ABSTRACT
CCR5-mediated cytotoxicity of staphylococcal bi-component toxins was investigated using human CCR5-expressing CHO cells. Cytotoxicity of rim domain loop-exchange mutants between LukE and Hlg2 indicated that loop-4 of LukE is essential for cytotoxicity in combination with LukD. Interestingly, Hlg2 showed LukF-dependent CCR5-mediated cytotoxicity, suggesting that the F-components of toxins also play a role in the cell-specific cytotoxicity.
KEYWORDS:
Staphylococcus aureus produces several types of bi-component β-barrel pore-forming toxins (β-PFTs), which requires two components for pore formation. The β-PFTs leukocidin (Luk) and γ-hemolysin (Hlg) share an F-component (LukF), and show leukocytolytic and hemolytic activity in combination with their specific S-components, LukS and Hlg2, respectively, suggesting the importance of S-components in cell specificity. Almost all S. aureus isolates harbor a gene cluster for Hlg/Luk [Citation1]. In addition, several leukocidin variants, Panton-Valentine leukocidin (PVL; LukF-PV and LukS-PV) [Citation2] and LukED [LukD (F-component) and LukE (S-component)] [Citation3,Citation4] have been identified. Of these, Hlg and LukED show hemolytic activity in human erythrocytes, whereas other leukocidins do not cause hemolysis.
The F- and S-components of Hlg/Luk are secreted as water-soluble monomers with similar β-strand–rich structures. After binding to the surface of target cells, each component aligns alternately and forms an octameric beta-barrel pore [Citation1]. Leukocidin and its variants are considered to function via the same mechanism. At the binding step, the interaction between the rim domain of the toxin components and the target cell surface is essential, and a phosphatidylcholine (PC)-binding site in the rim domain of LukF and other F-components is involved in interacting with the erythrocyte surface and α-hemolysin [Citation1]. PC-binding site of LukF consists of W177, E182, and R198, and these residues directly interact with the choline head of PC or sphingomyelin in the plasma membrane [Citation5–Citation7].
Conversely, the loop structures in the rim domain of S-components have been investigated as candidate factor(s) that interact with target cell, because S-components lack the PC-binding site. Four loop structures have different amino acid sequences in the rim domain of Hlg2 and LukE, and these loops are involved in erythrocyte binding ( and Figure S1) [Citation8]. Hlg2 and LukE have been suggested to recognize the same receptor on the human erythrocytes via a different site of Duffy Antigen Receptor for Chemokines (DARC) [Citation9]. Moreover, Hlg2 and LukE also share the CXC chemokine receptor 1 (CXCR1) and CXCR 2 on neutrophils [Citation10,Citation11], suggesting that the loops of Hlg2 and LukE have the ability to recognize multiple different receptor molecules belonging to G protein-coupled receptor family. Thus, the interaction of various combinations of S-component loops with extra-cellular loops of receptor proteins on the target cell membrane is a topic of interest in studies investigating the cell recognition mechanism of S-component.
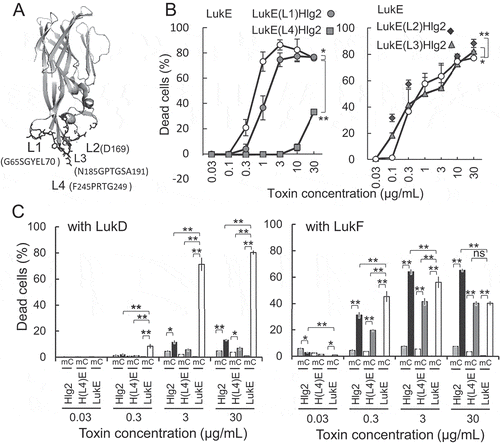
Figure 1. Effect of S-component loop-replacement mutation on cytotoxicity in human CCR5-expressing CHO cells.
