ABSTRACT
Streptococcus mutans is a bacterium found in human oral biofilms (dental plaques) that is associated with the development of dental caries. Glucosyltransferases (GTFs) are key enzymes involved in dental plaque formation, and compounds that inhibit their activities may prevent dental caries. We developed a screening system for GTF-inhibitory activities, and used it to profile 44 types of herbal tea extracts. Lemon myrtle (Backhousia citriodora) extract exhibited the highest GTF-inhibitory activity, with an IC50 for GTF in solution of 0.14 mg mL−1. Furthermore, lemon myrtle extracts had the third-highest polyphenol content of all tested extracts, and strongly inhibited S. mutans biofilm. Interestingly, lemon myrtle extracts did not inhibit cell growth.
Graphical Abstract
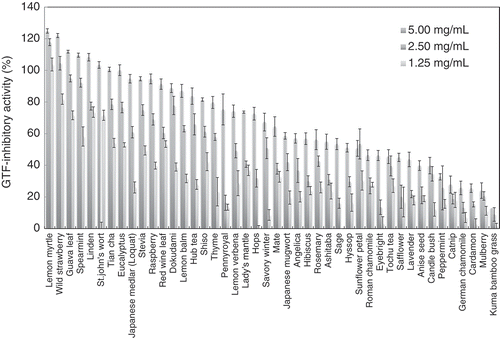
Glucosyltransferases-inhibitory activities of 44 types of herbal tea extracts.
Streptococcus mutans (S. mutans) is a Gram-positive bacterium that contributes to the pathogenesis of dental caries [Citation1–Citation3]. This is since an adhesive glucan produced by S. mutans leads to the formation of oral biofilms (dental plaques). Glucan production depends on glucosyltransferases (GTF; EC 2.4.1.5), which catalyze the hydrolysis of sucrose to form fructose and glucose; these sugars are then transferred to glucose residues of acceptor sugars, such as sucrose and nigerooligosaccharide[Citation4].
S. mutans produces three different GTFs: GTF-I, GTF-S, and GTF-SI. GTF-I and GTF-S catalyze the synthesis of only insoluble and soluble glucans, respectively, whereas GTF-SI can synthesize both types of glucans [Citation2,Citation4,Citation5]. GTF-SI is essential for S. mutans sucrose-dependent adhesion, which engenders biofilm formation on smooth surfaces[Citation2]. These findings suggest that a GTF-SI inhibitor would be a useful therapeutic strategy for preventing dental caries.
Several potential anti-caries compounds have been characterized in various foods [Citation6–Citation11]. Japanese green tea polyphenols inhibited S. mutans growth, adhesion, and glucan synthesis activity [Citation6,Citation8]. Furthermore, apple, oolong tea, and hop bract polyphenol inhibited glucan synthesis activity in S. mutans [Citation7,Citation10,Citation11]. These findings indicate that polyphenol compounds are important modulators of adhesion, biofilm formation, and GTF activity in S. mutans.
Many herbal teas contain large amounts of polyphenols, which have many functions, including anti-bacterial [Citation12–Citation15], anti-viral [Citation15] anti-cancer[Citation16], and anti-inflammatory activities[Citation17]. However, there are few reports regarding the GTF-inhibitory activity or anti-biofilm properties of herbal tea. In this study, we developed an assay for GTF inhibition using a recombinant GTF-SI derived from S. mutans, and determined the GTF-inhibitory activities of 44 individual food herbal tea extracts. Among them, extracts from lemon myrtle (Backhousia citriodora) exhibited the highest GTF-inhibitory activity.
Material and methods
Preparation of herbal tea extract
Forty-four kinds of herbal tea were purchased from Yuwn Co., Ltd (Tokyo, Japan) (Table S1). Ten grams of herbal tea was boiled for 10 min in 100 mL of distilled water. The extract was filtered through a bleached cotton cloth, and the filtrate was then lyophilized in DC800 freeze-dryer (Yamato Co., Tokyo, Japan) before use in experiments.
Bacteria and cell growth
S. mutans strain MT8148 was obtained from the RIKEN BioResource Center (Tsukuba, Japan) and grown in brain heart infusion broth (Nissui Pharmaceutical, Tokyo, Japan) under static culture conditions at 37°C for 18 h.
