ABSTRACT
Aurea Helianthus (AH), also known as wild confederate rose or golden sunflower, is a curative herb. It has been used as a medicinal material in China due to its anti-inflammatory, immune regulatory, and anti-oxidant activities. However, its melanogenic effect on skin has not been sufficiently investigated. In this study, we tested whether AH has melanogenic inhibitory activities for the development of effective skin whitening agent. The extract showed inhibition of melanin synthesis and reduced the oxidation of 3, 4-dihydroxyphenilalanine (DOPA) to o-dopaquinone. Additionally, AH downregulated the levels of microphthalmia-associated transcription factor (MITF), tyrosinase and tyrosinase related proteins (TRPs), suggesting that AH has inhibitory effects on melanogenesis. Analysis of the components of AH showed that it contained paprazine and trans-N-feruloyltyramine (FA). We confirmed that the effect of AH resulted from paprazine and FA. Therefore, AH might have potential as an effective candidate for skin whitening.
Graphical Abstract
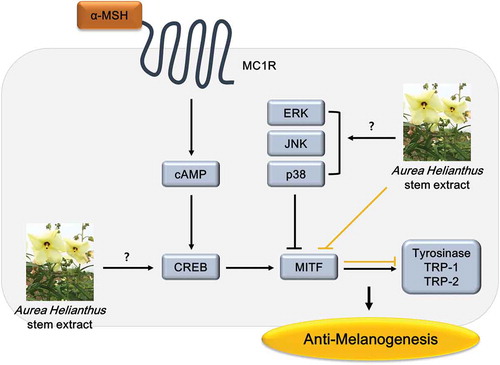
Aurea Helianthus extract inhibits cellular melanin production by downregulating MITF level and futher reducing tyrosinase, TRP-1 and TRP-2.
The color of the skin, hair and eyes of mammals depends on the amount of melanins produced by melanocytes under the skin. Melanin plays a major physiological defense role against solar irradiation. However, repeated exposure to ultraviolet radiation and ageing, hypersynthesis of melanin can induce pigmented spots such as melasma and freckles [Citation1,Citation2].
Melanins are synthesized within membrane-bound organelles termed melanosomes containing three major pigment enzymes: tyrosinase, TRP-1 which is, the most abundant glycoprotein in melanocytes, and dopachrome tautomerase (DCT), also known as TRP-2 [Citation1,Citation3]. Melanins are produced by tyrosinase and two TRPs through a series of metabolism [Citation4,Citation5]. Melanins can be produced in two distinct types: eumelanin and pheomelanin. Eumelanins are dark colored (black-to-brown) and highly polymerized while pheomelanins are yellow-to-reddish-brown and less polymerized [Citation3]. It has been reported that tyrosinase is needed for the synthesis of both types of melanins while TRP-1 or TRP-2 is needed to produce eumelanins.
Tyrosinase catalyzes the tyrosine to DOPA, followed by production of DOPAquinone. TRP-2 stimulates the rearrangement of dopachrome to form 5, 6-dihydroxyindole-2-carboxylic acid (DHICA), and TRP-1 oxidizes DHICA to produce indole-5, 6-quinon-carboxylic acid. TRP-1 and TRP-2 also function in the biosynthesis of melanin downstream of tyrosinase. The tyrosinase, TRP-1 and TRP-2 are tightly regulated by MITF. MITF is the important transcription factor in the regulation of tyrosinase gene expression and is related with the pigmentation and proliferation of melanocytes [Citation6]. A large number of effectors such as α-melanocyte-stimulating hormone (α-MSH), UV irradiation, lipopolysaccharide, cholera toxin, placental total lipid fraction, lupeol can stimulate melanogenesis [Citation7–Citation10]. When α-MSH binds to melanocortin 1 receptor (MC1R) on the cell surface, it regulates cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) to induce phosphorylation of cAMP response element binding protein (CREB) and increase MITF expression. Upregulated MITF activates tyrosinase, TRP-1 and TRP-2, and then they regulate conversion of tyrosine to dopaquinone and accumulation of melanin pigments [Citation11,Citation12]. Therefore, inhibitors of MITF or tyrosinase activity, regulators of proteins related to melanogenesis, antagonists to α-MSH and inhibitors of N-glycosylation have been continuously investigated to develop skin whitening materials [Citation13–Citation15].