a) Side view of the LukE monomer (pdb:3ROH). Positions of rim domain loops are depicted. b) Cytotoxicity of LukE mutants with loops substituted with the corresponding loops of Hlg2. Cytotoxicity in human CCR5-expressing CHO cells was measured after 1-h incubation with various concentrations of both LukE mutants and LukD (in the ratio 1:1) as indicated in the figure. c) Cytotoxicity of S-components and Hlg2 Hlg2 loop4 mutant substituted with LukE loop4 was measured in combination with LukD and LukF. Bars indicate SD, with n = 3. Statistical significance is displayed as ns (not significant), *p < 0.05, and **p < 0.01 using two-way ANOVA with Bonferroni post hoc test correction for multiple comparison or Student t test where appropriate. Statistical analyses were performed using Excel 2011 (Microsoft, Redmond, WA) with the add-in software Statcel 4 (OMS publishing, Tokorozawa, Japan). Abbreviations: m, mock/CHO-K1 cells; C, hCCR5/CHO-K1 cells; H(L4)E, Hlg2(L4)LukE.
However, only LukED is known to show lymphocyte cytotoxicity, and a C-C chemokine receptor type 5 (CCR5) has been identified as the human T lymphocytes receptor of LukE [Citation12,Citation13]. On the other hand, CCR5 has not been reported as a receptor for Hlg2. Therefore, in this study, we investigated whether the rim domain loops of LukE and Hlg2 are involved in CCR5-mediated cytotoxicity.
We previously showed that the rim loops of Hlg2 and LukE, especially loop-4, play an important role in erythrocyte binding, and loop-1 and -2 of both the components assist the binding of these components [Citation9]. At that time, we constructed rim domain loop exchange mutants between LukE and Hlg2 ( and Figure S1), according to previously described methods [Citation9]. Briefly, mutations were introduced in LukE and Hlg2 expression plasmids using the PrimeSTAR® mutagenesis basal kit (TaKaRaBio, Shiga, Japan) and the recombinant proteins with N-terminus His6-tags were expressed in Escherichia coli BL21(DE3) cells. Toxins were purified using a His-Trap HP column (GE Healthcare Biosciences AB, Uppsala, Sweden), and their secondary structures were confirmed by CD spectrum analyses using Jasco J-720W1 Spectropolarimeter (JASCO, Tokyo, Japan).
CCR5 is also one of the co-receptors involved in human immunodeficiency virus (HIV)-1 infection [Citation14]. Interaction between HIV-1 glycoprotein gp120 and the host cell co-receptor is one of the targets for anti-viral drugs development, and a drug-screening system have been designed accordingly [Citation15]. Thus, we tried to use human CCR5-expressing Chinese hamster ovary-K1 (hCCR5/CHO-K1) cell system according to the method described by Maeda et al [Citation15] to assess the CCR5-mediated cytotoxicity of a series of rim loop exchange mutants between LukE and Hlg2 described above. The cells (5 × 104 cells/well) were inoculated in Ham’s F-12 medium (Wako Pure Chemical Ind., Ltd., Osaka, Japan) supplemented with 10% fetal calf serum (FCS; HyClone, Logan, UT), and 100 U/mL penicillin and 100 µg/mL streptomycin in the presence of 5 µg/mL blasticidin S hydrochloride, plated in a 96-well black plate, and incubated for 18 – 24h at 37°C in 5% CO2. Then, the medium was removed by decantation, 90 µL of 0.1% BSA/PBS was added to each well, and cells were incubated for 10 min at 37°C in 5% CO2. The S-components and their mutants were mixed with same amounts of F-components in 0.1% BSA/PBS, 10 µL of this toxin mixture was added to each well, and cells are incubated at 37°C in 5% CO2 for 60 min. Cell death was measured using the CytoTox-ONETM (Promega, Fitchburg, WI) system. As expected, cell death was observed by incubation of the hCCR5/CHO-K1 cells with equal amounts of LukE and LukD, whereas neither LukE alone with the hCCR5/CHO-K1 nor LukE and LukD with mock/CHO-K1 showed cytotoxicity. Hence, we judged that this system is available to assess the effect of rim loops of LukE on the CCR5-mediated synergistic cytotoxicity of LukED.
