ABSTRACT
Polyinosinic-polycytidylic acid (PIC), a double-stranded RNA that induces innate immunity in mammals, is a candidate immunopotentiator for pharmaceuticals. The potency and adverse effects of PIC are strongly correlated with the nucleotide length, and the inability to precisely control the length in PIC production limits its practical use. Length extension during the annealing process is the major factor underlying the lack of control, but tuning the annealing conditions is insufficient to resolve this issue. In this study, we developed a novel method to produce accurate nucleotide length PIC at an industrial scale. The length extension was significantly suppressed by the assembly of multiple short polyinosinic acid molecules with one long polycytidylic acid molecule. A newly developed PIC, uPIC100-400, demonstrated a reproducible length and better storage stability than that of corresponding evenly structured PIC. Human dsRNA receptors exhibited equivalent responsiveness to uPIC100-400 and the evenly structured PIC with the same length.
Graphical Abstract
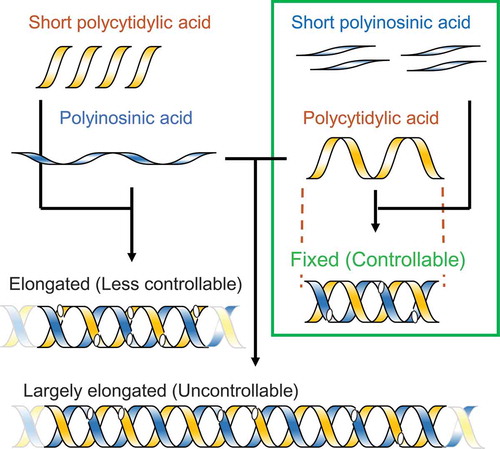
Precise control method for poly I:C nucleotide length
Polyinosinic-polycytidylic acid (PIC) is a double-stranded RNA (dsRNA) consisting of two homopolyribonucleotides, polyinosinic acid (poly-I) and polycytidylic acid (poly-C), with intermolecular nucleotide-paired interactions, forming a stable double-helix conformation [Citation1]. This particular dsRNA structure is associated with viral infections and is detected by antigen-presenting cells in mammals [Citation2,Citation3]. After endosomal sorting via raftlin and clathrin-associated endocytosis, dsRNAs are detected by the toll-like receptor 3 (TLR3) [Citation4], and dsRNAs sorted in the cytoplasm are detected by either retinoic acid inducible gene-I (RIG-I) or melanoma differentiation-associated protein 5 (MDA5) [Citation5]. Detection of these dsRNAs by the ligands of mammalian cells initiates signal transduction, leading to the synthesis of type-I interferon (IFN) and inflammatory cytokines, which activate innate immunity and, generally, necroptosis [Citation6,Citation7]. A representative artificial dsRNA, PIC, has a strong immunopotentiator activity [Citation8,Citation9].
PIC acts as a vaccine adjuvant for infectious diseases in animal models. Hasegawa and co-workers demonstrated in both mice and non-human primates that the addition of 3- to 10-fold volumes of PIC against the antigen enables the H5N1 and H1N1 (pdm09)-inactivated influenza vaccine to induce mucosal immunity in the respiratory tract [Citation10,Citation11]. Another group reported that intranasal PIC administration improves protection by replicating influenza vaccines via enhanced dendritic cell function and T cell immunity [Citation12]. In a study of Ebola vaccines, the subcutaneous vaccination of the combination of Ebola immune complexes and PIC resulted in an equivalent survival rate in a mouse model to that achieved with an alternative viral vector vaccine candidate [Citation13]. The application of PIC as an adjuvant for cancer therapeutic vaccines is also in progress [Citation14–Citation19]. Furthermore, preconditioning with a low dose of PIC prior to brain surgery induces tolerance to subsequent ischemia-reperfusion injury, resulting in neuroprotection against stroke [Citation20,Citation21].
However, numerous reports have indicated that the administration of more than 1 mg PIC/kg body weight exacerbates symptoms of some diseases in animal models, e.g., in chronic obstructive pulmonary disease [Citation22], asthma [Citation23], pancreatitis [Citation24], neurodegenerative diseases [Citation25], neuropsychiatric disorders [Citation26], and necrotizing enterocolitis [Citation27] in newborn mice. These negative pharmacological effects of PIC are related not only to its direct action, but also to its side effects, due to overproduced IFN and inflammatory cytokines by cells [Citation25,Citation28–Citation30].
To suppress the negative effects of active pharmaceutical ingredients and maximize the pharmacological effect, dose optimization is essential. In the case of PIC, establishing a quality standard with respect to the nucleotide length of the double-stranded molecule (NLDS) and proper manufacturing control are indispensable because the strength and duration of the biological activity of PIC are correlated with the NLDS [Citation31,Citation32]. For instance, the intensity and duration of IFN induction observed in mice after the intraperitoneal administration of PIC are correlated with its NLDS [Citation32]. Furthermore, the lethal pharmacological toxicity of PIC is also strongly correlated with an NLDS between 8.2 and 16 S (r = −0.991). It should be noted that 8.2–16 S corresponds to 0.8–5.6 kbp using a formula for the conversion of the sedimentation coefficient to the molecular mass of double-stranded nucleotides [Citation33]. The bone marrow toxicity of PIC in mice is also dependent on the NLDS; toxicity was observed in mice for PICs of 13–24 S (3.1–18 kbp), but not for PICs of 8–9 S (0.8–1.0 kbp) [Citation34].
These NLDS-dependent biological activities of PIC are reflected in the biokinetics of PIC after administration. In fact, PIC administered to mammals is subjected to the hydrolysis of 3′,5′-phosphodiester linkages by a mixture of ribonucleases contained in the blood or body fluid before it is detected by antigen-presenting cells. Although dsRNA is less susceptible to double-strand breaks by ribonucleases than single-stranded RNA, the nucleotide length is inevitably shortened by constant exposure to ribonucleases [Citation35]. Because there is a lower size limit of dsRNA to generate TLR3-, RIG-I-, and MDA5-mediated signal transduction, the duration of PIC signal transduction depends on its NLDS.
A clustering analysis of human TLR3 has revealed that the lower limit of the NLDS for TLR3 recognition is 40–50 bp [Citation36,Citation37]. In the case of helicases, i.e., RIG-I and MDA5, the double helix structure is required to initiate signal transduction, except for some 5′-triphosphate single-stranded RNAs; there is a certain lower length limit for their recognition because both helicases could not detect endonucleases generating short dsRNAs of 15–25 bp [Citation38].
Despite its influence on the strength and duration of the biological activity of PIC, the NLDS of commercially available PIC reagents varies among manufacturers. This variance in the NLDS can be determined by high-resolution analyses using size exclusion chromatography (SEC) (Supplementary Fig. 1). PIC has not been prepared with a successfully controlled NLDS to date according to our survey, indicating that practical methodology to manage the NLDS in PIC production is needed.