Aurea Helianthus is, an annual plant. It is a rare and curative herb in the family of Malvaceae and genus of Abelmoschus. It is also called wild confederate rose or golden sunflower. It grows up to 2 m tall. Its yellow flowers can be 16–18 cm in diameter. AH flowers or leaves are rich in collagen. They have been used for the treatment of various pain, detoxification, anti-inflammation, and anti-fever effects [Citation16]. However, the whitening effect of AH has not been sufficiently investigated. Therefore, at this study, we determined the whitening potential of AH on melanin synthesis and the expression of melanogenesis related proteins.
Materials and methods
AH stem ethanol extract
Aurea Helianthus stems (100 g) were extracted in 70% (v/v) ethanol for 5h at 60°C. The ethanol extract was filtered and then evaporated under a vacuum at 60°C. After evaporation, the AH stem extract (7 g) were obtained and used for test on B16 melanoma cells.
Cell culture
B16 melanoma cells were purchased from American Type Culture Collection (USA) and cultured in Dulbecco’s modified eagle’s medium (Welgene, Korea) supplemented with antibiotics (Gibco, USA) and 10% fetal bovine serum (Gibco, USA) at 37°C with 5% CO2 atmosphere.
Measurement of melanin content
B16 cells were stimulated with α-MSH (Sigma, USA) at 100 nM and treated with various concentrations (1, 10, 25, 50, 100, 250 and 500 μg/mL) of AH for 72h. After washing with phosphate-buffered saline (PBS), cells were dissolved with 200 μL of 1 N NaOH. The absorbance was measured using an ELISA reader (Tecan, Sunrise) at 450 nm. Arbutin was used 100 μg/mL concentration as positive control.
Measurement of cellular tyrosinase activity
The L-DOPA oxidation activity of tyrosinase was estimated by spectrophotometer as previously reported [Citation17,Citation18]. B16 cells were seeded in 6 well plates and allowed to attach for 24h. Then, cells were treated with AH for 24h, washed with PBS, collected in an Ep tube. After centrifuged briefly Pro-prep protein extraction solution (iNtRON, Korea) were added to each tube. All tubes were incubated on ice, and then were centrifuged at 13,000 rpm for 5min at 4°C. The protein concentrations were determined by Qubit® Protein Assay kit (Invitrogen, USA). The supernatant containing 10 μg total protein was added to each well in a 96 well plate, and then 100 μL of 1 mg/mL L-DOPA in 0.1 M sodium phosphate buffer (pH 6.8) was mixed with protein. The mixture incubated at 37°C for 2h. The dopachrome formation from the reaction mixture was measured as the increase of absorbance at 475 nm.
HPLC analysis of AH stem ethanol extract
Chromatographic analysis of AH stem extract was performed using HPLC utilizing a Shiseido Capcell pak 4.6 X 250 mm C18 column at a flow rate 1.0 mL/min. An elution system was consisted of 20% acetonitrile and 0.1% trifluoroacetic acid in water. And the injection volume was 20 µL and PDA analysis was performed at 280 nm.
Cell viability assay
Cell viabilities were determined by 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma, USA) assay. Briefly, 2.5 mg/mL MTT in PBS was added to wells to 24 well or 6 well plates and incubated at 37°C for 4h, followed by the additional of DMSO (Duksan, Korea). The absorbance was measured at 570 nm. Absorbance readings were subtracted from the value of blank wells. Cell viabilities were calculated as a percentage of control absorbance.