Here, we found that substitution of loop-4 of LukE (F245PRTG249) with that of Hlg2 (V240TRHR244) [LukE(L4)Hlg2] markedly decreased its hCCR5/CHO-K1 cytotoxicity compared to wildtype LukE in combination with LukD, indicating that loop-4 of LukE is involved in the synergistic cytotoxicity of LukE and LukD (). Furthermore, substitution of LukE loop-1 (G65SGYEL70) with that of Hlg2(K65YPY68) [LukE(L1)Hlg2] showed only slight reduction of cytotoxicity, suggesting that loop-1 assists the cytotoxicity. Tam et al. [Citation16] used an in-frame deletion mutant of the amino acid segment K64GSGYE69 of the LukE rim domain and showed that this region is required for CCR5 targeting and cytotoxicity. Almost this entire segment except K64, which is conserved among the S-components, overlapped with loop-1 sequence. However, only slight reduction in cytotoxicity was observed by substitution of LukE loop-1 with Hlg2 loop-1, indicating the exchangeability of loop-1 between LukE and Hlg2 with respect to CCR5-mediated cytotoxicity. Therefore, the loss of CCR5-mediated cytotoxicity observed after deletion of loop-1 region may not indicate a direct interaction between Loop-1 and the receptor molecule. In contrast, LukE loop-2 [LukE(L2)Hlg2; D169N point mutation] and loop-3 [LukE(L3)Hlg2; N185GPTGSA191 was substituted with T183GPAA187] substitution mutations with those of Hlg2 partly increased cytotoxicity at low doses (0.03–0.1 µg/mL).
Considering the results of our previous study on hemolytic activity [Citation9], we hypothesized that LukE loop-4 is essential for both hemolysis and CCR5-mediated cytotoxicity. Therefore, we next investigated the effect of LukE loop-4 on cytotoxicity in hCCR5/CHO-K1 using an Hlg2 mutant with the LukE loop-4 segment [Hlg2(L4)LukE; V240TRHR244 was substituted with F245PRTG249] (). Unexpectedly, both Hlg2 mutant Hlg2(L4)LukE and original Hlg2 showed considerably low cytotoxicity at the concentration of 30 µg/mL in combination with LukD. However, this value was slightly higher than the cytotoxicity of LukE, Hlg2, and Hlg2(L4)LukE, in combination with LukD, in mock/CHO cells, which do not express human CCR5, suggesting that [Hlg2(L4)LukE and Hlg2 show CCR5-mediated cytotoxicity. Previously, we showed that the hemolytic activity of loop-4 exchange mutants between Hlg2 and LukE differed with the F-component present. Hemolytic activity was observed in the LukE mutant with Hlg2 loop-4 [LukE(L4)Hlg2], in combination with LukF, but not LukD [Citation9]. Therefore we investigated the CCR5-mediated cytotoxicity of Hlg2(L4)LukE in combination with LukF. Indeed, the cytotoxicity of Hlg2(L4)LukE was markedly higher in combination with LukF, than that with LukD. This activity was CCR5-dependent, because LukE, Hlg2 and Hlg2(L4)LukE did not show cytotoxicity in mock/CHO cells. Surprisingly, CCR5-dependent cytotoxicity of Hlg2 was also increased in combination with LukF, although Hlg cytotoxicity in human lymphocytes has not yet been reported. These results suggest that LukF also contributes to the CCR5-mediated cytotoxicity of Hlg2, in its original state and when its loop-4 is substituted with that of LukE.
