ABSTRACT
Two variants of the sword bean (Canavalia gladiata), namely the white sword bean (WSB) and the red sword bean (RSB), are known. The MgCl2 concentration-dependent canavalin solubility showed different behavior among the extracts from distinct beans prepared by distinct pretreatments. Pretreatment and bean selection are important factors for use in food chemical and biochemical experiments.
Sword beans (Canavalia gladiata) have long been eaten in the Asian tropics and subtropics [Citation1,Citation2]. The yield ability of sword beans is comparable to that of soybeans, under optimal agricultural management conditions [Citation1]. Sword beans are relatively resistant to pests and diseases [Citation3]. From a nutritional perspective, the dried beans contain approximately 62% carbohydrate, 26% protein, and 3% fat [Citation4]. The agricultural and nutritional characteristics show the potential for utilizing sword beans in processed foods. Previously, we reported the physicochemical characteristics of sword bean proteins [Citation5,Citation6] and gelation of sword bean extract [Citation7], both of which are important for making processed foods. However, more scientific knowledge is essential to begin using sword beans to make processed foods.
Sword bean is classified into two variant species, namely the white sword bean (WSB; Canavalia gladiata var. alba MAKINO) and red sword bean (RSB; Canavalia gladiata var. gladiata). For the present study, dried WSBs and RSBs were purchased from Morika Kometen (Nara, Japan). RSBs were larger and heavier than WSBs (Supplemental Table 1). In our previous studies, dried WSBs were used for protein extraction after water absorption by soaking [Citation5,Citation6]. Dried WSBs absorbed sufficient water for extraction purposes after soaking in 10 volumes of distilled water for 18 h at 20°C [Citation5]. However, dried RSBs absorbed a little amount of distilled water, in contrast to dried WSBs. The water-absorbing property of RSBs is consistent with that reported by Une et al. [Citation8]. Despite the difference in color, size, weight, and water-absorbing property, most previous reports did not describe which beans were studied. When extracted substances from WSBs show different properties relative to the same substances extracted from RSBs, such a non-descriptive approach might impede a proper scientific understanding. The different capacities for water absorption require different pretreatments between WSBs and RSBs when preparing processed foods and cooking. The different pretreatments may have an influence on the extracted substances. A comparison between WSBs and RSBs is important for the scientific understanding of sword beans and the development of the processed foods from sword beans.
Previously, we reported that the solubility of canavalin, which is a major storage protein of sword bean with a molecular mass of 47.6 kDa [Citation9,Citation10] and is classified as a 7S seed globulin or legume vicilin [Citation11], is controlled by the MgCl2 concentration in the crude extract from WSBs [Citation6]. The properties of extracted proteins are useful indicators for comparing WSBs and RSBs. To extract proteins from beans in water, we prepared untreated, drilled, and milled beans. Drilled beans were prepared by drilling four diagonal points using a 1-mm-diameter drawing pin (clear push pin, Moritoku, Osaka, Japan) to a depth of approximately 1 mm. Untreated WSBs, drilled WSBs, and drilled RSBs were ground in eight volumes (v/w) of distilled water following soaking for 18 h in distilled water with a hand blender (CSB-77JBSTRW, Cuisinart, CT, USA) on ice for 5 min. Milled beans were prepared by grinding dried beans for 3 min with a grinder (Force Mill, Y-308B, Osaka Chemical Co., Ltd., Osaka, Japan), and then also ground in eight volumes (v/w) of distilled water in the same manner without soaking in advance. Suspensions were separated into extracts and residues by sieving through a cotton cloth. These yields were quantitatively estimated, as summarized in . The yield of extract from drilled beans was significantly larger than that from untreated WSBs and milled beans (). The collection rate was over 80% with either type of beans (). The lower yield appeared to be related to water absorption by the cotton cloth. In addition, the collection rate of drilled beans was higher than that of untreated and milled beans (). The finer ground particles of drilled beans might have resulted in the higher collection rate because it was comparatively easier for the fine particles to go through the cotton cloth.
Table 1. Comparison of extract weights, protein concentrations and quantities between beans prepared with different pretreatments.