Construction of GTF-SI expression plasmids
S. mutans genomic DNA was extracted using a NucleoSpin Tissue Kit (Macherey-Nagel, Duren, Germany). The open reading frames of GTF-SI were amplified from S. mutans genomic DNA using the primer sets shown as follows: SmGTF-SI-full-F (5′ -AAGGAGATATACATATGGAAAAGAAAGTACGTTTTAA-3′), pET24b/SmGTF-SI-full-R (5′- GGTGGTGGTGCTCGAGAAATCTAAAGAAATTGTCAA-3′), pET24b/SmGTF-SI-∆V-F (5′- AAGGAGATATACATATGGTCAAAAATATCAGAAAAGTGAACGGT-3′), and pET24b/SmGTF-SI-∆V-R (5′- GGTGGTGGTGCTCGAGACCATCAAATACCAATCCAGTTACA-3′). Amplified DNA fragments were purified and then introduced into a pET24b (Novagen, San Diego, CA, USA) using an In-Fusion® HD Cloning Kit (Takara Bio, Shiga, Japan). The sequence of the inserted region in the pET24b was verified by DNA sequencing with ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Expression and purification of his-tagged recombinant GTF-SI
Escherichia coli strain BL21 StarTM (DE3) pLysS (Invitrogen, Carlsbad, CA, USA) that had been transformed with pET24b/SmGTF-SI-full or pET24b/SmGTF-SI-∆V was grown in 50 mL of Luria-Bertani broth that contained 50 µg mL−1 of kanamycin and 34 µg mL−1 of chloramphenicol. A culture was then transferred to 1 L of Overnight Express Instant TB Medium (Novagen, San Diego, CA, USA), and bacteria were grown at 37°C for 24 h. The cells were then harvested by centrifugation at 6,000 × g for 10 min, and cell pellets were kept frozen at −20°C. Recombinant E. coli cells (10 g) harboring pET24b/SmGTF-SI-full or pET24b/SmGTF-SI-∆V were suspended in 50 mL of 50 mM potassium phosphate buffer (pH 7.4) that contained 500 mM NaCl, 45 mM imidazole, 10 mM 2-mercaptoethanol and 10% (v/v) glycerol (buffer A), sonicated (10 kHz) using 20-sec strokes at 30-sec intervals, and then centrifuged at 15,000 × g for 15 min. The extract was loaded on to a Ni Sepharose 6 Fast Flow (GE Healthcare, Chicago, IL, USA) column equilibrated with buffer A. The column was washed with 10 mL of buffer A and eluted with a 5 mL of 50 mM potassium phosphate (pH 7.4) that contained 500 mM NaCl, 500 mM imidazole, 10 mM 2-mercaptoethanol and 10% (v/v) glycerol. Eluted fractions were dialyzed against 50 mM potassium phosphate buffer at 4°C for 24 h and then stored at −20°C.
SDS-PAGE
SDS-PAGE was performed in a 10% (w/v) polyacrylamide slab gels using a Tris/Gly buffer system according to Laemmli’s method[Citation18]. The gels were stained with CBB Stain One (Nakalai Tesque, Kyoto, Japan).
Enzyme assay
To evaluate easily and efficiently GTF activity, we measured glucose production from sucrose (sucrase activity) during a GTF reaction. Sucrase activity of GTF was assayed at 37°C for 1 h in a 100-µL reaction mixture that contained 50 mM potassium phosphate buffer (pH 6.6), 50 mM sucrose, and the enzyme. A reaction was initiated by adding sucrose and stopped by boiling. Glucose formation was determined with a Glucose CII-test Wako Kit (Wako Pure Chemical, Osaka, Japan), according to the manufacturer’s instructions. Absorbance was measured at 505 nm using a microtiter plate reader (Sunrise Rainbow RC-R, TECAN, Männedorf, Switzerland). One unit of enzyme activity is defined as the amount of enzyme that produces 1 µmol of glucose per min.