There are two issues with controlling the NLDS in PIC production. First, it is difficult to control the nucleotide lengths of poly-I and poly-C molecules that determine the NLDS of PIC. Enzymatic synthesis by polyribonucleotide nucleotidyltransferase (PNPase) is the sole method for the preparation of both poly-I and poly-C with sufficient lengths by excluding structural isomers with 2′,5′-phosphodiester linkage, although PNPase is a bifunctional enzyme that catalyzes both polymerase and exonuclease reactions [Citation39,Citation40]. In addition, PNPase is activated under weak alkaline conditions that concomitantly allow the non-enzymatic hydrolysis of phosphodiester linkages of synthesized polyribonucleotides. These complicated reactions influence the nucleotide lengths of poly-I and poly-C preparations. Second, high-molecular-mass PICs, exceeding predicted values, are obtained in the annealing process by the assembly of multiple poly-I and poly-C molecules. The traditional annealing method for PIC production involves mixing equimolar poly-I and poly-C molecules at room temperature [Citation41]. Using this method, the NLDS of prepared PIC is 5 to 10 times longer than the corresponding length of pre-annealed poly-I and poly-C molecules [Citation9,Citation32]. The impact of this extended structure on the NLDS of PIC is much greater than the alterations in the nucleotide lengths of poly-I and poly-C molecules used in the annealing process. Thus, suppressing the extension of NLDS is the main target for the management of the NLDS in PIC production. NLDS extension is slightly suppressed by the heating and cooling treatment of the mixture of poly-I and poly-C molecules [Citation9]. Although several notes regarding the heating temperature before cooling have been provided, the degree of suppression of the NLDS extension by the treatment and its reproducibility have not been described [Citation9,Citation42].
Quality standards for active ingredients in pharmaceuticals ensure the equivalence of quality specifications between production lots [Citation43]. To develop PIC that meets this requirement, it is necessary to establish a methodology for controlling the NLDS of this homo-polymeric dsRNA. We developed a novel method for suppressing and thereby precisely controlling the extension of the NLDS of PIC.
We propose that an uneven length combination of poly-I and poly-C molecules is advantageous for industrial scale PIC production. This uneven structure detailed in this paper not only prevents NLDS extension during the annealing process, but also maintains the NLDS during storage. We further demonstrate the impact on the secondary structure of PIC as well as its in vitro bioactivity.
Materials and methods
Preparation of PIC
All solutions, except for those with high-molecular-mass compounds, were passed through an ultrafiltration membrane to avoid ribonuclease contamination. Poly-I and poly-C were enzymatically synthesized without a template by PNPase from 5′-inosinic acid diphosphate and 5′-cytidylic acid diphosphate, respectively, according to previously described protocols [Citation42]. The PNPase was purchased from Unitika (Osaka, Japan). The PNPase reactions were performed in 40 mL or 250 mL volume at 50°C, and poly-I and poly-C of various lengths were obtained by changing the pH condition (pH 8.0–9.0) and reaction time (5–24 h). PIC was prepared by annealing poly-I to poly-C, either by mixing and maintaining at 20–25°C [Citation41] or by mixing and cooling after heating [Citation42]. The mixture ratio of poly-I to poly-C was 52:48, according to the peak absorbance at 260 nm (OD260), as determined by observing the decrease in OD260 after mixing poly-I with poly-C [Citation1]. The mixture was suspended at a concentration of ~ 10 OD260 units in 40 mL or 500 mL of 0.02 M HEPES buffer (pH 7.0) containing 0.03 M sodium chloride, heated to 60–70°C, and cooled by allowing it to stand at 20–25°C. Depending on the annealing conditions, the time required to cool to 30°C was between 40 min and 3 h. Poly-I, poly-C, and PIC were purified by ethanol precipitation followed by dialysis against 0.03 M sodium chloride using an Amicon ultra centrifugal filter units Ultra-15 (NMCO 10,000, EMD Millipore, Darmstadt, Germany) until the electrical conductivity becomes constant. For large-scale preparation (approximately 2 g), purification was performed using ion-exchange chromatography [Citation44], ultrafiltration dialysis using 10,000 NMCO membrane, and freeze-drying.
Determination of the nucleotide length of RNAs
Nucleotide lengths of poly-I, poly-C, and PIC were determined by the OD260 SEC data. To perform SEC, a Prominence GPC system, a UV-VIS detector (SHIMADZU, Kyoto, Japan), and a TSKgel G5000PWxl column (TOSOH, Tokyo, Japan) were used. The mobile phase was an aqueous solution consisting of 0.15 M disodium sulfate and 0.01 M Tris-sulfate buffer (pH 7.0). All test samples were appropriately diluted with the mobile phase prior to loading on the SEC column. The molecular size markers for SEC were DNA fragments of 0.1–4.0 kbp produced by polymerase chain reaction, a 24-mer oligonucleotide, and cytidine diphosphate. The Prominence GPC system outputs the weight average molecular mass and the molecular mass at the peak OD260 of test samples. Although the data processing functionality of the Prominence GPC system links the OD260 chromatogram to the molecular mass, the OD260 values of single-stranded RNA and dsRNA are magnified by the number of nucleotides and nucleotide pairs, respectively [Citation45,Citation46]. Therefore, the weighted average of the nucleotide length (Lw) was recalculated using the GPC system slice data and the following equation:
In this equation, and
are the correlative signal intensities at OD260 and the molecular mass, respectively, of slice n output from the Prominence GPC system.
is the molecular mass of the structural unit of the corresponding polymer. The
values of poly-I, poly-C, and PIC are 330, 305, and 635, respectively. The nucleotide length at the absorbance peak at 260 nm (Lp) was obtained by dividing the molecular mass at the absorbance peak at 260 nm by
.
Effects of pH, salt concentration, and polynucleotide concentration on annealing
The effects of pH (pH 6.5–7.5), sodium chloride concentration (0–0.15 M), and polynucleotide concentration (1–10 OD260 units) on the annealing of poly-I and poly-C were examined in 40 mL of 0.05 M HEPES buffer. The prepared mixtures were heated to 70°C and cooled to 20–25°C. The NLDS distribution of the samples was analyzed by SEC.
Non-enzymatic hydrolysis of poly-I and poly-C
Alterations of the Lw of poly-I and poly-C in solution were analyzed. Poly-I or poly-C was dissolved in 0.05 M Tris-buffered saline solution (pH 7.0‒9.2) or 0.05 M HEPES-buffered saline solution (pH 6.5‒7.0) at a concentration of ~ 30 OD260 units and a volume of 1 mL was added to test tubes (1.5 mL). Samples were incubated in a 50°C, 60°C, and 70°C bath. At each sampling time, one sample was selected and its Lw was analyzed by SEC.