The protein concentration of extracts was determined using the Bradford method with reagents from Bio-Rad Laboratories Inc. (CA, USA), using bovine serum albumin as a standard. The protein concentrations are summarized in . The protein concentration in untreated WSBs was higher than that in other WSBs and RSBs. In addition, the protein concentration in drilled beans was lower than that in milled beans. Protein quantities were also calculated by multiplying the protein concentration by the volume of the extract (). The quantity of extracted proteins in milled RSBs was lowest, and approximately half of that in untreated WSBs. When comparing WSBs and RSBs prepared using the same pretreatment, the quantity of proteins extracted from RSBs was lower than that of WSBs. However, the protein quantity in drilled WSBs was almost the same as that in untreated WSBs. These results show that proteins were extracted from untreated WSBs and drilled WSBs with similar efficiency and that the efficiency in milled beans was lower than that of untreated and drilled beans. In addition, the quantity of extracted protein from untreated WSBs was almost equivalent to that from drilled WSBs, but significantly higher than that from milled WSBs (p < 0.05). The composition of extracted proteins was analyzed by SDS-PAGE (Supplemental Figure 1). The protein patterns were nearly identical among the extracts from beans prepared by different pretreatments.
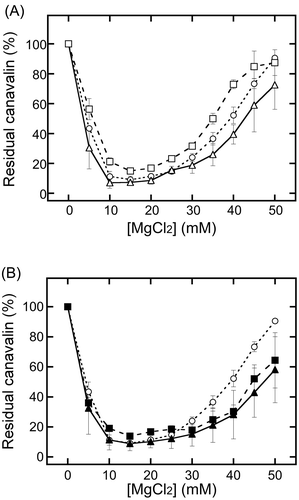
Canavalin solubility is controlled by the divalent cation concentration in a crude extract [Citation6]. To investigate the influence of different pretreatments, MgCl2 was added to each extract in the range of 0–50 mM. The mixtures were incubated at 25°C for 15 min, followed by incubation on ice for 5 min, and then divided into insoluble and soluble phases by centrifugation at 9,100 × g for 20 min at 4°C. To compare the effects of different pretreatments in detail, the intensity of residual canavalin bands was quantified from the results of SDS-PAGE analysis using Image J software (National Institutes of Health, Bethesda, MD) [Citation13] (). The soluble canavalin ratio was expressed as a percentage of the canavalin band intensity for soluble phases with MgCl2 relative to that for soluble phases incubated with distilled water. Compared with canavalin in extracts from untreated WSB, canavalin in extracts from milled WSBs showed a lower insolubilization ratio in the range of 5–45 mM MgCl2 (, open squares). In contrast, canavalin from drilled WSBs showed a higher insolubilization ratio at a higher MgCl2 concentration than that of untreated WSBs (, open triangles). In addition, canavalin from drilled RSBs was insolubilized by a higher MgCl2 concentration than canavalin from untreated WSBs (, closed triangles). Compared to canavalin from untreated WSB, milled RSBs showed a decreased insolubilization ratio at a lower MgCl2 concentration but an increased insolubilization ratio at a higher MgCl2 concentration (, closed squares). The different behaviors for the insolubilization indicate that the different bean pretreatments influenced canavalin solubility based on the MgCl2 concentration. Interestingly, the standard deviations for drilled and milled beans were larger than those for untreated WSBs. The difference in the standard deviation implies that extracts from drilled and milled beans reduce the repeatability of protein extraction compared to untreated WSB extracts.
Figure 1. Comparison of canavalin solubility changes.
The canavalin solubility in the extracts was analyzed by SDS-PAGE after addition of MgCl2 to WSB and RSB extracts. The proportion of residual canavalin in the supernatant was estimated from the band intensity using Image J software. The data for WSBs (A) and RSBs (B) were plotted. The open circles indicate data for untreated WSBs that were only treated by soaking. The open triangles indicate drilled WSBs. The open squares represent milled WSBs. The closed triangles indicate drilled RSBs. The closed squares represent milled RSBs. Data are expressed as the average ± standard deviation of three independent experiments.
