multiple paralogous genes encoding the chaperones Ssa1/Hsp70 and Hsp82/Hsp90 in S. cerevisiae, including four SSA subfamily genes (SSA1, SSA2, SSA3, and SSA4) and two HSP90 genes (HSC82 and HSP82). However, interestingly, there was only one corresponding gene orthologous to each chaperone in Aspergillus fungi, including A. oryzae; these were AO090012000995 (SSA1/HSP70 ortholog) and AO090102000620 (HSP82/HSP90 ortholog) in A. oryzae (Supplementary Figure S2). In addition, the orthologous gene of the yeast STI1 was found as AO090038000562 in A. oryzae. According to the proposed model in the yeast [Citation9,10,16], Hsp70 and Hsp90 directly interact with Mal63. Therefore, we first examined the interaction of MalR with these orthologous chaperones. To address this possibility, Co-IP assays were conducted by using the co-expression strains, wherein C-terminally c-Myc-tagged Hsp70 (Hsp70-Myc) or Hsp90 (Hsp90-Myc) was expressed together with 3 × FLAG-tagged MalR (FLAG-MalR). After the pre-culture in the casamino acid-containing medium, FLAG-MalR was immunoprecipitated with both Hsp70-Myc and Hsp90-Myc, suggesting that MalR was initially associated with both Hsp70 and Hsp90 under non-inducing condition. Interaction of MalR with Hsp70 or Hsp90 was observed after incubation for 20 min following maltose addition, whereas that with Hsp90 was undetectable after 30-min incubation (Figure ). Furthermore, after a minimum incubation period of 60 min, no interaction of MalR with Hsp70 and Hsp90 was detected (Figure ). On the contrary, following the addition of glucose, the binding of MalR to Hsp70 and Hsp90 was clearly observed after 60-min incubation. Moreover, the association of MalR with Hsp70 and Hsp90 remained even after 120 min of glucose addition (Figure ). Similarly, after isomaltose addition, the interaction of MalR with Hsp70 was retained during the 120-min incubation period, although that with Hsp90 mostly disappeared by that time point (Figure ). These results indicated that incubation in the presence of inducer resulted in the release of MalR protein from its complex with both Hsp70 and Hsp90. Judging from the results of Co-IP analyses, Hsp90 chaperone dissociated earlier from the chaperone complex of MalR than Hsp70 when grown under inducing condition. Furthermore, the interaction of MalR with Hsp90 was observed to be stable in the 24-h incubation period in casamino acid medium, but not 2 h after glucose or isomaltose addition, which suggests that the non-inducing sugars such as glucose and isomaltose could also induce instability in the chaperone complex of MalR and Hsp90.
Figure 1. The interaction of FLAG-MalR with Hsp70-Myc and Hsp90-Myc under inducing condition in the presence of maltose. Strains were cultivated in liquid MM containing 1% casamino acid without sugars for 24 h at 30 °C and then transferred to MM supplemented with 0.1% maltose. Co-IP assays were conducted using Anti-FLAG Antibody Beads. Cell lysates (10 μg of total intracellular proteins) and 10 μL of Co-IP supernatants were subjected to SDS-PAGE and immunoblotting (IB) with anti-FLAG and anti-c-Myc antibodies. Reciprocal Co-IP analysis was performed using Anti-c-Myc Antibody Beads. Samples derived from the strains expressing tagged protein(s) are indicated by a plus sign (+) in lanes. Co-IP experiments were repeated at least twice and found reproducible. The data presented here are those from one of the two representative experiments.
