ABSTRACT
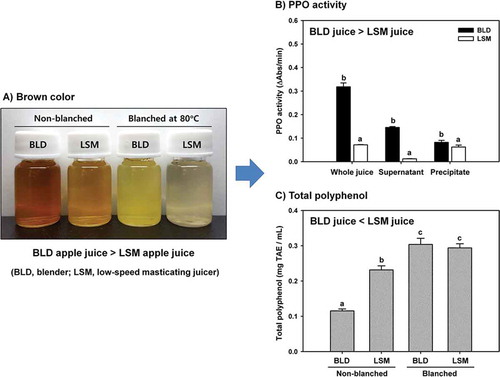
The aim of this study was to investigate the effect of juicer type (blender or LSM household juicer) on the browning reaction of apple juice and evaluate the remaining antioxidant activity in the juice. The blender apple juice showed a darker brown color and 4.5 times higher PPO activity than LSM apple juice. This result suggested that the blender caused severer damage to plastids in cells leading to leakage of PPO into the juice than the LSM juicer. The total polyphenol and flavonoid content of LSM apple juice was approximately 2 times higher than that of blender apple juice because polyphenols and flavonoids can be used as substrates by PPO. The antioxidant activity of LSM juice was higher than that of blender juice. Together, these results suggested that the LSM juicer is superior to the blender for preparation of fresh apple juices due to the minimization of enzymatic oxidation reactions.
Abbreviations: LSM: low-speed masticating; PPO: polyphenol oxidase; ABTS: 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); DPPH: 2,2-diphenyl-1-picrylhydrazyl
GRAPHICAL ABSTRACT
Graphical abstract: Low-speed masticating household juicer (LSM) minimized the enzymatic oxidation reactions in fresh apple juice compared with blender (BLD).
Recently, consumer health-oriented consumption patterns have made a significant impact on the beverage market. While the overall juice market is shrinking, the premium juice market is expanding every year [Citation1]. Premium juice is distributed through the non-thermal treatment of fresh fruit juice, which contains a large amount of healthy functional ingredients, and is made without additives or chemical preservatives. However, due to concerns regarding the rapid deterioration of fresh fruit juices, consumers prefer home-made juices prepared directly from the desired fruits and vegetables. As such, the home appliance market for household juicers, such as a blenders and a low-speed masticating (LSM) juicers, is also expanding consistently [Citation2–Citation4]. In case of the blender, the metal blade crushes the materials by rotating at high speeds of 5,000 to 30,000 rpm. Hence, the nutrients are liable to be lost and the juice tends to be deteriorated due to the heat generated by the blade. In contrast, the LSM juicer was developed to complement the disadvantages of the blender and does not use a method of crushing but one of squeezing by way of a screw rotating at a comparatively low speed of 80 rpm. Since heat generation is minimized by this system, there is little nutrient loss, and oxidative stress can be reduced [Citation3,Citation4].
Apples are a widely distributed fruit throughout the world and contain phenolic compounds, organic acids, dietary fiber, and vitamin C [Citation5–Citation7]. They are one of the most preferred fruits by consumers. Apples also contain polyphenol oxidase (PPO; EC 1.10.3.1), which catalyzes enzymatic browning reactions when the damaged surface of an apple comes in contact with oxygen molecules [Citation8,Citation9]. PPO oxidizes o-diphenols into o-quinones which rapidly polymerize to form brown pigments (melanin). In an intact cell, polyphenols, which are a substrate of PPO, are located in the vacuoles and the enzyme exists in the plastid or chloroplast. Therefore the browning reaction does not occur since substrate and enzyme remain physically separated [Citation10]. However, when cell damage occurs, the organelles are broken and the browning reaction is initiated in the presence of oxygen. When apple juice is prepared using a blender, the tissues of apple can be easily damaged due to the physical forces generated by the rotating blades, therefore the apple juice becomes dark and turbid due to the enzymatic browning reaction, which is easily observed with the naked eye [Citation11].
There have been several studies on the composition and bioactivity of the ingredients in apples [Citation5,Citation12,Citation13], however, studies of the effect of the juicing method on the browning reaction when preparing fresh apple juice at home are currently limited. Therefore, in this study, we compared the PPO activity of apple juice prepared using an LSM juicer with that prepared using a blender, and measured the color change by browning reaction, polyphenol contents, and antioxidant activities. From the results, we suggest the LSM juicer as a desirable juicing method to prepare fresh apple juice that is rich in useful bioactive compounds and has high sensory quality.
