ABSTRACT
The products from the thermal reaction of chlorogenic and caffeic acids, which is a model process of roasting coffee beans, exhibited xanthine oxidase (XO) inhibitory activity. From caffeic acid, six inhibitory phenylindanes were identified, and a new phenylindane displayed the highest inhibitory activity among them. The activity of these phenylindanes may contribute to XO inhibition-related functions of roasted coffee beverages.
Coffee beans are known to contain large amounts of chlorogenic acid and its isomers (3 ~ 8% of green beans) as polyphenol constituents, which exert various biological activities including antioxidant, hepatoprotective, and hypoglycemic activities [Citation1], and might be responsible for health promoting effects of coffee beverages [Citation2]. It should be noted that coffee beverages consumed by humans are made from roasted coffee beans and not from raw beans (green beans), which are dry seeds of the tropical Rubiaceae plant. The roasting of coffee beans consists of a high-temperature thermal treatment at around 200ºC. During roasting, the characteristic color, aroma, and taste of coffee are developed. This suggests that the constituents of green coffee beans are converted to other compounds under the thermal process. We previously found xanthine oxidase (XO) inhibitory activity, which may relate to the prevention of gout in coffee consumers by reducing uric acid in their plasma [Citation3], only in roasted coffee beans [Citation4] where several XO inhibitors were identified [Citation5,Citation6]. However, other non-polar inhibitors, which were suggested to exist in roasted coffee beans [Citation5], have not yet been identified because of the high complexity of the non-polar constituents of roasted coffee beans. Stadler and coworkers [Citation7] identified two phenylindanes from the thermal treatment of caffeic acid. Later, Frank and coworkers [Citation8] found that such phenylindanes existed in roasted coffee beans. These phenylindanes should be characteristic non-polar compounds of roasted coffee beans. Therefore, we attempted to isolate such phenylindane derivatives from the thermal reaction of coffee bean constituents and examine their XO inhibitory activity.
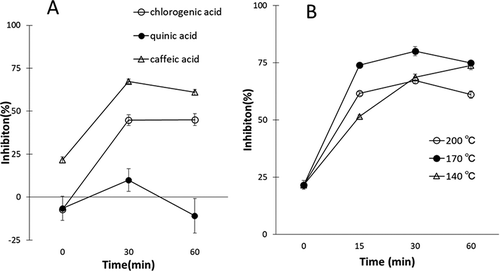
In a screw-capped test tube (i.d. 8 mm, L. 100 mm) were placed 10 mg of chlorogenic acid (Carbosynth, Compton, UK), caffeic acid (Kanto Chemicals, Tokyo, Japan), or quinic acid (MilliporeSigma, St. Louis, USA) with methanol (200 µL) and 400 µL of phosphate buffer (500 mmol/L, pH 6.0, from Na2HPO4 and KH2PO4). After removing the solvent in vacuo, the tube was heated in a metal block bath. After cooling the tube, methanol (1 mL) was added and the mixture was centrifuged at 2000 rpm for 5 min at 25ºC to give a supernatant. After evaporation of the solvent from the supernatant, the XO inhibitory activity was measured using a previously reported method [Citation4]. ) shows the XO inhibitory activity of the products from the thermal reaction at 200ºC of chlorogenic acid, caffeic acid, and quinic acid. The product mixture obtained from heating chlorogenic acid expressed XO inhibitory activity at the concentration of 0.3 mg/mL, whereas that obtained from quinic acid, whose structure is contained in chlorogenic acid, did not show significant XO inhibitory activity for 1 h of reaction time (X-axis of )) under employed conditions. Although caffeic acid, which is also a structure contained in chlorogenic acid, displayed weak XO inhibitory activity, the product mixture obtained from its thermal treatment exhibited enhanced inhibitory activity and the activity was stronger than that of the product mixture of chlorogenic acid ()). These results indicate that the thermal reaction of caffeic acid (140ºC ~) produces efficient XO inhibitors, which are expected to contain phenylindanes according to Stadler [Citation8]. ) shows the XO inhibitory activity of the products obtained from thermal reaction of caffeic acid at three different temperatures (reaction time is expressed in X-axis). The 170ºC reaction showed maximal XO inhibition efficiency at short time within 30 min, and then the activity gradually decreased. In contrast, the 140ºC reaction increased XO inhibition continuously for 1 h until almost the same maximal activity. Therefore, the temperature of 140ºC was chosen for the large-scale reaction because it was easy to monitor the reaction progress by HPLC analysis.
Figure 1. Panel A, XO inhibitory activity of thermal reaction products (0.3 mg/mL) from chlorogenic acid, caffeic acid and quinic acid at 200ºC. Scale of X-axis expresses reaction time. Data are expressed at the mean ± SD (n = 3). Panel B, XO inhibitory activity of thermal reaction product (0.3 mg/mL) from caffeic acid at the different temperatures. Scale of X-axis expresses reaction time. Data are expressed at the mean ± SD (n = 3).