Subcellular localization of GFP-MalR with mutations in putative NLSs
In the previous study, MalR was constitutively localized in the nucleus [Citation7]. Hence, the association of MalR with Hsp70/Hsp90 chaperons occurred in the nucleus, but MalR was released from the chaperone complex in the nucleus under inducing conditions. To examine whether the association/dissociation of MalR with the chaperones occur regardless of its nuclear localization, we constructed GFP-MalR mutants with mutation in the putative NLSs. Previously, a basic amino acid cluster, RRK, located within the zinc finger motif of MalR was predicted as a putative NLS (Figure (a)) [Citation7]. The replacement of these amino acid residues by alanine resulted in the diffusion of the GFP fluorescence in the cytoplasm, although the fluorescence was still observed in the nucleus, suggesting the existence of another sequence that can function as an NLS (Figure (b)) [Citation7]. The search result of PSORT II prediction program (https://psort.hgc.jp/form2.html) identified another putative sequence, PTERARR, in the C-terminal region of MalR (Figure (a)). In the present study, putative NLSs located within the zinc finger motif and in the C-terminal region were designated as NLS1 and NLS2, respectively. To examine the effect of mutations in these putative NLSs on the MalR intracellular localization, arginine and lysine residues within the sequences were replaced with alanine residues (Figure (a)). Similarly in GFP-MalRNLS1m with mutation in NLS1, the GFP fluorescence in GFP-MalRNLS2m with mutation in NLS2 was also diffused in the cytoplasm as compared with that in GFP-MalRWT (Figure (b)). However, GFP-MalRNLS2m could be retained in the nucleus, because the α-amylase activity was observed in the strain with GFP-MalRNLS2m construct by the halo assay on the starch-containing medium (Supplementary Figure S3). On the contrary, in GFP-MalRNLS1/2m with mutation in both NLS1 and NLS2, the fluorescence was observed in the cytoplasm, but rarely in the nucleus (Figure (b)). However, there exists a possibility that the MalR mutant still existed in the nucleus because mutation in NLS1 within the zinc finger motif could result in the loss of DNA-binding activity and the α-amylase activity could not be examined in the strains with NLS1 mutation. Nevertheless, these results suggested that mutations in both NLS1 and NLS2 can lead to a severe loss of nuclear localization of MalR and that both sequences can actually function as NLSs.
Figure 2. The interaction of FLAG-MalR with Hsp70-Myc and Hsp90-Myc under non-inducing conditions in the presence of 0.1% glucose or isomaltose. Strains were grown and Co-IP analysis was performed as described in Figure 1. Co-IP experiments were repeated at least twice and found reproducible. The data presented here are those from one of the two representative experiments.
Interaction of MalR mutants with chaperone proteins Hsp70 and Hsp90
Considering that mutation in both NLS1 and NLS2 resulted in the loss of MalR nuclear localization, we examined the interaction of the mutated MalR with the chaperone complex. FLAG-MalRNLS1/2m was also associated with both Hsp70 and Hsp90 before sugar addition, as observed in the MalR wild-type (FLAG-MalRWT) (Figure ). Interactions with both the chaperones were observed 10 min after the addition of the inducer maltose, although the association with Hsp90 was abolished after 20-min incubation, which is slightly earlier than that observed for FLAG-MalRWT. In contrast, the association with Hsp70 was retained even after 60-min incubation, but it disappeared after 90-min incubation. On the contrary, under non-inducing condition, the association of MalR with both Hsp70 and Hsp90 was observed for 120-min incubation following glucose addition (Figure ). The stability of the chaperone complex of MalRNLS1/2m appeared to be slightly different from that of MalRWT, which may be attributed to the conformational change of MalR caused by the alteration of the amino acid residues in both NLSs. To examine this possibility, further analysis would be required using the co-expression strains, wherein FLAG-MalRNLS1m or FLAG-MalRNLS2m was expressed together with either Hsp70-Myc or Hsp90-Myc. Nonetheless, these results suggested that the subcellular localization of MalR does not significantly affect the chaperone complex formation under both non-inducing and inducing conditions.
Figure 3. The subcellular localization of the wild-type and NLS-mutated MalR proteins. (A) Strategy for mutations in NLS1 and NLS2 in the MalR protein. Conserved cysteine residues in the Zn2Cys6 finger motif region (dotted underline) are indicated in bold. (B) GFP fluorescence of the wild-type and NLS-mutated GFP-MalR after the addition of sugars with thiamine. The hyphae grown in liquid MM with 1% casamino acid as the sole carbon source for 12 h at 30 °C were dipped in fresh MM containing 1% maltose with thiamine at a final concentration of 10 μM. The microscope images were obtained with a confocal laser fluorescence microscope at a magnification of 1,000×(mycelium) or 3,000×(hyphal tip).