Materials and methods
Materials
Apples (Malus domestica Borkh. cv. Red Fuji) used in this study were obtained from a farm located in Miryang, Korea in December 2016. (+)-Catechin, Folin & Ciocalteu’s phenol reagent, 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of reagent grade.
Apple juice preparation
After removal of the peel and seeds of the apple, the flesh was cut into ten equal parts, and the equal amount of apple (230 g) was allocated into each juicer. The sample temperature was 20°C. Apple juice was prepared using a blender (HM-1600 PB; Hanil electric Co., Ltd., Seoul, Korea) or an LSM juicer (HH-SBF11; Hurom Co., Ltd., Gimhae, Korea) according to the manufacturer’s instructions at 20°C after adding distilled water corresponding to 20% of sample weight, and then filtered with a cheese cloth. The time of operation for the blender was 30 s at a blade speed of 8,800 rpm. The operation time for the LSM juicer was 30 s at an auger rotating speed of 80 rpm. After the filtrate was centrifuged for 20 min at 500 g and 4°C, the supernatant was collected and the precipitate was resuspended with an equal volume of distilled water as that of juice sample. Apple juice samples were prepared immediately prior to experimentation. Apple slices after blanching for 10 min at 80°C were used instead of fresh apple for a control.
Physicochemical analyses
Titratable acidity (TA) and pH of apple juice were measured using a pH meter (Model 420; Thermo Scientific Orion, Waltham, MA, USA). The TA was determined by diluting 20 mL of a juice sample with 80 mL of distilled water to a final volume of 100 mL, and titrating with 0.1 N NaOH to pH 8.3. Total soluble solids (°Brix) were measured using a digital refractometer (PAL-α; Atago Co., Ltd., Tokyo, Japan) at 20°C.
Color change measurement
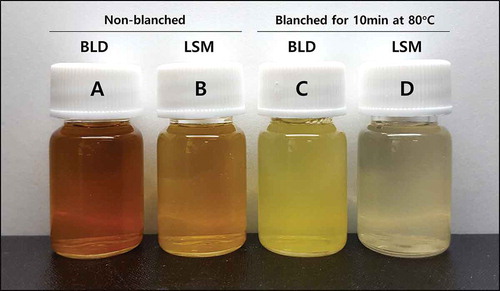
In order to measure the color change to dark brown in apple juice due to an enzymatic browning reaction, 1 mL of apple juice supernatant was taken to measure L (lightness), a (redness), and b (yellowness) values using a color difference meter (CT-301; Konica Minolta, Inc., Tokyo, Japan). The color was measured 25 min after preparing of apple juice.
PPO activity assay
The activity of PPO in apple juice was assayed according to Murata’s method with a modification [Citation14]. Catechin solution (10 mM) in working buffer (0.05 M McIlvaine buffer, pH 4.5) and apple juice filtered with a cheese cloth (AJft) were used as substrate and enzyme sample, respectively. A supernatant (AJsp) and a precipitate (AJpt) after centrifugation of AJft were also used for enzyme samples in addition of AJft. To check for an effect of detergent, Triton X-100 was added to the enzyme solutions (AJft-T, AJsp-T, and AJpt-T, respectively) at a final concentration of 0.05%, and vortexed gently. For preparation of a reaction mixture, 142.5 μL of working buffer and 150 μL of substrate were transferred to a 96-well microplate. Then, 7.5 μL of enzyme sample was added to the mixture and the enzyme reaction proceeded at 37°C for 10 min. The absorbance at 390 nm was measured using a microplate reader (powerwave XS; BioTek, Winooski, VT, USA) in kinetic mode with a time interval of 25 s. The PPO activity was calculated from a slope of straight line plotted with absorbance vs. reaction time. One unit (U) of PPO is defined as the amount of enzyme which is able to increase the absorbance by 1.0 per min under the above reaction conditions.