Thus, a large-scale caffeic acid-phosphate buffer salt mixture was prepared as described [caffeic acid (10 g) was dissolved in 100 mL of methanol and 400 mL of 500 mmol/L Na2HPO4-KH2PO4 (pH 6.0) and then evaporated to dryness]. The solid mixture was heated in a stainless reactor (i.d. 14 cm; h. 15 cm) under a N2 atmosphere at 140ºC for 90 min. After cooling, the reaction mixture was extracted twice with 1L of methanol. This procedure was repeated ten times (in total 100 g of caffeic acid were treated). After removal of the methanol from the extract, the residue was used for the isolation of the products. Part of the residue (84 g) was subjected to Amberlite XAD-7 column chromatography eluted with increasing percent of methanol (50% to 100%) in water, which produced 8 separate fractions. Fraction 3 (208 mg out of 6.5 g), which was eluted with 60% methanol in water, was purified by preparative HPLC under the following conditions [column, Cosmosil 5C18-AR-II (250 × 20 mm i.d.); solvent, 1% acetic acid in H2O–CH3CN = 85:15; flow rate, 9.6 mL/min; detection, 280 nm]. Products 1 (3 mg), 2 (3 mg), 3 (2 mg), and 4 (6 mg) were isolated from the peaks at retention times: 34 min, 39 min, 24 min, and 28 min, respectively. Products 5 (87 mg) and 6 (134 mg) were isolated from fraction 6 (an eluted fraction with 75% methanol in water) using Sephadex LH-20 column chromatography and subsequent HPLC purification [column, Cosmosil 5C18-AR-II (250 × 20 mm i.d.); solvent, 1% acetic acid in H2O–CH3CN = 75:25; flow rate, 9.6 mL/min; detection, 280 nm; collected peaks, retention time 43 min (product 5) and 47 min (product 6)].
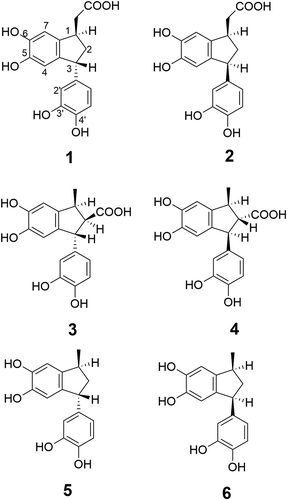
Product 5 showed a molecular-related ion peak at m/z 295 in the ESI-MS. The 1H NMR of 5 showed two sets of aromatic protons, one at 6.48 (dd, J = 7.8 and 1.8 Hz), 6.53 (d, J = 1.8 Hz), and 6.69 (d, J = 7.8 Hz) ppm, and another one at 6.68 (brs) and 6.42 (brs) ppm, indicating the presence of a tri-substituted and a tetra-substituted benzene rings. Geminal coupled protons were observed at 2.09 and 2.19 ppm, which were both coupled with the protons at 4.16 and 3.22 ppm. The proton at 3.22 ppm was also coupled with the methyl protons at 1.23 ppm. These data indicated that 5 is a phenyl-substituted indane derivative. From the comparison of the 1H NMR analytical data (chemical shifts and coupling constants), we concluded that 5 is the 1,3-trans isomers of Stadler’s phenylindanes [Citation7]. Product 6 showed the same molecular-related ion at m/z 295 and very similar 1H NMR data to those of 5. Typical differences were observed in the chemical shifts and coupling constants of the protons at 1-, 2-, and 3-positions of the indane structure, which indicate that 6 is the cis isomer of 5 [Citation7] as shown in .
Products 3 and 4 showed similar 1H NMR data to those of 5 and 6. The comparison of the 1H NMR spectra of 3 and 4 revealed that one proton signal at corresponding to the 2-methylene was lacking and another proton signal was shifted to higher frequency (3.29 ppm) in the spectrum of 3. The negative ESI-MS showed a molecular-related ion at m/z 271.0986, which indicated that 3 had the molecular formula C17H16O6. These data suggested the presence of a carboxylic acid group at the 2-positon. The m/z value of 653.1634 observed in the ESI-MS was assigned to a characteristic carboxylic acid cluster ion [2M‒2H+Na]−. The relative stereochemistry of the three substituted non-aromatic carbons of the indane was determined to be relative 1S, 2R, 3S according to an NOE observed from 1-methyl protons to the proton at the 3-proton and a very strong NOE observed from 2-H to the proton at the 2-position of 3-phenyl group in the NOE differential spectra of 3. Although product 4 shows a similar 1H NMR spectrum of 3, some differences are observed in the chemical shifts and coupling constants of protons at 1-, 2-, and 3-positons. Moreover, the observed NOEs from 1-methyl protons to the protons at 2- and 2′-positions suggested that relative stereochemistry is 1S, 2S, 3R. The structures of 3 and 4 are shown in . The planar structure of 3 and 4 was already reported as a forming aid obtained from coffee in a US patent by Martine and coworkers [Citation9].