Structural analysis of RNAs
The hydrophilic interactions between double strands and the double helical structure of PIC were analyzed by temperature dissociation curves and circular dichroism (CD) spectra [Citation46]. The melting temperature (Tm) and the complete dissociation temperature were determined from the temperature dissociation curve of the absorbance at 260 nm, which was measured using a TMSPC-1 system (SHIMADZU, Kyoto, Japan). CD spectra of poly-I, poly-C, and PIC were determined at 25°C using a Model 430 CD spectrometer (AVIV Biomedical, Lakewood, NJ, USA). Sample preparation for both analyses was performed by dissolving RNAs in phosphate-buffered saline (PBS) with pH 7.4 and adjusting the concentration to 0.5 OD260 units.
Storage stability test
For the storage stability test, uPIC100-400 and PIC400-400CA were dissolved in 1× PBS at a concentration of 20 OD260 units and 10 mL was added to heat-sterilized 20-mL glass vials, which were then sealed. The two production lots of both PICs were subjected to 25°C and 40°C storage conditions for 4 months. Two vials from each lot were opened for SEC analysis at each sampling point.
TLR3 recognition test by human TLR3 reporter cells
The HEK-BlueTM hTLR3 Cell System (InvivoGen, San Diego, CA, USA) was used for the test of human TLR3 recognition at the recommended conditions. Cultures were maintained at 37°C in 5% CO2 using DMEM supplemented with 10% fetal bovine serum (FBS) and the corresponding antibiotics for the cell line. In our experiments, the cells were grown to pre-confluence on a collagen-coated twelve-well plate, and the culture medium was changed to 1 mL of serum-free DMEM with antibiotics after three washes with serum-free DMEM. PICs were applied at 0.002 OD260 units (approximately 0.1 µg) in each well. After 2 hours of cultivation, 0.1 mL of low-IgG fetal bovine serum (Life Technologies Japan, Tokyo, Japan) was added to each well, and cultivation was continued for 20 hours. The levels of secreted alkaline phosphatase (SEAP) induced by the signaling pathway from TLR3 were determined using QUANTI-BlueTM (InvivoGen). Cell viability was determined using the Premix WST-1 Cell Proliferation Assay System (Takara-Bio, Shiga, Japan).
RIG-I and MDA5 recognition test using the human lung fibroblast cell line, MRC-5
The human lung fibroblasts, MRC-5 (RCB0218) [Citation47], were obtained from the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. Cultures were maintained at 37°C in 5% CO2 using DMEM supplemented with 10% FBS. The induction of IFN-β by sorting PIC into the cytoplasm was performed by transfecting PIC into cells using cationic liposomes [Citation5]. In our experiments, MRC-5 was grown to pre-confluence in a twelve-well plate, and the culture medium was changed to 1 mL of serum-free DMEM after three washes with serum-free DMEM. Then, 50 μL of the mixture of either 0.006 OD260 units (approximately 0.3 µg) or 0.02 OD260 units (approximately 1 µg) of PIC with a cationic liposome LyoVecTM (InvivoGen Inc.) was suspended in each well. After 2 hours of cultivation, 0.1 mL of low-IgG FBS (Life Technologies Japan) was added to each well, and cultivation was continued for 20 hours. The IFN-β concentration in the culture was determined using the human-IFN-β ELISA Kit (Kamakura Techno-Science, Kanagawa, Japan). Cell viability was determined using the Premix WST-1 Cell Proliferation Assay System (Takara-Bio).
Statistical analysis
The statistical significance of differences between groups was determined using the Student’s t-test. p-values <0.05 were considered significant.
Results
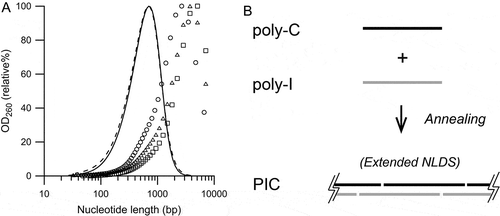
Nucleotide length distributions of poly-I, poly-C,poly-i, poly-c, and PIC
The target nucleotide lengths of the prepared PIC were set to 0.7 kbp, the nucleotide length (Lp) at the peak OD260 value because PIC with an Lp of 0.7 kbp corresponds to 8 S by applying the centrifugal sedimentation coefficient–molecular mass conversion formula [Citation33], which is not only low in toxicity [Citation32,Citation34], but also rich in the molecules with more than 50 bp in length recognized by TLR3 [Citation36,Citation37]. Preparation of PIC with an Lp of 0.7 kbp is theoretically achieved by the annealing of poly-I with an Lp of 0.7 kb and poly-C with an Lp of 0.7 kb. These poly-I and poly-C molecules were prepared by the PNPase reaction, and the NLDS distributions are shown in . Both poly-I and poly-C with an Lp of 0.7 kb had an Lw of 0.4 kb. The NLDS distribution of the PIC prepared by mixing poly-I and poly-C molecules is also shown in . This PIC, named PIC400-400, had a broad NLDS distribution, characterized by an Lp of 3.4 ± 0.7 kbp (n = 20); this was approximately 5 times longer than the nucleotide lengths of the poly-I and poly-C molecules. The Lw of PIC400-400 was not calculated because the majority of the NLDS distribution exceeded the range of the SEC column. The NLDS distribution of PIC400-400 never shifted to a shorter range by heating at 37°C for several hours. These findings suggested that PIC400-400 was assembled with multiple poly-I and poly-C molecules, as illustrated in .
Figure 1. Distribution of the nucleotide lengths of poly-I, poly-C, and PIC prepared without heat treatment.
(A) Representative Lp 0.7 kb poly-I molecules (dashed line), Lp 0.7 kb poly-C molecule (solid line), and PIC referred to as PIC400-400 (symbols). Three PIC400-400 preparations with the same set of poly-I and poly-C molecules are plotted with different symbols. (B) Schematic diagram of the extended NLDS in PIC400-400.