GTF-inhibitory activity was assayed at 37°C for 1 h in a 100-µL reaction mixture that contained 50 mM potassium phosphate buffer (pH 6.6), 50 mM sucrose, 10 mU of GTF, and herbal tea extract. GTF-inhibitory activity was calculated as follows: GTF-inhibitory activity (%) = [1 – (produced glucose level by GTF reaction in the presence of herbal tea extracts in the buffer – produced glucose level by GTF reaction in the presence of herbal tea extracts in the sucrose free-buffer)/produced glucose level by GTF reaction in the absence of herbal tea extracts in the buffer] × 100. For the lemon myrtle extract with the highest GTF-inhibitory activity, inhibition curves (concentration versus activity; concentrations ranged from 0.05 mg mL−1 to 0.625 mg mL−1) were plotted, and 50% inhibitory concentrations (IC50; the concentrations of lemon myrtle extract required to inhibit enzymatic activity by 50%) were calculated from regression lines.
Determination of polyphenol contents
Polyphenol contents in the herbal tea extracts were determined with use of Folin-Ciocalteu reagent by the method of Slinkard and Singleton[Citation19] with some modifications. Results were expressed as mg of ferulic acid equivalent per g of herbal tea extract. Briefly, 2 µL of approximately diluted samples and a standard solution of ferulic acid were added to microtiter plate wells (96-well, flat-bottom, Fukaekasei, Kobe, Japan) containing 160 µL of distilled water and 8 µL of ethanol. A reagent blank of distilled water was prepared. Folin-Ciocalteu reagent (10 µL) was added to the mixture and shaken. After 5 min, 20 µL of a 10% Na2CO3 solution was added with mixing and then allowed to stand for 1 h. The absorbance was measured at 760 nm using the microtiter plate reader (Sunrise Rainbow RC-R).
Adherence assay for quantifying biofilm formation
An adhesion assay was performed as described by Matsunaga et al [Citation20]. with some modifications. This assay was based on measuring the degree of cellular attachment due to biofilm formation on the surface of the microtiter plate well. A pre-cultured S. mutans suspension (20 µL) was pipetted into microtiter plate wells (96-well, flat-bottom, catalog # 1–1601-05, As one, Osaka, Japan) that contained 70 µL of brain heart infusion broth and 10 µL of herbal tea extracts, and statically cultured at 37°C for 24 h. The plates were then gently washed twice with distilled water, and any adherent bacterial cells were stained with 1% (w/v) crystal violet. The stain was then dissolved in ethanol, and optical density was measured at 595 nm using the microtiter plate reader (Sunrise Rainbow RC-R). Relative biofilm formation (%) at a given herbal tea extract concentration as compared with the amount produced in the absence of herbal tea extract was determined.
Statistical analyses
Results are given as mean ± standard deviations (SD). Statistical analyses were performed using Microsoft Excel software (Redmond, WA, USA).
Results and discussion
Construction of a screening system for GTF-inhibitory compounds
The crystal structures of S. mutans GTF-SI and Lactobacillus reuteri GTF180, a homolog of S. mutans GTF-SI, have been determined [Citation21–Citation23]. These GTFs comprised five separate domains: A, B, C, IV, and V. Domains A, B, and C are reportedly catalytic domains, domain V is a glucan-binding domain, and domain IV appears to be a “hinge” between domains B and V.
We found that only small amounts of the full-length recombinant GTF-SI protein could be obtained in our expression system (data not shown). Since domains IV and V of this protein are not essential for enzymatic activity[Citation24], we produced a domain V-truncated GTF-SI protein (GTF-SI ∆V) in E. coli cells and purified it by nickel-affinity chromatography. This recombinant protein was produced at high efficiency (Figure. S1). A protein band corresponding to the recombinant GTF-SI ∆V, which correlated with the molecular mass (103.7 kDa, respectively) calculated from the deduced amino acid sequence of cloned GTF-SI and the His-tag sequence in the pET24b vector, were observed. The purified protein exhibited sucrase activity (2.66 ± 0.08 µmol min−1 mg−1 protein).
We used a pseudotetrasaccharide acarbose (a potent inhibitor of GTF-SI), to validate our GTF inhibitor screen. shows that enzyme activity was indeed inhibited with increasing concentrations of acarbose.
Table 1. Effect of acarbose on GTF activity.