Extraction of total RNA and cdna synthesis
Total RNA was extracted by using TriZol (Invitrogen, USA) following the manufacturer’s protocol. RNA concentrations were determined using Qubit® RNA BR Assay kit (Invitrogen, USA). High Capacity RNA to cDNA kit (Applied Biosystems, USA) was then used for cDNA synthesis following the manufacturer’s protocol. The cDNA was stored at −20°C until use.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Using Power SYBR Green PCR Mix (Applied Biosystems, USA) following the manufacturer’s protocol, qRT-PCR reactions were performed in triplicates. Primers for the amplification of MITF (F: 5ʹ-CAGGCTAGAGCGCATGGACT-3ʹ, R: 5ʹ-CGATTCACCAGATCAGGCG-3ʹ), tyrosinase (F: 5ʹ-TATAAACTCAGTGTTTCCCTTTATCACAA-3ʹ, R: 5ʹ-TCTATGATGACATGAAGTGGCAAA-3ʹ), TRP-1 (F: 5ʹ-GTTCAATGGCCAGGTCAGGA-3ʹ, R: 5ʹ-CAACGCAGCCACTACAGCA-3ʹ), TRP-2 (F: 5ʹ-TGGCTCACTCCTTCCTGAATG-3ʹ, R: 5ʹ-CAAACACAGGGTCGTTGGCT-3ʹ) and GAPDH (F: 5ʹ-GGCATCTTGGGCTACACTGAG-3ʹ, R: 5ʹ-GGAAGAGTGGGAGTTGCTGTTG-3ʹ) were purchased from Cosmogene Tech. Mouse GAPDH was used as an endogenous control gene. qRT-PCR reactions were performed on a 7300 Real Time PCR System (Applied Biosystems, USA). The mRNA expression levels of MITF, tyrosinase, TRP-1 and TRP-2 were evaluated relative to the levels of GAPDH, and α-MSH was considered as 100%.
Immunoblot analysis
Cells were lysed with Pro-prep protein extraction solution (iNtRON, Korea). After incubation, cell lysates were centrifuged at 13,000 rpm for 5min at 4°C, and the supernatants were collected into fresh tubes. For immunoblot analyses, proteins were separated on 10% Bis–Tris gels and transferred to nitrocellulose membranes (Invitrogen, USA). The membranes were incubated with MITF, tyrosinase, TRP-1, TRP-2, and GAPDH antibodies (Santa Cruz, USA) at 4°C overnight. After washing, the membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (Bio-rad, USA) at room temperature for 1h. Blots were developed by using Westsave Gold (Abfrontier, Korea) according to the manufacturer’s instructions. Protein levels were analyzed using Luminograph II and CSAnalyzer4 program (Atto, Japan).
Results
Cytotoxicity of AH extract in B16 melanoma cells
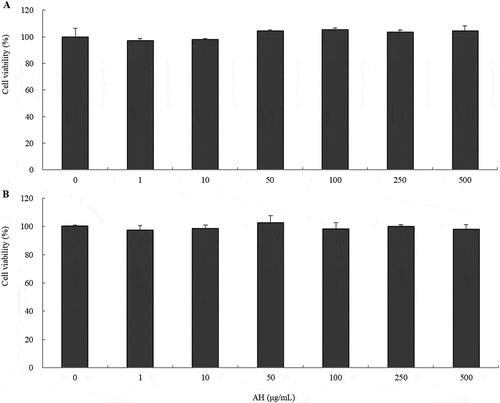
To determine whether AH exerted cytotoxic effects on B16 melanoma cells, MTT assay was performed first. The viability of B16 cells treated with AH at concentrations between 0 and 500 μg/mL was measured at 24h or 72h after exposure. Cells were approximately viable 100% compared to the control after treatment with all concentrations of AH ().
Effects of AH extract on cellular melanin synthesis
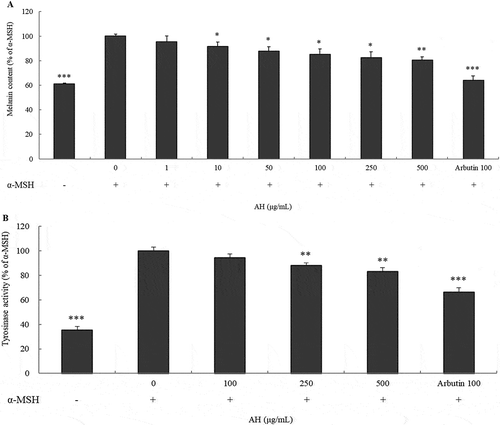
Tyrosinase is a multifunctional, copper-containing enzyme. This enzyme mediates L-tyrosine to L-DOPA and L-DOPA to o-dopaquinone. And it has known as a key enzyme in melanin biosynthesis [Citation19]. Accordingly, inhibition of cellular tyrosinase activity and melanin production would be effective measurement for investigating the skin whitening agent. To examine AH affects to melanogenesis, we measured cellular melanin content and tyrosinase activity. As shown in , after treatment with AH cellular melanin content was decreased by approximately 20% in B16 melanoma cells upon α-MSH stimulus (p < 0.01). AH reduced the conversion of L-DOPA to dopachrome in a dose-dependent manner (panel B in ). These findings suggested that AH showed a potential effect on anti-melanogenesis by inhibition of melanin synthesis.