Total polyphenol content
The total polyphenol content in apple juice was measured according to the method described by Wolfe et al. with some modification [Citation15]. For sample preparation, 1 mL of the juice was added to 3 mL of methanol, and mixed vigorously. After allowing the mixture to stand in a dark place for 1 h, it was centrifuged at 2,500 g for 10 min at 4°C. The supernatant was taken and used as a sample. In a 96 well plate, 120 μL of sample and 60 μL of 50% (v/v) Folin & Ciocalteu’s phenol reagent were mixed, and after 3 min, 120 μL of 2% (w/v) Na2CO3 was added to the mixture and held at room temperature for 30 min. Methanol was used instead of sample as a blank. The absorbance was measured at 750 nm using a microplate reader. The total polyphenol content was calculated from the calibration curve obtained using tannic acid as a standard, and expressed as milligrams of tannic acid equivalents per 1 mL of juice (mg TAE/mL).
Total flavonoid content
The content of total flavonoid in juice was determined according to the method described by Zhishen et al. with a slight modification [Citation16]. The sample was prepared with the same method as that for total polyphenol content analysis. The absorbance was measured at 510 nm using a microplate reader and the total flavonoid content was calculated from the calibration curve obtained using catechin as a standard. The content was expressed as milligrams of catechin equivalents per 1 mL of juice (mg CE/mL).
ABTS decolorization activity
In order to evaluate the antioxidant activity of apple juice, ABTS decolorization activity was measured according to the method described by Re et al. with a slight modification [Citation17]. The sample was prepared with the same method as that for total polyphenol content analysis. The ABTS reagent was prepared by mixing 2.45 mM potassium persulfate solution with 7 mM ABTS solution at a ratio of 1:1 (v/v), and stored in a dark place for 16 h. Before use, the reagent was diluted until the absorbance at 734 nm was 0.80 ± 0.02. In a 96 well plate, 20 μL of the sample was added to 200 μL of ABTS reagent, and left to stand for 4 min in a dark place. After 4 min, the absorbance was measured at 734 nm using a microplate reader. Methanol was used as a blank instead of sample. The ABTS decolorization activity of the sample was calculated using the calibration curve obtained from Trolox standard from the absorbance difference (ΔAbs.) between a sample and a blank, and expressed as milligrams of Trolox equivalents per 1 mL of juice (mg TE/mL).
DPPH radical scavenging activity
The scavenging activity of DPPH radical was assayed according to the method described by Fukumoto and Mazza with a slight modification [Citation18]. The sample was prepared with the same method as that for total polyphenol content analysis, and diluted to the proper concentration with methanol. Twenty microliter of the sample was mixed with 200 μL of the DPPH reagent in a 96 well plate and then allowed to stand in a dark place. After 30 min, the absorbance was measured at 517 nm using a microplate reader. A blank was prepared in the same way using methanol instead of the sample. The DPPH radical scavenging activity (EC50) was calculated using the concentration of juice (μL juice/mL) required for scavenging 50% of DPPH radical.
Statistical analysis
All experiments were carried out in triplicate, and the data were presented as mean ± SD. Comparison of the data among the experimental groups was performed using Tukey’s range tests (SPSS, ver. 19), and a significant difference was accepted at a probability of 5% (p < 0.05).
Results and discussion
General properties of apple juice
The general properties of apple juices prepared using household methods are shown in . The yield of the LSM juicer was 76.2%, which was lower than that for a blender due to a different mechanism of juice extraction [Citation4]. Total soluble solids were a slightly higher in blender apple juice than in LSM apple juice. The juicing methods did not make any significant difference on the pH or the TA of the juice.
Table 1. General properties of apple juice prepared using a blender (BLD) or a low-speed masticating (LSM) household juicer.
Color changes in apple juice
Changes in color of the apple juice prepared using a blender or an LSM juicer are shown in . The pictures were taken just after juice extraction. The color of the juice turned brown or dark brown due to the enzymatic browning reaction during juice extraction as expected ()) [Citation11]. The brown color of the blender apple juice appeared quite darker than that of the LSM juice, indicating that the browning reaction was more active in the blender than in the LSM juicer. However, the color of juice prepared with blanched apples did not change, which means that PPO was responsible for the color change since the enzyme was inactivated by heating treatment at a blanching step (,d)). Color change was also determined using a color difference meter (). As compared with blanched apple juice, L values were decreased (to darker), and both of a and b values were increased (to redder and yellower) significantly in non-blanched juice. Among the non-blanched juice, the blender juice showed lower L value and higher a and b values than the LSM juice. This result is in agreement with the color changes of apple juice as shown in .
Table 2. Color values of apple juice prepared using a blender (BLD) or a low-speed masticating (LSM) household juicer.