Impact of annealing conditions on the extension of double-stranded PIC
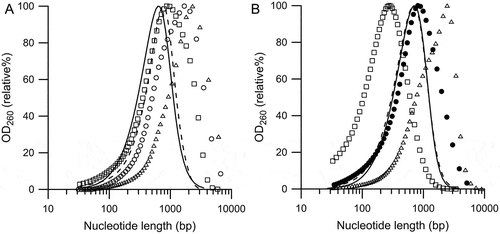
Efforts to tune the annealing conditions to suppress NLDS extension are needed. The application of gentle cooling after heating is a general technique to align the double strands of both DNA and dsRNA. Three temperatures spanning the complete dissociation temperature were selected for the heating temperature, followed by cooling at room temperature. Because the Tm and the complete dissociation temperature of PIC400-400 were 63°C and 64°C, respectively, the heating temperatures were 60°C, 70°C, and 80°C. Nucleotide length distributions after the heating and cooling treatments in the annealing of the poly-I and poly-C molecules (Lw 0.4 kb) are shown in . The nucleotide length distributions were influenced by the heating temperature itself, and not by whether the temperature was higher or lower than the complete dissociation temperature, although these had a reduced suppressive effect on NLDS extension. In addition to the temperature conditions, other parameters related to the stacking interactions between base pairs, i.e., the pH, salt concentration, polynucleotide concentration, were evaluated, but manipulations of these parameters did not successfully suppress the NLDS extension of PIC400-400. The operation with the most substantial effect on the NLDS of PIC400-400 was slowing the cooling rate, in addition to the heating temperature. A cooling operation at a constant rate of 2°C/h after heating to 70°C suppressed NLDS extension (). The PIC400-400 prepared by this heating and cooling operation was named PIC400-400CA and had an Lp of 0.8 ± 0.0 kbp and an Lw of 0.4 ± 0.0 kbp (n = 10). The NLDS distribution of PIC400-400CA was reproducible. However, the operation of cooling at 2°C/h after heating at 60°C, instead of 70°C, failed to suppress the NLDS extension. The other operation of cooling at 2°C/h after heating at 80°C, instead of 70°C, yielded a much shorter NLDS than the nucleotide lengths of the poly-I and poly-C components. These results indicated that the NLDS of PIC400-400CA was obtained by appropriately stopping the thermal decomposition of the strands in the annealing process.
Figure 2. Effect of the heating temperature and cooling conditions on NLDS in the PIC400-400 annealing process.
(A) Representatives prepared by cooling at room temperature after heating to 60°C (open triangle), 70°C (open circle), 80°C (open square), and pre-annealed components, i.e. Lw 0.4 kb poly-I (dashed line) and Lw 0.4 kb poly-C (solid line), the target NLDS after annealing. (B) Representatives prepared by cooling at 2°C/h after heating to 60°C (open triangle), 70°C (PIC400-400CA; closed circle), 80°C (open square), and pre-annealed components, i.e. Lw 0.4 kb poly-I (dashed line) and Lw 0.4 kb poly-C (solid line), the target NLDS after annealing.
Heat stability of 3ʹ,5ʹ-phosphodiester linkages of poly-I and poly-C molecules
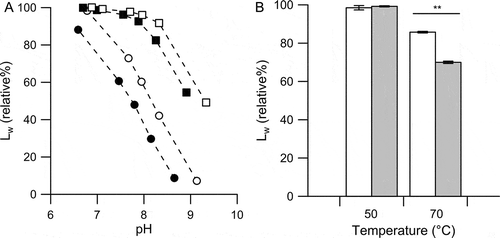
Single-stranded RNA is more susceptible to cleavage by non-enzymatic hydrolysis than single-stranded DNA because the 3ʹ,5ʹ-phosphodiester bond of RNA is subjected to nucleophilic attack by the neighboring 2ʹOH group of ribose [Citation48]. This non-enzymatic cleavage of RNA is accelerated in alkaline conditions. In a comparison of the hydrolysis resistance properties, poly-I molecules were more easily cleaved than poly-C molecules at the same pH and the same temperature (). The Tm and complete denaturation temperature of PIC400-400CA were 63°C and 64°C, respectively, which were equivalent to those of PIC400-400. For a cooling rate of 2°C/h from 70°C in the annealing process of PIC400-400CA, 3 hours were required to reach the temperature necessary for the formation of the double helix. The nucleotide lengths of poly-I and poly-C molecules retained at 70°C after 3 hours were significantly different ().
Figure 3. Characteristics of the non-enzymatic cleavage of poly-I and poly-C molecules.
(A) Effect of pH on the cleavage of poly-I (closed symbols) and poly-C (open symbols) molecules at 50°C (closed and open squares) and 70°C (closed and open circles); percentages of Lw values retained after 2 h of incubation are plotted. (B) Effect of temperature on the cleavage of poly-I (gray bars) and poly-C (open bars) molecules in the solution at pH 7.0; percentages of Lw values retained at 50°C and 70°C after 3 h of incubation are plotted. Bars and error bars are the averages and SEM, respectively, of three samples. Significant differences between poly-I and poly-C samples determined by Student’s t-tests are marked (**p < 0.01).
Impact of the nucleotide length of poly-I on double strand formation with poly-C
To determine the relationship between the nucleotide length of the poly-I molecule and NLDS extension of PIC in the annealing step, three short poly-I molecules, with Lw values of 0.2 kb, 0.1 kb, and 0.05 kb, were annealed with the poly-C molecule with an Lw of 0.4 kb, and NLDS was examined. In the annealing procedure, samples were cooled at room temperature after heating at 60°C or 70°C in order to avoid strand shortening by exposure to high temperatures. A combination of the Lw 0.2 kb poly-I and Lw 0.4 kb poly-C molecules, referred to as uPIC200-400, showed slight suppression of NLDS extension as compared to that before heating. The suppression of NLDS extension was not sufficient in both heating at 60°C and 70°C (). For the combination of Lw 0.1 kb poly-I and Lw 0.4 kb poly-C molecules, named uPIC100-400, NLDS extension was sufficiently and reproducibly suppressed by cooling after heating to 60°C (Lp 0.7 ± 0.0 kbp, Lw 0.4 ± 0.0 kbp (n = 10)) (). This NLDS distribution closely resembled that of the assembled poly-C molecule, with the same Lp and Lw values. Increasing the heating temperature to 70°C shortened the NLDS of uPIC100-400 by approximately 5%. The combination of Lw 0.05 kb poly-I and Lw 0.4 kb poly-C molecules, named uPIC50-400, also sufficiently suppressed NLDS extension, even without heating (). An additional detailed examination of the length of poly-I molecules annealing to Lw 0.4 kb poly-C molecules by cooling at room temperature after heating to 60°C was performed. Sufficient suppression of NLDS extension using uPIC100-400 was obtained for Lw 0.13 kb poly-I molecules, but Lw 0.14 kb poly-I molecules resulted in a slightly elongated PIC with an Lw of 0.4 kbp and Lp of 0.8 kbp. These results indicated that the length of the poly-I molecule with an Lw of 0.13 kb was the upper limit to sufficiently reduce the NLDS extension when Lw 0.4 kb poly-C was used by cooling at room temperature after heating to 60°C.
Figure 4. Effect of assembling PIC with uneven length combinations of poly-I and poly-C molecules on the NLDS extension.