The ESI-MS data showed peaks at m/z 315.0897 (C17H15O6 [M‒H]−) and 653.1628 (C34H30O12Na [2M‒2H+ Na]−) for product 1, and 315.0902 (C17H15O6 [M‒H]−) and 653.1629 (C34H30O12Na [2M‒2H+ Na]−) for product 2. Moreover, similar 1H NMR data for both compounds indicated that they were stereoisomers of each other. The 1H NMR of 2 revealed the presence of a 1,3,4-tri-substituted benzene and a 1,3,4,6-tetra-substituted benzene similar to other isolated products. A proton network (CH-CH2-CH-CH2), which was identified from the COSY, suggested a two-substituted indane structure similar to that of 5 and 6. The chemical shift of a terminal proton at 4.03 ppm was assigned to a methine proton signal between two benzene rings, while the signals at 2.37 and 2.83 ppm, with coupling constants characteristic germinal protons, were assigned to protons adjacent to a carboxylic acid. The assignments were confirmed by the HMBC correlation between the methylene protons and a carbonyl carbon at 179 ppm (this carbon chemical shift was obtained from the F1-projection of the HMBC spectrum). Taking into consideration the above data, structure 2 was assigned as a newly identified compound: 1-hydroxycarbonylmethyl-3-(3,4-dihydroxy)phenyl-5,6-dihydroxyindane. The relative stereochemistry of the acetic acid group at the 1-position and the dihydroxylphenyl group at the 3-position was determined to be cis (structure 2 in ) from the NOESY of 2 (one NOE correlation between 1-CH2 and 2′-H, and other between 1-H and 3-H). The 1H NMR spectral data of 1 indicated that 1 is a stereoisomer of 2 concerning the 1- and 3-positions of the indane scaffold, which was deduced from a clear NOE correlation observed between the 1-methylene protons and the proton at 3-position. Thus, 1 was identified as a new compound with trans stereochemistry of the 1-hydroxycarbonylmethyl and 3-dihydroxyphenyl groups as shown in .
The XO inhibitory activity of the isolated phenylindanes (concentration: 200 μmol/L) was measured by a previously reported procedure [Citation4], which is based on the quantitative HPLC analysis of produced uric acid, the data are summarized in . While caffeic acid showed almost no activity at the measured concentration, isolated phenylindanes exerted stronger activity than caffeic acid. Especially newly identified phenylindane 1 had the most potent activity (62% inhibition at 200 μmol/L) among them. Pyrogallol (IC50 0.73 μmol/L) and chlorogenic acid 1,5-lactones (IC50 210 ~ 360 μmol/L) isolated from roasted coffee beans have been identified as XO inhibitors. Although the phenylindanes identified in this work have moderate XO inhibitory activity comparing with potently active pyrogallol, they are non-polar inhibitors produced from caffeic acid, which may play a role in the XO inhibitory activity exerted by roasted coffee.
Table 1. XO inhibitory activity of identified phenylindanes (200 μmol/L) from thermal reaction product of caffeic acid.
Author Contribution
T. Masuda designed the research. Y. Fukuyama performed the experiments. K. Hidaka and A. Masuda analyzed the data and supported manuscript preparation. T. Masuda and Y. Fukuyama prepared the manuscript mainly.
Suppl.pdf
Download PDF (92.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Farah A, Donangelo CM. Phenolic compounds in coffee. Braz J Plant Physiol. 2006;18:23–36.
- Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123.
- Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum. 2007;56:2049–2055.
- Honda S, Miura Y, Masuda A, et al Identification of crypto- and neochlorogenic lactones as potent xanthine oxidase inhibitors in roasted coffee beans. Biosci Biotechnol Biochem. 2014;78:2110–2116.
- Honda S, Masuda T. Identification of pyrogallol in the ethyl acetate-soluble part of coffee as the main contributor to its xanthine oxidase inhibitory activity. J Agric Food Chem. 2016;64:7743–7749.
- Honda S, Fukuyama Y, Nishiwaki H, et al Conversion to purpurogallin, a key step in the mechanism of the potent xanthine oxidase inhibitory activity of pyrogallol. Free Radic Biol Med. 2017;106:228–235.
- Stadler RH, Welti DH, Stämpli AA, et al Thermal decomposition of caffeic acid in model systems: identification of novel tetraoxygenated phenylindan isomers and their stability in aqueous solution. J Agric Food Chem. 1996;4:898–905.
- Frank O, Blumberg S, Kunert C, et al Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J Agric Food Chem. 2007;55:1945–1954.
- Martine V, Leloup J, More F, et al Process of preparing a foaming aid and uses thereof. United States patent US 20150320071A1. 2015 Dec 10.