PPO activity in apple juice
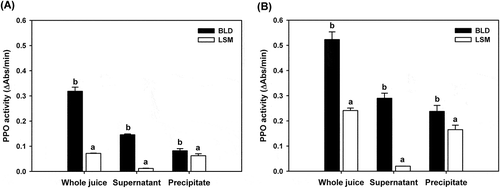
To evaluate the effect of juicing method on PPO activity in apple juice, enzyme activity was measured using catechin as a substrate since it is one of the major polyphenols contained in apples [Citation19]. As expected from the results of the color change analysis, the PPO activity of a blender juice was increased compared with LSM juice (). The PPO activity of the LSM juice was only 22.5% of that of blender juice. To determine the reason for this difference, the juices were centrifuged at a mild condition of 500 g in order to separate the precipitate containing undamaged plastid organelles. It was reported that the PPO is mainly contained in plastid organelles in apple cells [Citation10]. Since most polyphenols are stored in vacuoles, the enzymatic browning reaction would be accelerated when the intact cells are broken down during the juicing process, and the color of apple juice would turn dark brown. While the supernatant of blender juice (BLD-AJsp) showed considerable PPO activity corresponding to 45.7% of whole juice activity, only 17.8% of PPO activity was observed in the supernatant of LSM juice (LSM-AJsp) (). Thus, the PPO activity of LSM-AJsp was 8.2% of BLD-AJsp activity. In contrast, the difference between the PPO activity of the precipitates (LSM-AJpt and BLD-AJpt) was much less than that of the supernatants. This result indicated that the blender caused severe damage to plastids leading to leakage of PPO into the juice. Together, with the polyphenols discharged from the vacuoles, the enzymatic browning reaction was maximized, resulting in a fast color change to dark brown. On the contrary, the LSM juicer minimized the leakage of PPO and color change of apple juice. The destruction of plastids during juicing process was confirmed by measuring PPO activity after adding Triton X-100, a detergent for disintegration of cell membrane. As shown in , all the PPO activity was increased by adding Triton X-100. The PPO activity of BLD-AJsp doubled in the presence of Triton X-100, indicating that a considerable amount of damaged plastid debris remained in supernatant fraction since undamaged intact plastids could be removed as a pellet after centrifugation.
Figure 2. Polyphenol oxidase (PPO) activity of apple juice prepared using a blender (BLD) or a low-speed masticating (LSM) household juicer (A) in the absence of Triton X-100 and (B) in the presence of 0.05% Triton X-100. Fresh apple juices were filtered through gauze (whole juice), and centrifuged at 500 g for 20 min for the separation of supernatant from precipitate. Data are expressed as mean ± SD (n = 3). The data with different letters are significantly different (p < 0.05).
The different mechanisms of the juicers seem to be responsible for the PPO activity and color change of apple juice. High-speed metal blades in a blender can give a stronger shearing force on the material (apple) inside causing more damage to cells than the low-speed screw in an LSM juicer. Similar results were observed in a previous study on tomato juice [Citation3]. Indeed, the destruction of tomato cells was minimized when using an LSM juicer compared with a high-speed centrifugal juicer. In addition, the strong vortex formed in the blending process could increase the chances of PPO to meet oxygen molecules, which would accelerate the browning reaction. The key role of oxygen molecules in the browning reaction in apple juice was investigated by Kim et al. [Citation20]. The higher vacuum that was applied when preparing apple juice using a blender, the less brown color developed, indicating that oxygen is critical for browning reaction.