Figure 2. Inhibitory effect on melanogenic activity of AH.
Cellular melanogenesis was performed after incubation of B16 cells treated with α-MSH and AH for 24h. Absorbance was measured at 475 nm (a). Melanin formation in cells treated with AH for 72h was measured by using ELISA (b). The cells treated with α-MSH were considered as 100%. Results represent the mean ± S.D. of triplicate independent experiments. *: p < 0.05, **: p < 0.01, ***: p < 0.001.
Effects of AH extract on mrna and protein levels of melanogenesis related proteins
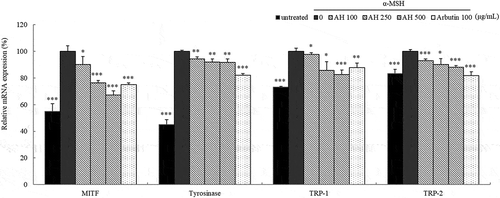
To determine whether AH affected mRNA expression of melanogenesis related proteins, B16 melanoma cells were exposed to AH at concentrations of 100, 250, 500 μg/mL for 24h and the mRNA expression levels were analyzed by qRT-PCR. Results are shown in . Treatment with AH significantly reduced the mRNA expression level of MITF, TRP-1 and TRP-2 compared to their levels in B16 melanoma cells treated with α-MSH only. And tyrosinase was slightly decreased after exposure.
Figure 3. Effect of AH on mRNA expression of MITF, tyrosinase, TRP-1 and TRP-2.
Total RNAs extracted from B16 melanoma cells treated with AH for 24h were subjected to qRT-PCR. The mRNA expression levels of MITF, tyrosinase, TRP-1 and TRP-2 were evaluated relative to the levels of GAPDH. The mRNA expression levels in cells stimulated with α-MSH were considered as 100%. Values represent the mean ± S.D. of three independent measurements. *: p < 0.05, **: p < 0.01, ***: p < 0.001.
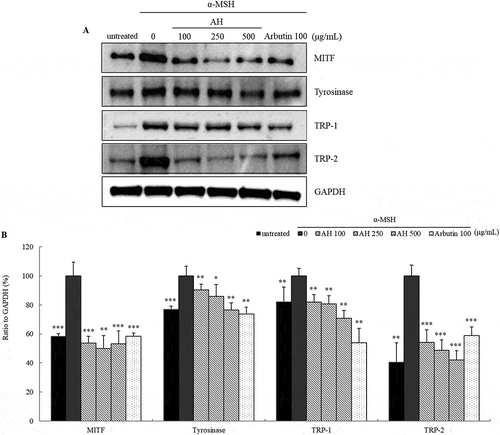
We also performed immunoblotting to examine the protein levels of MITF, tyrosinase, TRP-1 and TRP-2. Results are shown in panel A in . MITF was significantly decreased by 50.1% compared to α-MSH (p < 0.01). Tyrosinase, TRP-1 and TRP-2 protein levels were quite reduced by 76.4%, 72.3% and 42.2%, respectively, in a dose-dependent manner (panel B in ). These results suggested that AH might inhibit MITF and therefore, regulate the levels of tyrosinase, TRP-1 and TRP-2.
Figure 4. Effect of AH on the expression of melanogenesis related proteins. MITF, tyrosinase, TRP-1 and TRP-2 protein expression levels in B16 melanoma cells treated with AH for 72h were analyzed by immunoblotting (a). Relative protein expression levels were normalized to GAPDH levels (b). Total proteins extracted from cells stimulated with α-MSH were considered as 100%. Values are mean ± S.D. of triplicate experiments. *: p < 0.05, **: p < 0.01, ***: p < 0.001.