Effect of herbal tea extracts on GTF activity
Using this system, we measured the GTF-inhibitory activity of 44 kinds of herbal tea extracts. shows the GTF-inhibitory activity (%) achieved with increasing concentrations of herbal tea extracts. Unexpectedly, some herbal extracts showed the GTF-inhibitory activity more than 100%, indicating that the produced glucose by GTF reaction in the presence of herbal tea extract was less than that contained in herbal tea extract. Although the produced glucose from sucrose may be polymerized to glucan by GTF, the detail is largely unknown. This finding suggests that it is necessary to examine the GTF-inhibitory activity at various concentrations of test sample.
Table 2. Effect of herbal tea extracts on GTF activity.
Lemon myrtle extracts showed the highest GTF-inhibitory activity (124.9 ± 0.5% at 5.00 mg mL−1), followed by wild strawberry, guava, spearmint and linden extracts (122.0 ± 1.4%, 111.8 ± 1.3%, 109.6 ± 0.9%, and 108.3 ± 1.3%, respectively, at 5.00 mg mL−1).
GTF-inhibitory activity of some herbal tea extract had reached a plateau at 2.50 mg mL−1. The linear relationship between the GTF-inhibitory activity and the concentration of herbal tea extracts was not observed, suggesting the possibility that a variety of compounds contained in the herbal tea extracts is to inhibit GTF activity by various inhibition mechanisms. Described above, many researchers have reported that polyphenols inhibit GTF activity of S. mutans [Citation6–Citation8,Citation10,Citation11]. Therefore, we measured polyphenol contents of herbal tea extracts (). Lemon myrtle extracts had the third-highest polyphenol content of all extracts tested. Polyphenol contents in the top five GTF inhibitory extracts (lemon myrtle, wild strawberry, guava, spearmint, and linden) were 4.44 ± 0.34 mg g−1, 2.30 ± 0.14 mg g−1, 2.49 ± 0.04 mg g−1, 2.47 ± 0.04 mg g−1, and 1.69 ± 0.09 mg g−1, respectively.
Table 3. Polyphenol contents of herbal tea extracts.
Lemon myrtle extract inhibits S. mutans biofilm formation, but not cell growth
Next, we examined whether lemon myrtle, wild strawberry, guava, spearmint, and linden extracts inhibited S. mutans biofilm formation. While lemon myrtle, guava, and linden extracts inhibited biofilm formation (), we unexpectedly found that wild strawberry and spearmint actually promoted biofilm formation.
Table 4. Effect of herbal tea extracts on S. mutans biofilm formation.
The lemon myrtle polyphenolic-rich fraction is known to inhibit the activities of α-glucosidase, lipase, and angiotensin converting enzyme, which are key enzymes in the pathogenesis of metabolic syndrome[Citation25]. It has been reported that the lemon myrtle extract showed relatively higher total ORAC activity (3359.9 µmol trolox equivalents/g DW)[Citation26]. Here, we show that this extract is also a potent inhibitor of S. mutans GTF activity and biofilm formation (IC50 value for GTF was 0.14 mg mL−1).
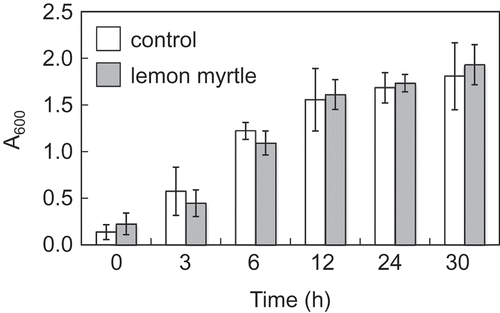
In addition to inhibition of GTF activity and biofilm formation, green tea polyphenols are reported to impede the growth of S. mutans[Citation8]. In contrast, we found that lemon myrtle extract had no significant effect on the growth of this bacterium ().
Figure 1. Effect of herbal tea extracts on S. mutans growth.
S. mutans was grown in 50 mL of brain heart infusion broth supplemented with 255 mg herbal tea extracts (final concentration was 5 mg mL−1) at 37°C for 30 h. The growth of S. mutans was quantified by measuring the optical density at 600 nm. All data are expressed as the mean ± SD (n = 6).