Total polyphenol contents in apple juice
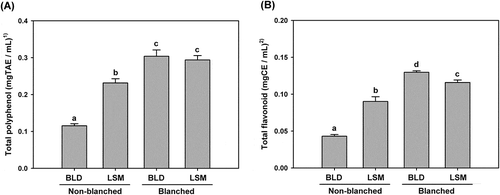
It is well known that apples contains high levels of polyphenols such as chlorogenic acid, catechin, epicatechin, and procyanidins, and these polyphenols are responsible for most of antioxidant activities in apples [Citation5,Citation12,Citation19]. Changes in total polyphenol content in apple juice prepared using a blender or an LSM juicer are shown in . The extraction method had a significant effect on the residual polyphenol contents in apple juice. The polyphenol content in the blender juice (0.12 ± 0.01 mgTAE/mL) was dramatically decreased to 40.0% of a blanched juice. In other words, 60.0% of total polyphenols were destroyed during the blending process. In the case of LSM apple juice, only 20.7% of total polyphenols were lost during the extraction and 79.3% of them remained in the juice. There was no difference in blanched apple juices between the blender and LSM juicer. The destruction of total polyphenols in apple juice seemed to be caused by an enzymatic browning reaction catalyzed by PPO since polyphenol is a substrate of the enzyme [Citation21]. The other substrate is oxygen (O2). Phenolic compounds are easily oxidized into quinones and then polymerized to form brown pigments in the presence of PPO, which leads to the loss of polyphenols [Citation22]. Therefore, higher PPO activity in apple juice causes more destruction of polyphenols. The tendency is shown in total polyphenol contents in apple juice and was very similar to that of PPO activity. As shown in , the PPO activity of blender apple juice was 4.6 times higher than that of LSM juice, and the destruction rate of total polyphenols in blender juice was higher than that of LSM juice. Moreover, exposure to oxygen is expected to be accelerated under grinding conditions due to the high-speed rotating blades in a blender. Therefore, the LSM juicer is considered to be advantageous for preparation of fresh apple juice rich in polyphenols as compared with the blender.
Figure 3. Antioxidant contents of non-blanched and blanched apple juices prepared using a blender (BLD) or a low-speed masticating (LSM) household juicer. (A) Total polyphenol content; and (B) total flavonoid content. 1)mg of tannic acid equivalent per mL of juice; and 2)mg of catechin equivalent per mL of juice. Data are expressed as mean ± SD (n = 3). The data with different letters are significantly different (p < 0.05).
Total flavonoid contents in apple juice
Catechin is one of the major flavonoids in apples, and has been known to have protective effects against cardiovascular disease and cancer [Citation23,Citation24]. The contents of total flavonoids in apple juice showed similarity to those of total polyphenols (). These results seem to be reasonable since flavonoids such as catechin can be used as substrates of PPO like polyphenols. The total flavonoid content in blender apple juice was 0.043 ± 0.002 mgCE/mL, which was less than half (47.8%) of that in LSM juice. While 66.9% of total flavonoids were destroyed by the blending process, only 22.4% were lost by the LSM juicer as compared with each control (blanched apple juice). Blanched apple juice showed higher total flavonoid contents than non-blanched juice, suggesting that PPO plays an important role in the destruction of flavonoids during preparation of juice.
Antioxidant capacities of apple juice
Antioxidant capacities of apple juice were evaluated by the ability to quench ABTS and DPPH free radicals by electron-transfer reaction. The results of the ABTS decolorization assay indicate that LSM apple juice contains stronger antioxidant activity than blender juice (). The ABTS radical quenching activity of LSM juice was 0.229 ± 0.004 mgTE/mL, which was 45.9% higher than that of blender juice. The antioxidant activities of the controls (blanched apple juice) were significantly higher than those of non-blanched juice. These results coincided with those of total polyphenol and flavonoid contents, which are known as major antioxidants in fruits. If PPO activity is high enough to consume considerable amounts of polyphenols and flavonoids in apple juice, it is reasonable to conclude that antioxidant activity is decreased due to lack of antioxidant contents.
Figure 4. Antioxidant activities of non-blanched and blanched apple juice prepared using a blender (BLD) or a low-speed masticating (LSM) household juicer. (A) ABTS radical cation decolorization activity; and (B) DPPH radical scavenging activity. 1)mg of Trolox equivalent per mL of juice; and 2)μL of apple juice per mL of reaction mixture. Data are expressed as mean ± SD (n = 3). The data with different letters are significantly different (p < 0.05).
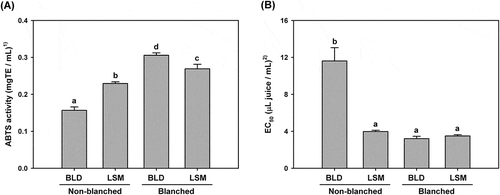
Antioxidant activity was also confirmed by analyzing DPPH radical scavenging activities of apple juice (). The half maximal effective concentration (EC50), which reduces 50% of initial DPPH radicals, of BLD juice was higher than other samples. The EC50 values did not show any significant difference among the LSM juice and controls. Since the lower EC50 indicated the stronger antioxidant activity, LSM juice was superior to BLD juice in terms of being an antioxidant-rich food.