To compare the insolubilization behavior between WSB and RSB, the residual canavalin was replotted for the drilled and milled beans (Supplemental Figure 2). With drilled beans, canavalin from WSBs showed almost same insolubilization ratio to that from RSBs, but showed a lower insolubilization ratio with a higher MgCl2 concentration than that from RSBs (Supplemental Figure 2A). In addition, in milled beans, compared to canavalin from RSBs, that from WSBs showed a lower insolubilization ratio with 5 mM MgCl2 and a lower insolubilization ratio with >25 mM MgCl2 (Supplemental Figure 2B). These results indicate that the insolubilization of canavalin from WSBs and RSBs was controlled by the MgCl2 concentration in different manners. Because the mechanism by which MgCl2 concentration controls canavalin solubility is unclear, it is difficult to discuss the differences observed among the different beans. Changes in protein structure may affect canavalin solubility at high MgCl2 concentrations. In addition, canavalin coexists with various bean substances in the crude extract. Inhibition of structural changes may result in differing solubilization.
In conclusion, proteins were extracted with high efficiency from untreated and drilled beans. The protein concentration, protein quantity, and SDS-PAGE data indicated that the use of untreated WSBs was favorable for protein extraction. Canavalin solubility was controlled by the MgCl2 concentration similar to that in our previous report [Citation6]. However, the behavior was somewhat different among extracts prepared with different pretreatments. These results indicate that the method used to prepare beans influences the protein properties in a crude extract. The bean variety and pretreatment are also important factors for using beans for food chemical experiments, biochemical experiments, and food industrial applications.
Author contributions
YA conceived and designed the experiments. YA and KN performed the experiments. KN and YA analyzed the data and wrote the paper. YA reviewed and edited the manuscript. KN and YA read and approved the manuscript.
Supplemental Material
Download PDF (580.5 KB)Acknowledgments
We wish to thank Suzuka Nada and Wakana Minami for their technical assistance. We would like to thank Editage (www.editage.jp) for English language editing.
Disclosure statement
No potential conflict of interest is reported by the authors.
Supplementary materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bressani R, Brenes RG, García A, et al. Chemical composition, amino acid content and protein quality of Canavalia spp. seeds. J Sci Food Agric. 1987;40:17–23.
- Siddhuraju P, Becker K. Species/variety differences in biochemical composition and nutritional values of India tribal legumes of genus Canavalia. Nahrung. 2001;45:224–233.
- Smartt J. Tropical pulses: Canavalia gladiata (Jacq.) D.C. (Sword bean). London, UK: Longman Group, Ltd.; 1976.
- Vadivel V, Janardhanan K. Nutrition and antinutritional characteristics of seven south India wild legumes. Plant Foods Hum Nutr. 2005;60:69–75.
- Nishizawa K, Masuda T, Takenaka Y, et al Precipitation of sword bean proteins by heating and addition of magnesium chloride in a crude extract. Biosci Biotechnol Biochem. 2016;80:1623–1631.
- Nishizawa K, Arii Y. Reversible changes of canavalin solubility controlled by divalent cation concentration in crude sword bean extract. Biosci Biotechnol Biochem. 2016;80:2459–2466.
- Nishizawa K, Arii Y. A crude sword bean (Canavalia gladiata) extract is gelated by cooling. Biosci Biotechnol Biochem. 2018;82:120–126.
- Une S, Nonaka K, Akiyama J. Effect of hull scratching, soaking, and boiling on antinutrients in Japanese red sword bean (Canavalia gladiata). J Food Sci. 2016;81:C2398–C2404.
- Yamauchi D, Nakamura K, Asahi T, et al. cDNAs for canavalin and concanavalin A from Canavalia gladiata seeds. Nucleotide sequence of cDNA for canavalin and RNA blot analysis of canavalin and concanavalin A mRNAs in developing seeds. Eur J Biochem. 1988;170:515–520.
- Takei Y, Yamauchi D, Minamikawa T. Nucleotides sequence of the canavalin gene from Canavalia gladiata seeds. Nucl Acid Res. 1989;17:4381.
- Sumner JB, Gralën N, Eriksson-Quensel IB. The molecular weights urease, canavalin, concanavalin B. Science. 1938;87:395–396.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;1970(69):680–685.
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with image. J. Biophot Int. 2004;:36–42.