(A) Representative uPIC200-400 prepared by cooling at room temperature after heating to 60°C (open circle), 70°C (open square), preheating (plus symbol), and pre-annealed components, i.e. Lw 0.2 kb poly-I (dashed line) and Lw 0.4 kb poly-C (solid line†). (B) Representative uPIC100-400 prepared by cooling at room temperature after heating to 60°C (n = 4; open symbols), preheating (plus symbols), and pre-annealed components, i.e., Lw 0.1 kb poly-I (dashed line) and Lw 0.4 kb poly-C (solid line†). (C) Schematic diagram of the structure of uPIC100-400. (D) Representative uPIC50-400 (plus and cross symbols) prepared by mixing at room temperature, and pre-annealed components, i.e., Lw 0.05 kb poly-I (dashed line) and Lw 0.4 kb poly-C (solid line†). (E) Representative ruPIC400-100 prepared by cooling at room temperature after heating to 60°C (open circle), 70°C (open square), preheating (plus symbol), and pre-annealed components, i.e., Lw 0.4 kb poly-I (dashed line†) and Lw 0.1 kb poly-C (solid line). (F) Representative ruPIC400-50 prepared by mixing at room temperature (plus and cross symbols), by cooling at room temperature after heating to 70°C (open square), and pre-annealed components, i.e., Lw 0.4 kb poly-I (dashed line†) and Lw 0.05 kb poly-C (solid line). The target NLDS distributions after annealing are marked with the symbol †.
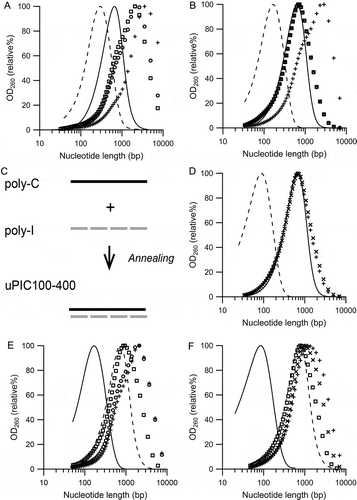
Two combinations with the reverse length ratio between poly-I and poly-C molecules were also examined. The combination of Lw 0.1 kb poly-C and Lw 0.4 kb poly-I molecules, named ruPIC400-100, was insufficient to suppress NLDS extension by cooling at room temperature after heating to both 60°C (Lp 1.9 ± 0.0 kbp, Lw 0.5 ± 0.0 kbp) and 70°C (Lp 0.9 ± 0.0 kbp, Lw 0.5 ± 0.0 kbp), which was significantly different from that of uPIC100-400 (p < 0.01) (). Another combination of Lw 0.05 kb poly-C and Lw 0.4 kb poly-I molecules, named ruPIC400-50, sufficiently suppressed NLDS extension by cooling at room temperature after heating to 70°C (). However, this combination did not show sufficient suppression before heating, unlike the results obtained for the NLDS distribution of uPIC50-400. Based on these results, even for the same length combinations of poly-I and poly-C molecules, the combination of relatively shorter poly-I and Lw 0.4 kb poly-C molecules apparently suppresses NLDS extension more substantially than the combination of relatively shorter poly-C and Lw 0.4 kb poly-I molecules.
The double helix structures of uPIC100-400, uPIC50-400, ruPIC400-100, ruPIC400-50, and PIC400-400CA
To examine the mechanism by which uPIC100-400, rather than ruPIC400-100, more effectively suppressed NLDS extension, CD spectra were analyzed (). The CD spectrum reflects the sum of each strand-specific secondary structure and the double helical structure of dsRNA, which is not affected by the nucleotide length [Citation46]. The CD spectrum of PIC in PBS (pH 7.4) has two peaks (245 nm and 275 nm) and two troughs (210 nm and 260 nm). The peak at 275 nm reflects the strand-specific secondary structure of PIC, and the combination of the 210 nm trough and the 245 nm peak reflects the right-handed double helix structure [Citation46]. The difference in CD spectra between poly-I and poly-C molecules was very clear, indicating a clear difference in the secondary structure between poly-I and poly-C; the poly-I molecule had very little secondary structure in physiological conditions, whereas the poly-C molecule showed obvious right helix formation [Citation49].
Figure 5. CD spectra for poly-I, poly-C, and PIC in phosphate-buffered saline (pH 7.4).
(A) CD spectra for poly-I (solid line), poly-C (dashed line), and PIC400-400CA (bold line). (B) CD spectra for uPIC100-400 (solid line), uPIC50-400 (dashed line), and PIC400-400CA (bold line). (C) CD spectra for uPIC400-100 (solid line), uPIC400-50 (dashed line), and PIC400-400CA (bold line).
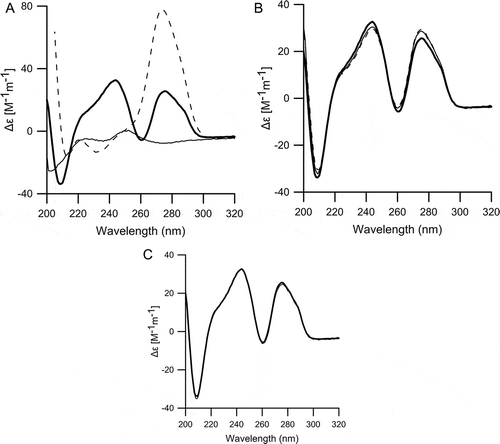
The signal at 245 nm and the two troughs of uPIC100-400 were slightly weaker than the corresponding peaks of PIC400-400CA (). The difference in these signals indicated looser double helix formation for uPIC100-400 than for PIC400-400CA. The increased signal at 275 nm for uPIC100-400 reflected the slight recovery of the inherent secondary structure of the poly-C molecule, in accordance with the looser double helix of uPIC100-400 than of PIC400-400CA. The CD spectrum of uPIC50-400 was very similar to that of uPIC100-400. The CD spectra of both ruPIC400-100 and ruPIC400-50 were similar to those of PIC400-400CA. The differences in secondary structure could not be determined from CD spectra ().
The highly ordered structure of dsRNA that exhibits the potency of the innate immune responses is reflected in the Tm values. The Tm values of not less than 60°C in the condition of 0.15 M Na+ are used as an empirical index of the highly ordered structure [Citation50]. The Tm values of PIC400-400CA, uPIC100-400, and ruPIC400-100 were 63°C, 62°C, and 61°C, respectively, which were matched to the index. On the contrary, the Tm values of both uPIC50-400 and ruPIC400-50 decreased to 58°C, which indicated that both uPIC50-400 and ruPIC400-50 were deficient in the binding strength of poly-I and poly-C molecules to take the highly ordered structure of dsRNA to exhibit the potency of the innate immune responses.
Storage stability of uPIC100-400 and PIC400-400CA
Storage stability of uPIC100-400 and PIC400-400CA in PBS solution (pH 7.4) following middle-term storage at 25°C and 40°C were assessed by SEC analysis. No changes were observed in the appearance of the solution, such as turbidity or pigmentation, and no increase was noted in low-molecular-weight compounds in the SEC analysis during the 4-month test period. The only detected change was a shift in the NLDS distribution toward shorter lengths. Time courses of the average NLDS in both storage conditions are plotted in .