Inhibition of paprazine and FA on cellular melanin production
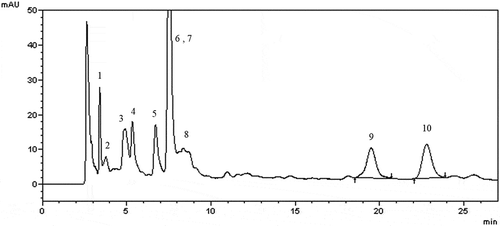
To investigate active ingredients contained in AH stem extract, we performed HPLC analysis (, Fig. S1). The AH was fractionated by solvent polarity, hexane, ethyl acetate, butanol and water to separate the components of the AH. Among them, ethyl acetate fraction was obtained by concentration gradient elution of chloroform/methanol mixture. And 10 compounds identified by NMR spectroscopy as (1) p-hydroxybenzoic acid, (2) β-hydroxypropiovanillone, (3) 3-hydroxy-4-methoxybenzoic acid, (4) vanillic acid, (5) p-hydroxybenzaldehyde, (6) vanillin, (7) p-coumaric acid, (8) scopoletin, (9) paprazine and (10) FA. Most of the separated materials were of the benzoic acid derivatives. Accurate analysis of these two substances was done by comparing HPLC peak with standard materials of paprazine and FA (Chengdu biopurify, China), and we were interested in these two compounds (Fig. S1). AH contained with paprazine (0.00758 mg/g) and FA (0.09637 mg/g). The standard materials of paprazine and FA were diluted to be used in following tests. It has been known that FA had inhibitory activity against tyrosinase, melanin production and inflammation [Citation20,Citation21]. FA contained in AH was lower concentration than using at previous study and was not sufficiently examined against the signaling pathways in the inhibition of melanin synthesis [Citation20]. In addition, the effect of paprazine, the other ingredient contained in AH, on melanogenesis has not been elucidated. Therefore, we investigated effective activity of paprazine and FA through melanogenesis related proteins.
Figure 5. HPLC analysis of AH stem ethanol extract.
The injection volume was 20 µL and PDA analysis was performed at 280 nm. The 10 compounds were separated through chromatographic purification and identified as (1) p-hydroxybenzoic acid, (2) β-hydroxypropiovanillone, (3) 3-hydroxy-4-methoxybenzoic acid, (4) Vanillic acid, (5) p-hydroxybenzaldehyde, (6) Vanillin, (7) p-Coumaric acid, (8) Scopoletin, (9) paprazine and (10) FA.
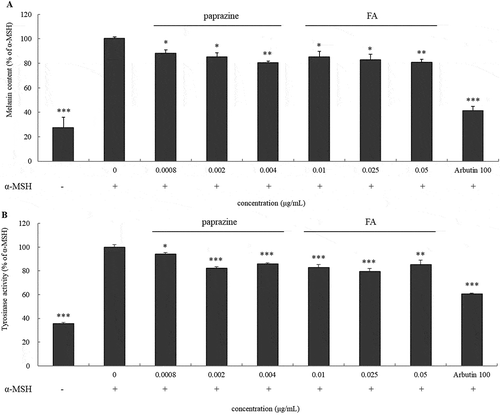
shows melanin content from B16 cells treated with paprazine or FA following concentrations contained in AH, and these compounds did not show cytotoxicity in the above range (data not shown). Both paprazine and FA diminished the melanin content by approximately 20% and reduced the conversion of L-DOPA to dopachrome (). These results indicated that these two ingredients exhibited a potent inhibitory effect against melanogenesis.
Figure 6. Inhibitory effect of paprazine and FA on melanin formation.
Cellular melanogenesis in B16 melanoma cells treated with paprazine or FA for 24h was analyzed by measurement of L-DOPA oxidation (a). Melanin content in cells treated with paprazine or FA for 72h was performed (b). The cells treated with α-MSH were considered as 100%. Values are mean ± S.D. of triplicate independent experiments. *: P < 0.05; **: P < 0.01, ***: p < 0.001.