Conclusions
From the above results, it is obvious that the remaining PPO activity in apple juice has a significant effect on the final antioxidant contents (such as total polyphenol and flavonoid) as well as antioxidant activity, and is affected greatly by the method of juice extraction. Since cells are easily broken apart by the blender, PPOs in the cell organelles are released into juice and utilize polyphenols and flavonoids as substrates, resulting in low antioxidant content and activity. Since fresh fruit juices are major sources of antioxidant in our diet, minimization of antioxidant destruction is very important, and therefore, the type of juicer is critical for making nutritious and healthy juice. In this study, the LSM juicer shows a great advantage over the blender for preparing apple juice with high antioxidant contents and activity by minimizing the PPO activity within the juice.
Authors’ contribution
All authors designed the experiments and discussed the results for the preparation of the manuscript. Shin-Young Park and Tae-Min Kang carried out the experiments and wrote the manuscript in consultation with Min-Ju Kim and Myo-Jeong Kim.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Corbo MR, Bevilacqua A, Petruzzi L, et al. Functional beverages: the emerging side of functional foods. Compr Rev Food Sci Food Saf. 2014;13:1192–1206.
- Uckoo RM, Jayaprakasha GK, Balasubramaniam VM, et al. Grapefruit (Citrus paradisi macfad) phytochemicals composition is modulated by household processing techniques. J Food Sci. 2012;77:C921–C926.
- Kim MJ, Kim JI, Kang MJ, et al. Quality evaluation of fresh tomato juices prepared using high-speed centrifugal and low-speed masticating household juicers. Food Sci Biotechnol. 2015;24:61–66.
- Kim MJ, Jun JG, Park SY, et al. Antioxidant activities of fresh grape juices prepared using various household processing methods. Food Sci Biotechnol. 2017;26:861–869.
- Eberhardt MV, Lee CY, Liu RH. Nutrition: antioxidant activity of fresh apples. Nature. 2000;405:903.
- Napolitano A, Cascone A, Graziani G, et al. Influence of variety and storage on the polyphenol composition of apple flesh. J Agric Food Chem. 2004;52:6526–6531.
- Spanos GA, Wrolstad RE, Heatherbell DA. Influence of processing and storage on the phenolic composition of apple juice. J Agric Food Chem. 1990;38:1572–1579.
- Janovitz-Klapp AH, Richard FC, Goupy PM, et al. Kinetic studies on apple polyphenol oxidase. J Agric Food Chem. 1990;38:1437–1441.
- Amiot MJ, Tacchini M, Aubert S, et al. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J Food Sci. 1992;57:958–962.
- Murata M, Tsurutani M, Hagiwara S, et al. Subcellular location of polyphenol oxidase in apples. Biosci Biotechnol Biochem. 1997;61:1495–1499.
- Sapers GM, Douglas Jr, FW. Measurement of enzymatic browning at cut surfaces and in juice of raw apple and pear fruits. J Food Sci. 1987;52:1258–1285.
- Lee KW, Kim YJ, Kim DO, et al. Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem. 2003;51:6516–6520.
- Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5.
- Murata M, Kurokami C, Homma S. Purification and some properties of chlorogenic acid oxidase from apple (Malus pumila). Biosci Biotechnol Biochem. 1992;56:1705–1710.
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237.
- Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000;48:3597–3604.
- Escarpa A, Gonzalez MC. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J chromatogr A. 1998;823:331–337.
- Kim AN, Kim HJ, Kerr WL, et al. The effect of grinding at various vacuum levels on the color, phenolics, and antioxidant properties of apple. Food Chem. 2017;216:234–242.
- Queiroz C, Mendes Lopes ML, Fialho E, et al. Polyphenol oxidase: characteristics and mechanisms of browning control. Food Rev Int. 2008;24:361–375.
- Richard-Forget FC, Rouet-Mayer MA, Goupy PM, et al. Oxidation of chlorogenic acid, catechins, and 4-methylcatechol in model solutions by apple polyphenol oxidase. J Agric Food Chem. 1992;40:2114–2122.
- Tijburg LBM, Mattern T, Folts JD, et al. Tea flavonoids and cardiovascular diseases: a review. Crit Rev Food Sci Nutr. 1997;37:771–785.
- Kohlmeier L, Weterings KG, Steck S, et al. Tea and cancer prevention: an evaluation of the epidemiologic literature. Nutr Cancer. 1997;27:1–13.