ABSTRACT
Rhodococcus erythropolis N9T-4 grows on an inorganic solid-state medium with no additional carbon and energy sources; however, it is unable to grow well in a liquid culture medium under the oligotrophic conditions. We examined submerged cultivations of N9T-4 using a polyurethane foam sponge to achieve approximately 10 times of the oligotrophic growth of the bacterium in the liquid culture medium.
Oligotrophs are microorganisms that grow under low-nutrient conditions. Kuznetsov defined a carbon source concentration of less than 10 mg/L as the requirement for isolation of oligotrophs; however, this is not a complete definition of the conditions required for the growth of these microorganisms [Citation1]. Like other extremophiles, oligotrophs are categorized into two groups: obligate and facultative oligotrophs. We proposed that oligotrophs could provide low-energy production systems for useful compounds and also be useful for in situ bioremediation. We have attempted to isolate oligotrophic bacteria from various environments. Intriguingly, we observed that many oligotrophs can be isolated from different environment with an inorganic minimum medium, containing no carbon and energy sources [Citation2], and suggested that such oligotrophs play an important role in the terrestrial ecosystem.
Rhodococcus erythropolis N9T-4, isolated from crude oil in the oil stockpile, is highly oligotrophic and can survive in a completely inorganic basal minimum (BM) medium with no additional carbon source [Citation3,Citation4]. Since this bacterium cannot grow in CO2-limiting conditions, it is suggested that it utilizes CO2 as a carbon source for its oligotrophic growth. However, none of the genes encoding the key enzymes involved in known biological CO2 fixation systems were found in the N9T-4 genome. Furthermore, we do not have to add any of the energy sources purposely, such as light and hydrogen gas, required by autotrophic microorganisms for the oligotrophic cultivation of N9T-4, suggesting that N9T-4 has a novel carbon metabolism and CO2 fixation system. Although we have extensively conducted genetic and biochemical analyses, the CO2 fixation and energy acquisition systems in N9T-4 are still unknown. Recently, random mutagenesis of the N9T-4 genome revealed that glyoxylate shunt and gluconeogenesis are essential for the oligotrophic growth of N9T-4 [Citation5]. Furthermore, aldA, encoding an aliphatic aldehyde dehydrogenase, was highly expressed in N9T-4 and is essential for its oligotrophic growth [Citation4,Citation6]. From these results, we suggested that C2 metabolism is an important clue to understand the oligotrophy of N9T-4. It has also been elucidated that N9T-4 can utilize trace amounts of ammonia in the atmosphere as the nitrogen sources, and we suggest that this bacterium can also utilize aldehyde and sulfur compounds in the atmosphere [Citation5,Citation7].
Under oligotrophic conditions, N9T-4 grows well on a solid-state medium solidified with agar or silica gel, but it grows very poorly in a liquid culture medium, which makes analysis of the physiology of N9T-4 very difficult. In addition, we had to scrape and collect the colonies on many agar plates to obtain the bacterial cells. Recently, we found that N9T-4 accumulates carbohydrates such as trehalose and glycogen in the cells under oligotrophic conditions [Citation8]. Especially, the N9T-4 strain Δppk2, in which a gene encoding inorganic polyphosphate kinase 2 was deleted, accumulated a larger amount of trehalose in the cells at a concentration of 0.54 g/g dry cell weight, whereas no trehalose accumulation was observed in the cells grown on the rich medium. We have proposed that trehalose-fermenting Saccharomyces cerevisiae should be used to produce bioethanol from trehalose in N9T-4 cells [Citation9]; that is, N9T-4 cells should be used as a bacterial biomass. For that purpose, a large number of bacterial cells should be obtained. Therefore, an effective cultivation method to obtain a large number of cells would be useful, not only for elucidation of the carbon metabolism mechanisms in N9T-4, but also for the industrial application of N9T-4 cells. In this study, we examined a submerged cultivation method of N9T-4 with a supporting material. We suggest that a polyurethane foam sponge was available to sustain the growth of the N9T-4 cells; we evaluated the cells by comparing the enzymatic activities of the cells grown on the solid-state medium with those of the cells grown on the newly established culture medium.
R. erythropolis N9T-4 was obtained from the NBRC culture collection (reference no. 110906). The BM medium, which was used to represent oligotrophic conditions, contained 0.1% NaNO3, 0.1% K2HPO4, 0.1% KH2PO4, 0.05% MgSO4・7H2O, 0.01% CaCl2・2H2O, and 0.1% vitamin mixture [Citation4] in deionized water (pH 7.0). Purified agar (1.5%, Nacalai Tesque, Kyoto) was used to prepare the solid-state medium. To obtain the inoculum, N9T-4 was cultivated on BM agar plates at 30°C for 5 days, and collected and suspended with 0.85% KCl. The cell suspension was inoculated into the BM liquid medium at an OD660 of 0.002.