The most abundant polyphenolic compound in lemon myrtle is ellagic acid; this is followed by quercetin, hesperetin, and myricetin[Citation25], suggesting that these polyphenols contribute to inhibition of GTF activity. Therefore, we measured the GTF-inhibitory activity of these polyphenols. Ellagic acid, quercetin, and myricetin clearly inhibited GTF activity, while hesperetin did not inhibit it ().
Table 5. Effect of ellagic acid, quercetin, hesperetin, and myricetin on GTF activity.
Ellagic acid showed anti-bacterial activity when added to cultures of Staphylococcus aureus, Bacillus subtilis, E. coli, Micrococcus luteus, and Staphylococcus epidermidis[Citation13]. Although lemon myrtle water extract has been reported to exhibit anti-microbial activity against S. aureus[Citation12], we did not observe it against S. mutans when concentrations up to 5 mg mL−1 of the extract were tested. We infer that higher concentrations of lemon myrtle extracts may be required to inhibit growth of this bacterium.
Xu et al [Citation27]. reported that epigallocatechin gallate (the major polyphenol in green tea) inhibited S. mutans biofilm formation via suppressing the transcription of GTF-encoding genes. Although our data suggests that lemon myrtle extract inhibits S. mutans biofilm formation via inhibition of GTF activity, we did not examine GTF expression in the present study. Thus, the precise mechanism(s) by which this extract blocks GTF activity require further elucidation.
Furthermore, epigallocatechin gallate inhibited the growth, acid production, and acid tolerance of S. muntans, and suppressed the transcript level of atpD, aguD, idh and eno[Citation27]. The genes, atpD and aguD, encode a part of the F1F0-ATPase and of the agmatine deiminase system, respectively. The genes, idh and eno, encode lactose dehydrogenase and enolase, respectively. These proteins involved in the virulence of S. muntans, suggesting that not only the inhibition of GTF activity but also those of other virulence factors is important to prevent dental caries. Although wild strawberry and spearmint extracts strongly inhibited GTF activity (), those did not inhibit the biofilm formation of S. mutans (). We infer that these extracts contain compounds that promote biosynthetic pathways leading to biofilm formation, or that stimulate S. mutans cellular growth. Alternatively, it seems likely that these extracts did not inhibit the other virulence factors of S. mutans.
It has been reported that several compounds which were not polyphenols inhibited the GTF activity, growth, and biofilm formation of S. mutans. tt-farnesol which is one of the sesquiterpene isolated from propolis inhibited the GTF activity and growth of S. mutans[Citation28]. Barley coffee melanoidin inhibited the biofilm formation of S. mutans[Citation29]. Naphthalene derivative [(4aS, 5R, 8aS) 5, 8a-di-1-propyl-octa-hydronaphthalen-1-(2H)-one] isolated from Trachyspermum ammi (Ajowan caraway) seeds inhibited the anti-biofilm and anti-adherence activity against S. mutans[Citation30]. It seems likely that the inhibition of GTF activity and biofilm formation of S. mutans of lemon myrtle, guava, and linden extracts involved in polyphenols and/or such compounds. Further experiments are needed to identify the compounds.
In conclusion, the results of presented study indicate that lemon myrtle extract is likely to prevent dental caries, due to its potent inhibition of S. mutans GTF activity and biofilm formation. We suggest that lemon myrtle extracts should be added to toothpaste, mouth wash, non-sugar gums, and candy, since it may block the adhesion of S. mutans to the tooth surface, thereby preventing dental caries and the onset of tooth decay.
Author contributions
Y.K., and N,K. developed a screening system for GTF-inhibitory activities. H.M., and Y.Y. analyzed GTF-inhibitory activities of herbal tea extracts. T.B., T.I., and A.I. contributed to the design of the project and discussed the results. Y.Y., A.I., and F.W. designed the experiments, interpreted the results, and wrote the manuscript. All authors commented on the manuscript and have approved the final version.
Supplemental Material
Download PDF (1.6 MB)Supplemental Material
Download PDF (77.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data can be accessed here.
References
- Fujiwara T, Terao Y, Hoshino T, et al. Molecular analyses of glcosyltransferase genes among strains of Streptococcus mutans. FEMS Microbiol Lett. 1998;161:331–336.