In conclusion, LukE mutant with the loop-4 of Hlg2 [LukE(L4)Hlg2] showed reduced cytotoxicity in hCCR5/CHO cells in combination with LukD, demonstrating the importance of LukE loop-4 in CCR5-mediated cytotoxicity with the presence of LukD. In addition, not only LukED but also Hlg showed cytotoxicity in hCCR5/CHO cells. Although LukED and Hlg have different amino acids in loop-4, these S-components share the target cell receptors, such as DARC on erythrocytes, CXCRs on neutrophils, and CCR5 on lymphocytes. To understand the cell specificity of staphylococcal bi-component β-PFTs, details of the multiple interactions between S-components and their receptor molecules need to be investigated.
Moreover, CCR5-dependent cytotoxicity of Hlg2 was affected by the F-components. These results suggest differences in the interaction of the S- and F-components on the target cell surface. Furthermore, the binding ability and stability of Hlg2 and/or LukF on the surface of lymphocytes may be essential for cytotoxicity, because Hlg cytotoxicity was not reported in human lymphocytes. Although cell specificity of the staphylococcal bi-component β-PFTs has been attribute to the S-components, our findings suggest that the cell specificity of these β-PFTs occurs via a highly complicated mechanism, for instance, interaction with S- and F-components, and stability of components on the target cell membrane. Some S. aureus clinical isolates have ability to produce several sets of β-PFTs, such as Hlg, Luk, LukED and PVL, and alternate combinations of S- and F-components of these β-PFTs may affect the virulence of bacterium.
Peng_et_al_Figure_S1.ppt
Download MS Power Point (381 KB)Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here
Additional information
Funding
References
- Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003.
- Prévost G, Cribier B, Couppié P, et al Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect Immun. 1995;3:4121–4129.
- Gravet A, Colin DA, Keller D, et al Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 1998;436:202–208.
- Morinaga N, Kaihou Y, Noda M. Purification, cloning and characterization of variant LukE-LukD with strong leukocidal activity of staphylococcal bi-component leukotoxin family. Microbiol Immunol. 2003;47:81–90.
- Olson R, Nariya H, Yokota K, et al Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat Struct Biol. 1999;6:134–140.
- Yokota K, Kamio Y. Tyrosine72 residue at the bottom of rim domain in LukF crucial for the sequential binding of the staphylococcal gamma-hemolysin to human erythrocytes. Biosci Biotechnol Biochem. 2000;64:2744–2747.
- Monma N, Nguyen VT, Kaneko J, et al Essential residues, W177 and R198, of LukF for phosphatidylcholine-binding and pore-formation by staphylococcal gamma-hemolysin on human erythrocyte membranes. J Biochem. 2004;136:427–431.
- Peng Z, Takeshita M, Shibata N, et al. Rim domain loops of Staphylococcal β-pore forming bi-component toxin S-components recognize target human erythrocytes in a coordinated manner. J Biochem. 2018;164:93–102.
- Spaan AN, Reyes-Robles T, Badiou C, et al Staphylococcus aureus targets the Duffy Antigen Receptor for Chemokines (DARC) to lyse erythrocytes. Cell Host Microbe. 2015;18:363–370.
- Reyes-Robles T, Alonzo F 3rd, Kozhaya L, et al Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe. 2013;14:453–459.
- Spaan AN, Vrieling M, Wallet P, et al The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun. 2014;5:5438.
- Alonzo F 3rd, Kozhaya L, Rawlings SA, et al CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55.
- Spaan AN, Van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15:435–447.
- Deng H, Liu R, Ellmeier W, et al Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666.
- Maeda K, Yoshimura K, Shibayama S, et al Novel low molecular weight spirodiketopiperazine derivatives potently inhibit R5 HIV-1 infection through their antagonistic effects on CCR5. J Biol Chem. 2001;276:35194–35200.
- Tam K, Schultz M, Reyes-Robles T, et al Staphylococcus aureus Leukocidin LukED and HIV-1 gp120 target different sequence determinants on CCR5. MBio. 2016;7:pii:e02024–16.