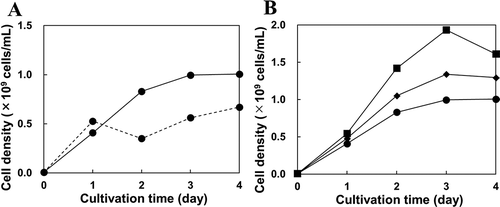
To examine the submerged cultivation of N9T-4, a commercial polyurethane foam sponge (6 × 6 cm-square) was placed at the bottom of a 500-mL beaker and soaked with 30 mL of the BM liquid medium inoculated with N9T-4 cells (Figure S1(a)). Cultivation was carried out at 30°C for several days under static conditions. As shown in (), the cell growth on the sponge with a height of 1.0 cm was better than that on the sponge with a height of 2.5 cm after cultivation for 4 days. Next, the culture volume was examined with the sponge with a height of 1.0 cm. We found that smaller culture volumes were more effective for the oligotrophic growth of N9T-4 cells, and growth was optimal in the 12 mL culture volume ()). Under optimum conditions (sponge size, 6 × 6 × 1.0 cm-rectangular parallelepiped; culture volume, 12 mL), the cell growth of N9T-4 in the BM liquid culture medium with a sponge and that in the BM liquid culture medium without a sponge were compared after 3 days of cultivation. We found that N9T-4 cell growth in the BM liquid culture medium with a sponge was approximately 2.5 times higher than that in the BM liquid culture medium without a sponge (Figure S2). The maximum culture volume retained on the sponge (6 × 6 × 1.0 cm-rectangular parallelepiped) was 12 mL, and the volume in excess of 12 mL exuded from the culture medium through the sponge. These results suggested that N9T-4 cells grew on the sponge, and contact with air was important for oligotrophic growth. As described above, it is thought that N9T-4 utilizes carbon, nitrogen, and sulfur sources in the atmosphere. Thus, the oligotrophy of N9T-4 could be closely associated with air, which is consistent with the results of this study and the difficulty of cultivation in a liquid medium.
Figure 1. Oligotrophic growth of N9T-4 in the submerged cultivation. (a) the cell growth of N9T-4 grown on 6 × 6 cm-square sponges with a height of 1.0 cm (solid line) and 2.5 cm (dotted line). The sponges were soaked with 30 mL of the BM liquid medium inoculated with N9T-4 cells (OD660 = 0.002) in a 500-mL beaker and cultivated at 30°C under static conditions. (b) 6 × 6 × 1.0 cm-rectangular parallelepiped sponges were soaked with 12 mL (square symbols), 20 mL (diamond symbols), and 30 mL (circle symbols) of the BM liquid medium inoculated with N9T-4 cells (OD660 = 0.002) and cultivated at 30°C under static conditions. For each experiment, 4 independent media were inoculated with different cell suspensions, and the sponge was withdrawn from one of the media every day, squeezed, and OD660 of the cell suspension was measured.
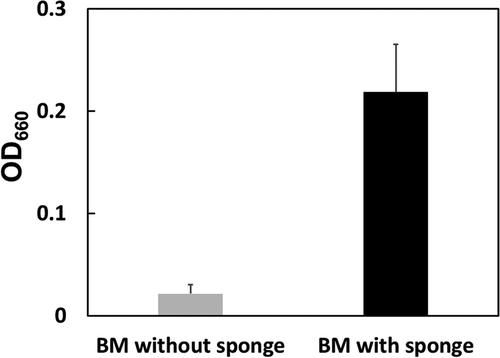
Next, we performed a scale-up test of the submerged cultivation with the sponge. A polyurethane sponge (20 × 30 × 1 cm-rectangular parallelepiped; density, 16–22 kg/m3; Softpren Industry Corp., Shizuoka) was set on a stainless tray (25 × 35 × 4 cm-rectangular parallelepiped) and soaked with 200 mL of the BM medium inoculated with N9T-4 cells (Figure S1(b)). After 5 days of cultivation at 30°C, the cell growth with and without sponge was compared. The results showed that the growth in the liquid culture medium with the sponge was enhanced approximately 10 times compared with that in the liquid culture medium without the sponge, whereas the OD660 of the large scale-cultivation decreased compared with that of the small-scale cultivation (). We also examined the concentration of each constituent in the BM medium, but no improvement in cell growth was observed (data not shown).
Figure 2. Scale-up of the submerged oligotrophic cultivation. N9T-4 cells (OD660 = 0.002) were inoculated into 200 mL of the BM liquid medium with or without the sponge (20 × 30 × 1.0 cm-rectangular parallelepiped) in a stainless tray. Cultivation was carried out at 30°C under static conditions. After cultivation, the sponge was squeezed and OD660 of the cell suspension was measured.