- Tamesada M, Kawabata S, Fujiwara T, et al. Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J Dent Res. 2004;83:874–879.
- Xie Z, Okinaga T, Niu G, et al. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol Microbiol. 2010;78:1431–1447.
- Komatsu H, Abe Y, Eguchi K, et al. Kinetics of dextran-independent α-(1→3)-glucan synthesis by Streptococcus sobrinus glucosyltransferase I. FEBS J. 2011;278:531–540.
- Binder TP, Côté GL, Robyt JF. Disproportionation reactions catalyzed by Leuconostoc and Streptococcus glucansucrases. Carbohydr Res. 1983;124:275–286.
- Otake S, Makimura M, Kuroki T, et al. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991;25:438–443.
- Nakahara K, Kawabata S, Ono H, et al. Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans Streptococci. Appl Environ Microbiol. 1993;59:968–973.
- Sakanaka S, Kim M, Taniguchi M, et al. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 1989;53:2307–2311.
- Signoretto C, Canepari P, Stauder M, et al. Functional foods and strategies contrasting bacterial adhesion. Curr Opin Biotechnol. 2012;3:160–167.
- Tagashira M, Uchiyama K, Yoshimura T, et al. Inhibition by hop bract polyphenols of cellular adherence and water-insoluble glucan synthesis of mutans streptococci. Biosci Biotechnol Biochem. 1997;61:332–335.
- Yanagida A, Kanda T, Tanabe M, et al. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J Agric Food Chem. 2000;11:5666–5671.
- Dupont S, Caffin N, Bhandari B, et al. In vitro antibacterial activity of Australian native herb extracts against food related bacteria. Food Control. 2006;17:929–932.
- Thiem B, Goślińska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75:93–95.
- Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1 Antimicrob Act Vitro Cytotoxicity Food Chem Toxicol. 2002;40:535–543.
- Wilkinson JM, Hipwell M, Ryan T, et al. Bioactivity of Backhousia citriodora: antibacterial and antifungal activity. J Agric Food Chem. 2003;51:76–81.
- Kwok BH, Koh B, Ndubuisi MI, et al. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766.
- Tan W, Lu J, Huang M, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6. DOI:10.1186/1749-8546-6-27
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enoll Vitic. 1977;28:49–55.
- Matsunaga T, Nakahara A, Minnatul KM, et al. The inhibitory effects of catechins on biofilm formation by the periodontopathogenic bacterium, Eikenella corrodens. Biosci Biotechnol Biochem. 2010;74:2445–2450.
- Ito K, Ito S, Shimamura T, et al. Crystallization and preliminary X-ray analysis of a glucansucrase from the dental caries pathogen Streptococcus mutans. Acta Cryst. 2010;F66:1086–1088.
- Ito K, Ito S, Shimamura T, et al. Crystal structure of glucansucrase from the dental caries pathogen Streptococcus mutans. Mol Biol. 2011;408:177–186.
- VujičIć-Žagar A, Pijning T, Kralj S, et al. Crystal structure of a 117 kDa glucansucrase fragment provides insight into evolution and product specificity of GH70 enzymes. Proc Natl Acad Sci U S A. 2010;107:21406–21411.
- Kralj S, Van Geel-Schutten GH, van der Maarel MJ, et al. Biochemical and molecular characterization of Lactobacillus reuteri 121 reuteransucrase. Microbiology. 2004;150:2099–2112.
- Sakulnarmrata K, Konczaka I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012;134:1011–1019.
- Konczak I, Zabaras D, Dunstan M, et al. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010;122:260–266.
- Xu X, Zhou XD, Wu CD. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob Agents Chemother. 2011;55:1229–1236.
- Koo H, Rosalen PL, Cury JA, et al. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–1309.
- Stauder M, Papetti A, Daglia M, et al. Inhibitory activity by barley coffee components towards Streptococcus mutans biofilm. Curr Microbiol. 2010;61:417–421.
- Khan R, Zakir M, Khanam Z, et al. Novel compound from Trachyspermum ammi (Ajowan caraway) seeds with antibiofilm and antiadherence activities against Streptococcus mutans: a potential chemotherapeutic agent against dental caries. J Appl Microbiol. 2010;109:2151–2159.