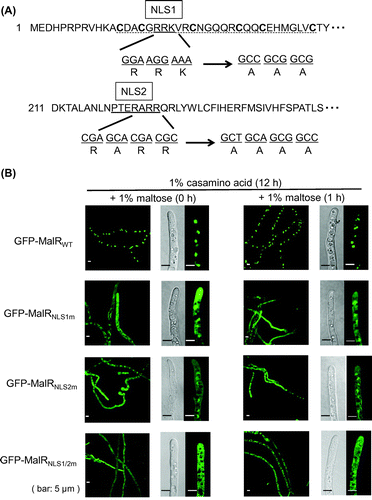
Figure 4. The interaction of FLAG-MalRNLS1/2m with Hsp70-Myc and Hsp90-Myc under inducing and non-inducing conditions. Co-IP analysis was performed as described in Figures and . Co-IP experiments were repeated at least twice and found reproducible. The data presented here are those from one of the two representative experiments.
Discussion
The production of amylolytic enzymes in A. oryzae is induced in the presence of starch or maltose, and the Zn2Cys6-type transcription factor AmyR is essential for this regulation [Citation4,5]. Another Zn2Cys6-type transcription factor, MalR, is also required for the production of amylolytic enzymes through the regulation of maltose transporter (MalP) and α-glucosidase (MalT), which are probably responsible for the conversion of maltose to isomaltose that can activate AmyR [Citation6,7]. The genes encoding MalP and MalT constitute the MAL cluster together with the MalR-coding gene malR, which is expressed constitutively as amyR [Citation6]. This suggests that MalR produced would be inactive under non-inducing conditions and become active under inducing condition in the presence of maltose. Chaperone complex similar to that of Mal63 – the yeast counterpart of MalR that forms a complex with Ssa1/Hsp70 and Hsp82/Hsp90 chaperones in the activation pathway [Citation9,10,16] – is predicted to form in the MalR activation.
In the present study, to examine the complex formation with the chaperones in the activation pathway of MalR, Co-IP analyses were performed with co-expression strains, wherein FLAG-MalR was expressed together with either Hsp70-Myc or Hsp90-Myc. MalR transcription factor was shown to form a stable complex with both Hsp70 and Hsp90 under non-inducing conditions, and, after the addition of the inducer maltose, the association of MalR with the chaperones was gradually abolished. The interaction with Hsp90 disappeared earlier than that with Hsp70. In contrast, the MalR–chaperone complex was stable in the absence of maltose as compared with that in the presence of maltose. Furthermore, the interaction mode of MalR with Hsp70 and Hsp90 chaperones was unaffected by its intracellular localization.
Although MalR formed a complex with Hsp70 and Hsp90, the interaction of MalR with the chaperones was apparently different from that with the yeast counterpart Mal63. Under non-inducing conditions, Mal63 associates with Ssa1/Hsp70 as an “early complex,” after which Hsp82/Hsp90 is recruited to the complex with the assistance of the co-chaperone Sti1/Hop to form a stable “intermediate complex” [Citation9,10]. In the presence of the inducer maltose, Ssa1/Hsp70 and Sti1/Hop are liberated from the intermediate complex and the resulting final complex consisting of Mal63 and Hsp82/Hsp90 is an active form, with the ability of DNA binding and transcription activation [Citation9,10]. On the contrary, for MalR activation, the occurrence of MalR–Hsp70 early complex was not evident because detection of such a chaperone complex was out of scope of our experiment. However, although the formation of the stable complex consisting of MalR, Hsp70, and Hsp90 was demonstrated under non-inducing condition, after the addition of inducer, Hsp90 was released first from the complex followed by Hsp70. The temporal order in the sequential release of these chaperones and the loss of the Hsp90 chaperone in the activation pathway of MalR distinctly differs from those observed for Mal63. The mode of MalR activation, wherein the Hsp70 and Hsp90 chaperones participate, was also different from that of the yeast Hap1 – an oxygen-sensing transcription factor. Hap1 initially forms a repressed complex with Hsp70 and their co-chaperones and is then bound to the inducer heme with its conformational change to bind Hap90, forming an active complex [Citation17–20]. While both Mal63 and Hap1 are associated with Hsp90 in their activation-competent state, MalR seems to be activated by its release from the Hsp90 chaperone, as described above. In addition, the subcellular localization of Mal63 has not yet been examined and we thus could not obtain any information about where the activation event occurs in Mal63. In contrast, we demonstrated that MalR was constitutively localized in the nucleus [Citation7] and the chaperone complex formation occurred regardless of its intracellular localization.