Effects of paprazine and FA on mrna and protein levels of melanogenesis related proteins
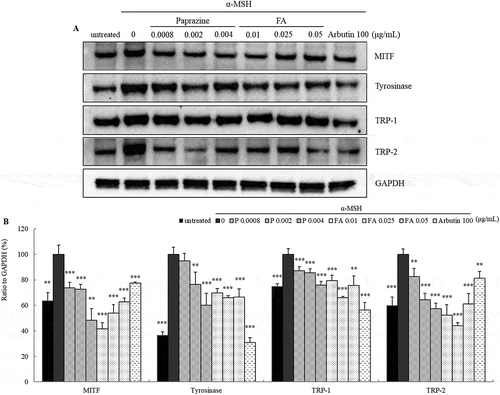
To examine whether the inhibition of melanogenesis by paprazine and FA is associated with changes of melanogenesis related proteins levels, B16 melanoma cells were exposed to paprazine or FA at concentrations contained in AH. The mRNA and protein levels of melanogenesis related proteins were measured by qRT-PCR () and immunoblotting (panel A in ). Treatment with paprazine effectively reduced the mRNA expression level of TRP-1 and decreased MITF and tyrosinase. However, TRP-2 was not affected by treatment with paprazine. FA decreased the mRNA levels of tyrosinase, TRP-1 and TRP-2. MITF was decreased only at 0.05 μg/mL. MITF, tyrosinase, TRP-1 and TRP-2 protein expression were significantly reduced by 48.2%, 60.1%, 75.8% and 57.3%, respectively, after exposure to paprazine. Also, FA effectively decreased MITF, tyrosinase, TRP-1 and TRP-2 protein levels by 41.7%, 65.9%, 65.9% and 44.0%, respectively (panel B in ). These findings indicated that the depigmentation effects of AH stem extract might be the result of paprazine and FA.
Figure 7. Effect of paprazine and FA on mRNA expression of melanogenesis related proteins.
Total RNAs extracted from B16 melanoma cells treated with paprazine or FA for 24h were subjected to qRT-PCR. The mRNA expression levels of MITF, tyrosinase, TRP-1 and TRP-2 were evaluated relative to the levels of GAPDH. The mRNA expression levels in cells stimulated with α-MSH were considered as 100%. Values represent the mean ± S.D. of three independent measurements. *: p < 0.05, **: p < 0.01, ***: p < 0.001.
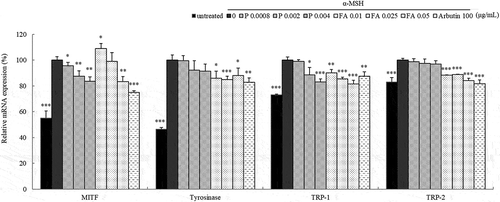
Figure 8. Inhibitory effect of paprazine and FA on the melanogenic proteins. Immunoblot was performed to investigate MITF, tyrosinase, TRP-1 and TRP-2 expression levels in B16 melanoma cells treated with paprazine or FA for 72h (a). Results were normalized to GAPDH expression (b). Total proteins extracted from cells stimulated with α-MSH were considered as 100%. Values are mean ± S.D. of triplicate independent experiments. **: p < 0.01, ***: p < 0.001.
Discussion
It has been reported that Aurea Helianthus physiological functions such as various pain-relieving, detoxification, and anti-inflammation effects [Citation16]. However, the whitening effect of AH has not been elucidated. We focused on the effect of AH on melanogenesis in this study by using measurement of α-MSH-induced melanin production and melanogenesis related proteins levels in cultured B16 melanoma cells. The α-MSH is used to induce MITF overexpression, followed by increase of tyrosinase and melanin content. AH showed an inhibitory effect on melanin formation in α-MSH-treated cells (). In addition, AH did not show cytotoxicity at all tested concentrations (1–500 μg/mL). These results indicated that AH was non-cytotoxic and the effective inhibitor of melanogenesis.
It has demonstrated that MITF increases tyrosinase synthesis and TRPs, TRP-1 and TRP-2. And melanin is produced by tyrosinase and two TRPs reaction [Citation5,Citation22]. Therefore, the components to downregulate MITF, tyrosinase and TRPs are used as ingredients of skin whitening. Our results demonstrated that AH significantly inhibited the transcriptions () and protein expressions () of MITF and its related proteins, tyrosinase, TRP-1 and TRP-2. These data suggested that the reduction of melanin content was because of downregulated MITF and its related proteins. Two components, paprazine and FA, identified by HPLC analysis of AH were tested for inhibitory effect on melanogenesis. At previous study, FA treatment decreased tyrosinase activity and melanin content, which inhibited tyrosinase protein expression [Citation20]. The depigmentation effect of paprazine, the other ingredient of AH, has not been known. Our study was confirmed that paprazine and FA inhibited melanin production (), as well as markedly reduced MITF protein expression and further downregulated tyrosinase, TRP-1 and TRP-2 (). At these results, we confirmed that the effect of AH was shown by paprazine and FA. Especially, these two components worked well even though they were much lower in concentration than arbutin.