Figure 6. Changes in the NLDS of uPIC100-400 and PIC400-400CA in PBS during storage.
Time course of the Lw values of uPIC100-400 (open circle) and PIC400-400CA (closed circle) at 25°C (panel A) and 40°C (panel B). Points and error bars are the averages and SEM, respectively, of the four samples. Significant difference between samples and Day 0 samples by Student’s t-tests are marked (*p < 0.05, **p < 0.01).
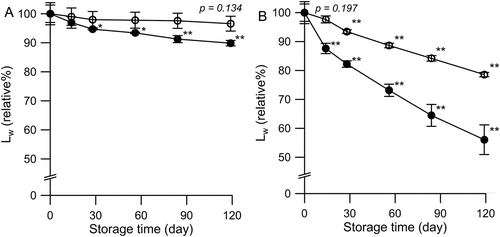
For PIC400-400CA, a significant decrease in the NLDS was observed at 25°C after Day 30 (p < 0.05). The initial Lw value (0.4 ± 0.01 kbp) of PIC400-400CA decreased to 89.9% ± 0.9% on Day 120. The decrease in NLDS was accelerated at 40°C, and the Lw value was reduced by almost half (56.1% ± 5.1%) on Day 120. On the contrary, the NLDS of uPIC100-400 did not decrease significantly at 25°C during the 4-month period (96.6% ± 2.6%, p = 0.134). A significant decrease in NLDS of uPIC100-400 was observed at 40°C after Day 30 (p < 0.01), although the decrease was not significant on Day 15 (p = 0.197). The Lw value reached 78.6% ± 0.7% on Day 120. The relative Lw% on Day 120 at both 25°C and 40°C storage conditions differed significantly between uPIC100-400 and PIC400-400CA (p < 0.01). These results indicated the better storage stability of uPIC100-400 than that of PIC400-400CA at both 25°C and 40°C storage conditions.
Effect of uPIC100-400 on the responsiveness of dsRNA receptors involved in innate immunity in humans
Potency to innate immune responses of uPIC100-400, PIC400-400CA, and ruPIC400-100 were assessed by the responsiveness of the dsRNA receptors involved in the signaling pathways of innate immunity. The ability to evoke TLR3 signaling was evaluated using a recombinant human TLR3 reporter cells to detect the intensity of TLR3 signaling by the titer of induced SEAP. Significant SEAP induction was observed in the 20-h cultures after the addition of uPIC100-400, PIC400-400CA, and ruPIC400-100 (p < 0.01) (). The SEAP titer in the uPIC100-400-stimulated culture was 7–10% lower than those of the others (p < 0.01).
Figure 7. Responsiveness of dsRNA receptors involved in human innate immunity to PIC400-400CA, uPIC100-400, and ruPIC400-100.
(A) Responsiveness of human TLR3 reporter cells after stimulation with 0.1 μg/mL PIC. Intensities of human TLR3 responsiveness are plotted as relative SEAP activity levels. (B) IFN-β induction in the human fibroblast cell line MRC-5 by sorting PIC to the cytoplasm. IFN-β titers of 0.3 μg/mL (open bars) and 1 μg/mL (gray bars) of PIC-stimulated cultures are shown. Bars and error bars are the averages and SEM, respectively, of three cultures. Significant difference between samples by Student’s t-tests are marked (**p < 0.01).
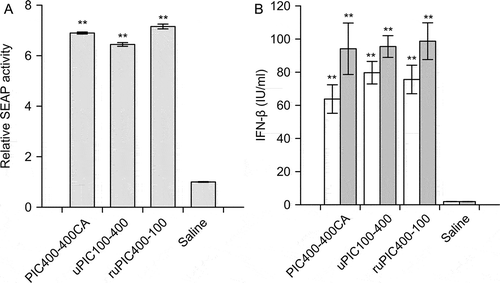
The ability to induce signaling from sensors in the cytoplasm, i.e., RIG-I and MDA5, was evaluated by the IFN-β titer in the culture after sorting PICs into the cytoplasm using cationic liposomes. The observed induction of IFN-β was dose-dependent, but significant differences in IFN-β levels were not observed among uPIC100-400, PIC400-400CA, and ruPIC400-100 (). Because IFN-β was not detected in the culture by adding either cationic liposomes alone or PIC alone, instead of transfecting a complex, IFN-β was initiated by signaling from either RIG-I or MDA5. The cell viability for MRC-5 decreased in a dose-dependent manner with PIC, but no significant difference was observed among uPIC100-400, PIC400-400CA, and ruPIC400-100.
Discussion
We demonstrated that assembling PIC with multiple short poly-I molecules and one long poly-C molecule improves the NLDS distribution. Precise control of the NLDS distribution in PIC production was realized by this uneven length structure for poly-I and poly-C molecules. Unexpected increase in the molecular mass of PIC during annealing is a common phenomenon [Citation9,Citation51], and is attributed to NLDS extension. Neither triple- nor multiple-stranded structures form; a double strand is the only secondary structure resulting from the annealing of poly-I and poly-C molecules at a neutral pH [Citation45]. Neither symmetric nor asymmetric loop structures are observed in PIC because both strands are full complementary homopolymeric sequences that are stabilized to maximize conformational entropy. It is generally understood that PIC exhibits a straight-chain double helix, at least following incubation at 37°C, for a short time, but exhibits an unstable multilooped double helix at 0°C [Citation52].
Although tuning the annealing conditions by slow cooling at 2°C/h after heating to 70°C resulted in the successful assembly of the poly-I and poly-C molecules to obtain PIC without NLDS extension, as observed for PIC400-400CA, the suppression of NLDS extension was achieved by the moderate non-enzymatic cleavage of the poly-I and poly-C molecules by long-term exposure to high temperatures. These PIC molecules probably had random cleavages in both the strands. Operating linear cooling at 2°C/h with accuracy is challenging in industrial-scale production because accurate temperature shifts are difficult to obtain in an industrial-scale tank owing to the lack of a uniform temperature distribution in the tank, which might limit the reproducibility of NLDS between production lots.
Applying cleavages to either strand of poly-I and poly-C before annealing was effective to reduce the NLDS extension. Neither long-term exposure to high temperatures nor operating linear cooling is essential for the reduction of the NLDS extension in the annealing of uneven length combinations of the poly-I and poly-C molecules, one of which was short and one of which was long. The shorter length of the short molecule was better to suppress the NLDS extension of PIC in the annealing, but the Lw 0.05 kb of poly-I and poly-C, the components of uPIC50-400 and ruPIC400-50, respectively, were short to maintain the Tm values of not less than 60°C that is the empirical index of dsRNA to activate innate immunity [Citation50].