Although the MalR protein was fully liberated from Hap90 30 min after the addition of maltose according to the Co-IP analyses, the expression of the MAL cluster genes (malP and malT) was detectable 10 min after maltose addition, as previously reported [Citation7]. This could be explained by the overexpression of malR under the control of the thiA promoter for enabling detection of protein–protein interaction by Co-IP analyses in the present study. Under physiological conditions, the transcription factor genes are generally expressed at low levels sufficient to regulate their controlling genes. In this study, MalR was overproduced by the thiA promoter in the absence of thiamine, and thus it was possible that the early release of a small amount of MalR from Hsp90 could not be detected by our Co-IP analyses. Even such a small quantity of activated MalR is sufficient to enhance the transcription of malP and malT. Northern blot analysis revealed that the expression levels of malP and malT reached their peak 25–30 min after the addition of maltose [Citation7]. This time point was consistent with that when most MalR protein was released from Hsp90. On the contrary, Hsp70 was released from MalR after Hsp90. Considering that Hsp70 is proposed to function as a repressor in Mal63 and Hap1 [Citation9,10,16], the role of Hsp70 in the transcription activation of MalR remains to be elucidated. In addition, we did not examine whether the co-chaperone encoded by AO090038000562, which is the counterpart of the yeast Sti1/Hop, was involved in the activation pathway of MalR. However, according to the observation in Mal63 that Sti1/Hop is liberated together with Ssa1/Hsp70 from the intermediate complex under inducing condition [Citation9], it is possible that Sti1 ortholog is associated with Hsp70 and Hsp90 in the intermediate complex and released together with these chaperones from MalR in the MalR activation pathway.
Under inducing condition in the presence of maltose, the MalR protein was released first from Hsp90 chaperone and then from Hsp70 chaperone. According to the general characteristics of the Hsp90 chaperone complex, the proteins associated with Hsp90 become notably unstable when their chaperone complex formation or interaction is impeded [Citation21–24]. In fact, in the yeast S. cerevisiae, the depletion of the Hsp90 chaperone resulted in a significant decrease in the Mal63 stability [Citation16]. In this context, we assumed that the MalR protein would be also destabilized under inducing condition because it was released from Hsp90 in the presence of maltose. Unexpectedly, however, the MalR protein appeared to remain stable even under inducing condition for a 90-min incubation period, during which MalR was liberated from Hsp90 (Figure , lane 6 in lysate). Furthermore, MalR mutants with mutations in NLSs were also stable in the presence of maltose in a 90-min incubation period. These results suggested that MalR was stable under conditions that prevented association with the Hsp90 chaperone and that the MalR stability was unaffected by its intracellular localization.
The next question in the activation pathway of MalR is regarding activation in the presence of the inducer maltose. In case of Mal63, it was hypothesized that the Mal63 protein binds to maltose and alters its conformation so that it can be converted to an active form [Citation9,10,16]. Similarly, MalR may bind to maltose for its transcription activation, but further analyses would be required to examine this possibility. Moreover, MalR seems to be competent for transcription activation by its release from the chaperone complex, although it has been unclear whether MalR has any interaction with other factors in its active form. For this purpose, we plan to examine the existence of some proteins that interact with MalR in the active form through tandem affinity purification (TAP). As described above, the fact that MalR remains stable even when released from Hsp90 under inducing condition would be advantageour for detecting such interacting proteins by the TAP method. In addition, we also plan to investigate whether the chaperone complex formation of MalR could be reversed and relevant experiments are under way to examine whether Hap70 and Hsp90 can associate with MalR when transferred from a maltose medium to a glucose-containing medium.
Author contributions
MT and KG conceived and designed the experiments. YK, KS, and MT conducted the experiments. MT, TS, and KG analyzed the data. MT and KG wrote the manuscript.
Disclosure statement
The authors declare that they have no conflict of interest.
Supplemental data
The supplemental data for this article is available online at https://doi.org/10.1080/09168451.2018.1447359
Funding
This work was supported by JSPS KAKENHI [grant number 25292044]; Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry; and Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry.
MalR-HSP_complex_MS_Suppl.zip
Download Zip (138.9 KB)References
- Machida M, Yamada O, Gomi K. Genomics of Aspergillus oryzae: learning from the history of koji mold and exploration of its future. DNA Res. 2008;15:173–183.10.1093/dnares/dsn020
- Tonomura K, Suzuki H, Nakamura N, et al. On the inducers of α-amylase formation in Aspergillus oryzae. Agric Biol Chem. 1961;25:1–6.