Several reports have been demonstrated that CREB phosphorylation upregulates MITF expression, and mitogen-activated protein kinases (MAPK) signaling pathways, extracellular responsive kinase (ERK), c-Jun N-terminal kinase (JNK) and p38, inhibit melanogenesis by inducing phosphorylation of MITF [Citation23,Citation24]. Further investigations are required to focus on the mechanism regulating MITF and the inhibitory effect on melanogenesis in human melanocytes. In conclusion, paprazine and FA contained in AH were effective inhibitors of melanogenesis by downregulating expression of MITF, tyrosinase, TRP-1 and TRP-2, and then decreased melanin production. Therefore, we suggest that AH could be used as a safe and an effective whitening agent in pigmented skin.
Supplementary_materials-h.docx
Download MS Word (250.1 KB)Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea. Grant No. HN14C0088
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data can be accessed here.
Additional information
Funding
References
- Bellei B, Maresca V, Flori E, et al. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem. 2010;285:7288–7299.
- Hill HZ, Li W, Xin P, et al. Melanin: a two edged sword. Pigment Cell Res. 1997;10:158–161.
- Kondo T, Vj H. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6:97–108.
- Del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–168.
- Hearing VJ. Determination of melanin synthetic pathways. J Invest Dermatol. 2011;131:E8–E11.
- Tuerxuntayi A, Liu YQ, Tulake A, et al. Kaliziri extract upregulates tyrosinase, TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC Complement Altern Med. 2014;14:1–9.
- Abdel Malek Z, Swope VB, Suzuki I, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci U S A. 1995;92:1789–1793.
- Fuller BB, Lunsford JB, Iman DS. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J Biol Chem. 1987;262:4024–4033.
- Halaban R, Lerner AB. The dual effect of melanocyte-stimulating hormone (MSH) on the growth of cultured mouse melanoma cells. Exp Cell Res. 1977;108:111–117.
- Hornyak TJ, Hayes DJ, Ziff EB. Cell-density-dependent regulation of expression and glycosylation of dopachrome tautomerase/tyrosinase-related protein-2. J Invest Dermatol. 2000;115:106–112.
- D’Mello SA, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:E1144.
- Choi MH, Shin HJ. Anti-melanogenesis effect of Quercetin. Cosmetics. 2016;3:1–16.
- Seiberg M, Paine C, Sharlow E, et al. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interation. Exp Cell Res. 2000;254:25–32.
- Jin KS, Oh YN, Hyun SK, et al. Vitis amurensis Ruprecht root inhibited α-melanocyte stimulating hormone-induced melanogenesis in B16F10 cells. Nutr Res Pract. 2014;8:509–515.
- Negroiu G, Branza Nichita N, Petrescu AJ, et al. Protein specific N-glycosylation of tyrosinase and tyrosinase-related protein-1 in B16 mouse melanoma cells. Biochem J. 1999;344:659–665.
- Dan LU, Rui BJIA. Research progress in Chinese medicinal material Aurea Helianthus. Chin J Drug Eval. 2015;2:2095–3593.
- Tomita Y, Maeda K, Tagami H. Melanocyte-stimulating properties of arachidonic acid metabolites: possible role in postinflammatory pigmentation. Pigment Cell Res. 1992;5:357–361.
- Hosoi J, Abe E, Suda T, et al. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 α, 25-dehycroxyvitamin D3 and retinoic acid. Cancer Res. 1985;45:1474–1478.
- Sung JH, Park SH, Seo DH, et al. Antioxidative and skin-whitening effect of an aqueous extract of Salicornia herbacea. Biosci Biotechnol Biochem. 2009;73:552–556.
- Efdi M, Ohguchi K, Akao Y, et al. N-trans-feruloyltyramine as a melanin biosynthesis inhibitor. Biol Pharm Bull. 2007;30:1972–1974.
- Jiang Y, Yu L, Wang MH. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: involvement of AP-1 and MAP kinase signalling pathways. Chem Biol Interact. 2015;235:56–62.
- Tu CX, Lin M, Lu SS, et al. Curcumin inhibits melanogenesis in human melanocytes. Phytother Res. 2012;26:174–179.
- Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69.
- Ko HH, Tsai YT, Yen MH, et al. Norartocarpetin from a folk medicine Artocarpus communis plays a melanogenesis inhibitor without cytotoxicity in B16F10 cell and skin irritation in mice. BMC Complement Altern Med. 2013;13:1–12.