The NLDS extension of PIC using combinations of short poly-I and long poly-C molecules was more easily suppressed with less heating compared with the reverse length combinations of long poly-I and short poly-C molecules, suggesting that the poly-C molecule takes advantage of the reference strand to fix the NLDS of PIC. This non-reversibility of the length combination can probably be attributed to the difference in secondary structures between the molecules. The poly-C molecule has an obvious right helical structure by strong stacking interactions between neighboring bases in the polymer [Citation46]. In contrast, the poly-I molecule has very little secondary structure in physiological conditions, and exhibits an intensive conformational transition to a sharp right-handed helix during annealing with the poly-C molecule [Citation49]. The shorter poly-I molecule exhibits less temporal conformational distortion at the initiation of base-to-base interactions with the poly-C molecule during annealing, and this may explain the smoother alignment of the combination of the short poly-I molecule and long poly-C molecule.
The significantly better storage stability of uPIC100-400 than that of PIC400-400CA might be attributed to the continuity of the poly-C strand. At the outset of the storage stability analyses, uPIC100-400 was suspected to be more susceptible to double-strand cleavage than PIC400-400CA because uPIC100-400 has nicks in its poly-I strand; however, our results did not support this prediction. The better storage stability of uPIC100-400 than that of PIC400-400CA is thought to be related to whether or not the poly-C strand in PIC is cleaved at the beginning of storage. The poly-C strand of uPIC100-400 hardly has nicks because uPIC100-400 does not require a certain amount of heating leading to the cleavage in the poly-C molecule during annealing. For comparison, PIC400-400CA requires sufficient heating to generate nicks not only in the poly-I molecule, but also in the poly-C molecule. Considering the observation that the heat stability of the poly-I molecule was lower than that of the poly-C molecule, the nicked positions in the poly-C strand of PIC, i.e., the positions maintaining the double strand with only the poly-I strand, are likely weak for preventing the non-enzymatic cleavage of the double strand. In other words, the continuity of the poly-C strand is presumed to be the key to improving the storage stability of PIC.
The storage stability of a substance is mainly inferred from the Arrhenius equation. Applying the rate constant for the 3′,5′-phosphodiester cleavage of RNA of 29 kcal/mol [Citation53] to dsRNA, the storage stabilities at 40°C on Day 15 and Day 30 correspond to 5 months and 10 months of stability at 25°C, respectively. The conserved NLDS of uPIC100-400 at 40°C on Day 15 is in accordance with the conserved NLDS at 25°C during the 4-month period. Coincidentally, the result on Day 30 at 40°C suggests a significant decrease in the NLDS within 10 months at 25°C. In addition, the results at 25°C during the 4-month period allows to make prediction of the 11-year stability at 5°C, although long-term experiments at 5°C are indispensable for the assessment.
The ability of uPIC100-400 to recognize dsRNA sensors was nearly the same as that of PIC400-400CA. Robust PIC-dependent TLR3 signaling was detected after applying each PIC, showing that the nicks in uPIC100-400 and ruPIC400-100 are not critical alterations in this compound class. Although uPIC100-400 showed an approximately 10% reduction in the initiation of TLR3 signaling compared with the other PICs, the difference in biological responsiveness was generally unremarkable. Both RIG-I and MDA5 are helicases located in the cytoplasm. The modulators involved in signal transduction from both sensors are the same, although functional differences between RIG-I and MDA5 are related to the nucleotide length of dsRNA [Citation38]. The ability to recognize these helicase-type sensors was determined by evaluating the induction of IFN-β in MRC-5 cultures. Apparent IFN-β production was observed only in the culture in which PIC was delivered into the cytoplasm using cationic liposomes, although MRC-5 reportedly expresses TLR3 [Citation54]. The observed IFN-β production is thought to reflect the innate immune response captured by the helicase-type dsRNA sensors. The lack of differences in IFN-β production by transfection with uPIC100-400, ruPIC400-100, and PIC400-400CA indicated that the nicks in uPIC100-400 and ruPIC400-100 did not affect helicase-type dsRNA sensor recognition.
In summary, the nucleotide length of PIC was controlled by applying an uneven length structure to the strands, i.e., PIC was assembled using multiple short poly-I molecules and one poly-C molecule. The ideal NLDS of this unevenly structured PIC was equal to the nucleotide length of the poly-C molecule. A successful representative of this unevenly structured PIC was uPIC100-400, which showed better storage stability than that of the corresponding evenly structured PIC400-400CA, indicating that the nicks in poly-I strands are not the primary cause of double-strand cleavage during storage. Both TLR3 and helicase-type dsRNA sensors of human cells recognized uPIC100-400 as well as PIC400-400CA. We believe that this uneven structure is a practical solution to manage the NLDS of PIC. The PICs with assured NLDS will contribute to precise analyses of the immunological events of this compound class.
Author Contributions
TN conceived and designed this study. TN, EY, and HF performed the experiments and analyzed the data. TN, TS, and KA wrote the manuscript. All authors have read and approved the final manuscript.
SupplementalFig1.pdf
Download PDF (138 KB)Acknowledgments
We thank Yumiko Morita for providing single-stranded RNAs and for valuable help with the experiments. We also thank Tomoumi Nagai for performing the storage stability tests and Nozomi Hayashida for help with cell culture experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Chamberlin MJ, Patterson DL. Physical and chemical characterization of the ordered complexes formed between polyinosinic acid, polycytidylic acid and their deoxyribo-analogues. J Mol Biol. 1965;12:410–428.
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7(2):131–137.
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75–86.
- Watanabe A, Tatematsu M, Saeki K, et al Raftlin is involved in the nucleocapture complex to induce poly(I:C)-mediated TLR3 activation. J Biol Chem. 2011;286(12):10702–10711.
- Kato H, Takeuchi O, Sato S, et al Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105.
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–825.
- Seya T, Shime H, Takaki H, et al TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. OncoImmunol. 2012;1(6):917–923.
- Hilleman MR, Lampson GP, Tytell AA, et al. Double-stranded RNAs in relation to interferon induction and adjuvant activity. In: Beers RF, Braun W, editors. Biological effects of polynucleotides. New York: Springer-Verlag; 1971. p. 27–44.
- Hamilton LD. Immunogenic polynucleotides. In: Beers RF, Braun W, editors. Biological effects of polynucleotides. New York: Springer-Verlag; 1971. p. 107–128.
- Ichinohe T, Watanabe I, Ito S, et al Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005;79(5):2910–2919.
- Ichinohe T, Ainai A, Ami Y, et al Intranasal administration of adjuvant-combined vaccine protects monkeys from challenge with the highly pathogenic influenza A H5N1 virus. J Med Virol. 2010;82(10):1754–1761.
- Perez-Giron JV, Belicha-Villanueva A, Hassan E, et al Mucosal polyinosinic-polycytidylic acid improves protection elicited by replicating influenza vaccines via enhanced dendritic cell function and T cell immunity. J Immunol. 2014;193(3):1324–1332.
- Phoolcharoen W, Dye JM, Kilbourne J, et al A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc Natl Acad Sci U S A. 2011;108(51):20695–20700.