- Yabuki M, Ono N, Hoshino K, et al. Rapid induction of alpha-amylase by nongrowing mycelia of Aspergillus oryzae. Appl Environ Microbiol. 1977;34:1–6.
- Gomi K, Akeno T, Minetoki T, et al. Molecular cloning and characterization of a transcriptional activator gene, amyR, involved in the amylolytic gene expression in Aspergillus oryzae. Biosci Biotechnol Biochem. 2000;64:816–827.10.1271/bbb.64.816
- Petersen KL, Lehmbeck J, Christensen J. A new transcriptional activator for amylase genes in Aspergillus. Mol Gen Genet. 1999;262:668–676.10.1007/s004380051129
- Hasegawa S, Takizawa M, Suyama H, et al. Characterization and expression analysis of a maltose-utilizing (MAL) cluster in Aspergillus oryzae. Fungal Genet Biol. 2010;47:1–9.10.1016/j.fgb.2009.10.005
- Suzuki K, Tanaka M, Konno Y, et al. Distinct mechanism of activation of two transcription factors, AmyR and MalR, involved in amylolytic enzyme production in Aspergillus oryzae. Appl Microbiol Biotechnol. 2015;99:1805–1815.10.1007/s00253-014-6264-8
- Hu Z, Gibson AW, Kim JH, et al. Functional domain analysis of the Saccharomyces MAL-activator. Curr Genet. 1999;36:1–12.10.1007/s002940050466
- Ran F, Bali M, Michels CA. Hsp90/Hsp70 chaperone machine regulation of the Saccharomyces MAL-activator as determined in vivo using non inducible and constitutive mutant alleles. Genetics. 2008;179:331–343.10.1534/genetics.107.084921
- Ran F, Gadura N, Michels CA. Hsp90 cochaperone Aha1 is a negative regulator of the Saccharomyces MAL activator and acts early in the chaperone activation pathway. J Biol Chem. 2010;285:13850–13862.10.1074/jbc.M109.040600
- Mizutani O, KudoY Saito A, et al. A defect of LigD (human Lig4 homolog) for nonhomologous end joining significantly improves efficiency of gene-targeting in Aspergillus oryzae. Fungal Genet Biol. 2008;45:878–889.10.1016/j.fgb.2007.12.010
- Gomi K, Iimura Y, Hara S. Integrative transformation of Aspergillus oryzae with a plasmid containing the Aspergillus nidulans argB gene. Agric Biol Chem. 1987;51:2549–2555.
- Yamada O, Lee BR, Gomi K. Transformation system for Aspergillus oryzae with double auxortophic mutations, niaD and sC. Biosci Biotechnol Biochem. 1997;61:1367–1369.10.1271/bbb.61.1367
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254.10.1016/0003-2697(76)90527-3
- Machida M, Asai K, Sano M, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161.10.1038/nature04300
- Bali M, Zhang B, Morano KA, et al. The Hsp90 molecular chaperone complex regulates maltose induction and stability of the Saccharomyces MAL gene transcription activator Mal63p. J Biol Chem. 2003;278:47441–47448.10.1074/jbc.M309536200
- Hon T, Lee HC, Hach A, et al. The Hsp70-Ydj1 molecular chaperone represses the activity of the heme activator protein Hap1 in the absence of heme. Mol Cell Biol. 2001;21:7923–7932.10.1128/MCB.21.23.7923-7932.2001
- Lan C, Lee HC, Tang S, et al. A novel mode of chaperone action: heme activation of Hap1 by enhanced association of Hsp90 with the repressed Hsp70-Hap1 complex. J Biol Chem. 2004;279:27607–27612.10.1074/jbc.M402777200
- Xin X, Lan C, Lee HC, et al. Regulation of the HAP1 gene involves positive actions of histone deacetylases. Biochem Biophys Res Commun. 2007;362:120–125.10.1016/j.bbrc.2007.07.156
- Lee HC, Zhang L. A unique mechanism of chaperone action: heme regulation of Hap1 activity involves separate control of repression and activation. Protein Pept Lett. 2009;16:642–649.10.2174/092986609788490113
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133.10.1177/153537020322800201
- Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477.10.1074/jbc.R800007200
- Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biophys Biochim Acta. 2012;1823:624–635.10.1016/j.bbamcr.2011.09.003
- Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114.10.1073/pnas.96.1.109