To evaluate the N9T-4 cells grown with the sponge, the activities of the key enzymes involved in the oligotrophic growth of N9T-4 were measured. N9T-4, grown in the submerged culture medium with a sponge and BM solid-state medium solidified with agar, was collected and washed three times with 0.85% KCl, and the OD660 was adjusted to 50. The cell suspension (500 µL) was disrupted with 0.1-mm zirconia beads using a beads beater (µT-01, TAITEC Corp., Saitama) at 4,200 rpm for 3 min. The disrupted cells were centrifuged at 4°C and 20,400 × g for 5 min, and the supernatant was used as a cell-free extract. Our previous proteomic analysis showed that an aliphatic aldehyde dehydrogenase (AldA) and methanol: N, N’-dimethyl-4-nitrosoaniline (NDMA) oxidoreductase (MnoA) were highly expressed under oligotrophic conditions. AldA activity was determined by the method previously described [Citation4]. It is known that MnoA has a remarkable formaldehyde dismutase activity, besides its methanol dehydrogenase activity with NDMA as an artificial cofactor [Citation10]. In this study, MnoA activity was measured as the formaldehyde dismutase activity as follows. The reaction mixture (100 µL) contained 10 mM potassium phosphate buffer (pH 7.0), 2 mM formaldehyde, and an appropriate amount of N9T-4 cell-free extract. The reaction was initiated by adding formaldehyde and further incubated for 30 min and 25°C. The concentration of formaldehyde in the reaction mixture was measured with the Formaldehyde-Test Wako (FUJI FILM Wako Pure Chemical Corp., Osaka). We found that the specific activities of the two enzymes in both cell-free extracts, prepared from the cells grown on the submerged culture medium with a sponge and the solid-state medium, were approximately equal in value (). These results confirmed that N9T-4 cells can be cultivated through the established method, with the same oligotrophic features as those we have examined.
Table 1. Oligotrophic enzyme activities in the cell-free extract of N9T-4 prepared from the cells grown on the submerged culture with sponge or the solid-state culture.
In this study, our established culture method remarkably improved the oligotrophic growth of N9T-4 in a liquid medium. This method also facilitated the collection of bacterial cells, and we could only obtain a high concentration of bacterial cells by squeezing the sponge. However, further investigations of the sponge material and the medium composition should be carried out to improve cell growth on the sponge.
Author Contribution
T.M. and N.Y. designed the experiments, discussed the results, and prepared manuscript. T.M. performed experiment.
Supplemental Material
Download PDF (37.2 KB)Supplemental Material
Download PDF (104.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kuznetsov S, Dubinina G, Lapteva N. Biology of oligotrophic bacteria. Annu Rev Microbiol. 1979;33:377–387.
- Yoshida N, Ohhata N, Yoshino Y, et al. Screening of carbon dioxide-requiring extreme oligotrophs from soil. Biosci Biotechnol Biochem. 2007;71:2830–2832.
- Yoshida N, Yagi K, Sato D, et al. Bacterial communities in petroleum oil in stockpiles. J Biosci Bioeng. 2005;99:143–149.
- Ohhata N, Yoshida N, Egami H, et al. An extremely oligotrophic bacterium, Rhodococcus erythropolis N9T-4, isolated from crude oil. J Bacteriol. 2007;189:6824–6831.
- Yano T, Yoshida N, Yu F, et al. The glyoxylate shunt is essential for CO2-requiring oligotrophic growth of Rhodococcus erythropolis N9T-4. Appl Microbiol Biotechnol. 2015;99:5627–5637.
- Yoshida N, Hayasaki T, Takagi H. Gene expression analysis of methylotrophic oxidoreductases involved in the oligotrophic growth of Rhodococcus erythropolis N9T-4. Biosci Biotechnol Biochem. 2011;75:123–127.
- Yoshida N, Inaba S, Takagi H. Utilization of atmospheric ammonia by an extremely oligotrophic bacterium, Rhodococcus erythropolis N9T-4. J Biosci Bioeng. 2014;117:28–32.
- Yano T, Funamizu Y, Yoshida N. Intracellular accumulation of trehalose and glycogen in an extreme oligotroph, Rhodococcus erythropolis N9T-4. Biosci Biotechnol Biochem. 2016;80:610–613.
- Zilli DM, Lopes RG, Alves SL Jr, et al. Secretion of the acid trehalase encoded by the CgATH1 gene allows trehalose fermentation by Candida glabrata. Microbiol Res. 2015;179:12–19.
- Bystrykh LV, Govorukhina NI, Van Ophem PW, et al. Formaldehyde dismutase activities in Gram-positive bacteria oxidizing methanol. J Gen Microbiol. 1993;139:1979–1985.