- Forte G, Rega A, Morello S, et al Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol. 2012;188(11):5357–5364.
- Seya T, Azuma M, Matsumoto M. Targeting TLR3 with no RIG-I/MDA5 activation is effective in immunotherapy for cancer. Exp Op Ther Tar. 2013;17(5):533–544.
- Galli R, Paone A, Fabbri M, et al Toll-like receptor 3 (TLR3) activation induces microRNA-dependent reexpression of functional RARbeta and tumor regression. Proc Natl Acad Sci U S A. 2013;110(24):9812–9817.
- Nagato T, Lee YR, Harabuchi Y, et al Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res. 2014;20(5):1223–1234.
- Ammi R, De Waele J, Willemen Y, et al Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs. Pharmacol Ther. 2015;146:120–131.
- Forghani P, Waller EK. Poly (I:C) modulates the immunosuppressive activity of myeloid-derived suppressor cells in a murine model of breast cancer. Breast Cancer Res Treat. 2015;153(1):21–30.
- Gesuete R, Packard AE, Vartanian KB, et al Poly-ICLC preconditioning protects the blood-brain barrier against ischemic injury in vitro through type I interferon signaling. J Neurochem. 2012;123 Suppl 2:75–85.
- Wang PF, Fang H, Chen J, et al Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3. J Immunol. 2014;192(10):4783–4794.
- Harris P, Sridhar S, Peng R, et al Double-stranded RNA induces molecular and inflammatory signatures that are directly relevant to COPD. Mucosal Immunol. 2013;6(3):474–484.
- Kimura G, Ueda K, Eto S, et al Toll-like receptor 3 stimulation causes corticosteroid-refractory airway neutrophilia and hyperresponsiveness in mice. Chest. 2013;144(1):99–105.
- Qu WM, Miyazaki T, Terada M, et al A novel autoimmune pancreatitis model in MRL mice treated with polyinosinic: polycytidylicacid. Clin Exp Immunol. 2002;129(1):27–34.
- Field R, Campion S, Warren C, et al Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun. 2010;24(6):996–1007.
- Aavani T, Rana SA, Hawkes R, et al Maternal immune activation produces cerebellar hyperplasia and alterations in motor and social behaviors in male and female mice. Cerebellum. 2015;14(5):491–505.
- Ginzel M, Yu Y, Klemann C, et al The viral dsRNA analogue poly (I:C) induces necrotizing enterocolitis in neonatal mice. Pediatr Res. 2016;79(4):596–602.
- Wright RJ, Visness CM, Calatroni A, et al Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182(1):25–33.
- Wang B, Koga K, Osuga Y, et al Toll-like receptor-3 ligation-induced indoleamine 2, 3-dioxygenase expression in human trophoblasts. Endocrinology. 2011;152(12):4984–4992.
- Dauletbaev N, Cammisano M, Herscovitch K, et al Stimulation of the RIG-I/MAVS pathway by polyinosinic: polycytidylicacid upregulates IFN-beta in airway epithelial cells with minimal costimulation of IL-8. J Immunol. 2015;195(6):2829–2841.
- Lampson GP, Field AK, Tytell AA, et al Relationship of molecular size of rIn: rCn(poly I:C) to induction of interferon and host resistance. Proc Soc Exp Biol Med. 1970;135(3):911–916.
- Machida H, Kuninaka A, Yoshino H. Relationship between the molecular size of poly I-poly C and its biological activity. Jpn J Microbiol. 1976;20(2):71–76.
- Studier FW. Sedimentation studies of the size and shape of DNA. J Mol Biol. 1965;11:373–390.
- Yano J, Oki T Nucleic acid derivative. JP 63-146233A1. 1988.
- de Clercq E. Degradation of poly(inosinic acid) - poly(cytidylic acid) [(I)n - (C)n] by human plasma. Eur J Biochem. 1979;93(1):165–172.
- Liu L, Botos I, Wang Y, et al Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320(5874):379–381.
- Luo J, Obmolova G, Malia TJ, et al Lateral clustering of TLR3: dsRNAsignaling units revealed by TLR3ecd:3Fabs quaternary structure. J Mol Biol. 2012;421(1):112–124.
- Kato H, Takeuchi O, Mikamo-Satoh E, et al Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205(7):1601–1610.
- Basilio C, Ochoa S. Enzymatic synthesis of polyribonucleotides. Methods Enzymol. 1963;6:713–718.
- Gillam S, Smith M. Use of E. coli polynucleotide phosphorylase for the synthesis of oligodeoxyribonucleotides of defined sequence. Methods Enzymol. 1980;65(1):687–701.
- Field AK, Tytell AA, Lampson GP, et al Inducers of interferon and host resistance, II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967;58:1004–1010.
- Torrence PF. Preparation of a synthetic polynucleotide interferon inducer. Methods Enzymol. 1981;78(Pt A):326–331.
- WHO guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines. WHO expert committee on biological standardization. Sixty-fourth report. Geneva: World Health Organization; 2014. p. 59–100.
- Kato Y, Yamasaki Y, Onaka A, et al Separation of DNA restriction fragments by high-performance ion-exchange chromatography on a non-porous ion exchanger. J Chromatogr. 1989;478:264–268.
- Michelson AM, Massoulié J, Guschlbauer W. Synthetic polynucleotides. Prog Nucleic Acid Res Mol Biol. 1967;6:83–141.
- Bloomfield VA, Crothers DM, Ignacio Tinoco J. Electonic and vibrational spectroscopy. Nucleic acids: structures, properties, and functions. Sausalito: University Science Books; 2000. p. 165–222.
- Billiau A, Edy VG, Heremans H, et al Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977;12(1):11–15.
- Li Y, Breaker RR. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2ʹ-hydroxyl group. J Am Chem Soc. 1999;121(23):5364–5372.
- Sarkar PK, Yang JT. Optical activity and the conformation of polyinosinic acid and several other polynucleotide complexes. Biochemistry. 1965;4(7):1238–1244.
- de Clercq E. Synthetic interferon inducers. Top Curr Chem. 1974;52:173–208.
- Machida H, Kuninaka A, Yoshino H. Effect of nucleosides on interferon production and development of antiviral state induced by poly I.poly C. Microbiol Immunol. 1979;23(7):643–650.
- Merigan TC, De Clercq E, Eckstain F, et al. Molecular requirements for synthetic RNA to act in interferon stimulation. In: Beer RFJ, Braun W, editors. Biological effects of polynucleotides. New York, NY: Springer; 1971. p. 67–78.
- Wolfenden R. Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes. Annu Rev Biochem. 2011;80:645–667.
- Okahira S, Nishikawa F, Nishikawa S, et al Interferon-β induction through toll-like receptor 3 depends on double-stranded RNA structure. DNA Cell Biol. 2005;24(10):614–623.
