ABSTRACT
We examined whether the stepwise oral immunotherapy (OIT) for 10 days ameliorates the severity of allergy and the biomarkers in an allergy mouse model. The OIT could not protect anaphylaxis symptoms after allergen challenges but promote the production of antibodies, especially allergen-specific IgA. It was suggested that this OIT influenced the function of immuno response against the allergen.
Abbreviations: EW: egg white; IFC: intraperitoneal food challenge; IFN-γ: interferon-γ; IL: interleukin; OVA: ovalbumin; OM: ovomucoid; OFC: oral food challenge; OIT: oral immunotherapy.
OIT, which aims to induce desensitization through continuous intake of food allergens, is a novel approach for the treatment of food allergies [Citation1]. Even if low-dose OIT, the build-up stage is increased the allergen dose under the hospital [Citation2]. Epstein et al reported that quality of life in a child with food allergy when initiating OIT is significantly affected, not only by the severity of previous reactions, but also by their tolerated starting dose [Citation3]. Moreover, some children with severe egg allergy feel nauseous after consuming cookies containing even a trace amount of egg [Citation4]. Thus, in the initial build-up period of OIT, the increase of allergen intake more than necessary should be avoided. Therefore, we examined whether the stepwise OIT encompassing less amount of allergen for 10 d, which assumed the initial build-up stage of OIT, ameliorates the severity of allergy and the allergic biomarkers in an allergic mouse model.
Protocols for the care and treatment of the experimental animals conformed to the Mukogawa Women’s University guidelines for the ethical treatment of laboratory animals (No. FSN-01–2016-03-A). Animals were maintained under specific-pathogen-free conditions and housed in individual cages at 22°C with 60% humidity under a 12 h light (08:00–20:00) and dark (20:00–08:00) cycle. Egg white (EW) was diluted 3 folds in water, mixed for 1 h, filtered through gauze, and freeze-dried. Diet was used as a 20% casein diet [Citation5].
The circumstantial experimental protocol is outlined in supplementary Figure 1 (see Biosci. Biotechnol. Biochem website) and the sensitization protocol was based on the method of Maeta et al [Citation5]. Twenty female Balb/c mice were systemically sensitized to EW and aluminum hydroxide gel (ImjectTM Alum Adjuvant; Thermo Fisher Scientific Inc., Waltham, MA, USA) by intraperitoneal injections, three oral allergen gavage. Ten female Balb/c mice were not systemically sensitized. At day −3 and −1, all mice were carried out oral food challenge (OFC). Sensitized mice (n = 10 per group) were divided into two group at day −1: (1) EW-sensitized; treated with OIT (OIT group) and (2) EW-sensitized; treated with a placebo (non-OIT group), and non-sensitized mice were categorized as the non-allergy group (n = 10). The protocol of stepwise OIT was modified by previous studies [Citation6,Citation7]. The EW allergic mouse model causes decrease in rectal temperature and diarrhea with oral administration of 40 mg EW [Citation5]. Moreover, in similar ovalbumin (OVA) allergic mouse model, oral administration of 20 mg OVA induced allergic diarrhea [Citation8]. Thus, the maximum ingested amount of stepwise OIT decided less than 20 mg EW. Mice in the OIT group were orally administered 1.0 mg EW/d during days 1–2, 2.0 mg EW/d during days 3–4, 4.0 mg EW/d during days 5–6, 8.0 mg EW/d during days 7–8, and 16 mg EW/d during days 9–10 using a feeding needle (Fuchigami Co. Ltd., Kyoto, Japan). The non-allergy and non-OIT groups were orally administered saline. On day 12, the plasma samples of mice were collected from the submandibular vein under isoflurane anesthesia [Citation9]. All the mice were subjected to OFC on day 13, and the intraperitoneal food challenge (IFC) on day 14 according to the protocol explained in the following paragraphs. At the experimental end point (day 16: non-allergy group, day 19: non-OIT and OIT groups), five mice from each group were sacrificed under isoflurane anesthesia to collect plasma samples and to harvest the spleen. Plasma and spleen lymphocytes culture supernatant were stored at −40°C.
OFC and IFC were performed according to the published methods [Citation5]. OFC involved the oral challenge with 40 mg EW in 200 μL saline to all the mice that were fasted from the previous night. At the time points before and 15 min after OFC, the rectal temperature was measured using a thermometer (KN-91-AD-1687-M; Natsume Seisakusyo Co. Ltd., Tokyo, Japan). Additionally, the frequency of diarrhea in each group was recorded for 30 mins after OFC. IFC included the intraperitoneal injection of 200 μg EW in 200 μL saline to all the mice. At the time points before and 30 min after IFC, the rectal temperature was measured using a thermometer.
The spleen lymphocytes were cultured according to the protocol of Maeta et al [Citation5]. The plasma levels of OVA- and ovomucoid (OM)-specific IgE and IgA were determined by capture enzyme-linked immunosorbent assay [Citation5].
Values are presented as mean ± SE [Citation10]. Statistical differences with p < 0.05 were considered significant. Each analysis was presented in the form of tables. The GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA) was used for all the analyses.
We observed that there were no differences in the average change in rectal temperature after OFC at day −3 and −1 among the non-OIT and OIT groups {Mean ± SE (n = 10); −0.5 ± 0.2 vs. −0.6 ± 0.1}. After OFC at day 13, the change in rectal temperature and the rate of diarrhea occurrence were comparable between the non-OIT and OIT groups (). In addition, the change in rectal temperature after IFC was not significantly different between the non-OIT and OIT groups (). Therefore, it was suggested that the stepwise OIT for 10 d failed to improve the severity of allergic response. However, Leonard et al reported that stepwise OIT for 14 d, which the maximum daily amount was 50 mg EW protein/d, induced oral desensitization in allergic mouse model [Citation7]. Itoh et al reported that all of 6 subjects with egg allergy could reach one whole heated egg within only a few weeks [Citation11]. Furthermore, Garcia Rodriguez et al indicated that 20 (86.9%) of the 23 patients with egg allergy was able to ingest one whole cooked egg within a week [Citation12]. In these OIT studies, patients with egg allergy even in the initial build-up stage took in far large dose of the allergen beyond the initial threshold before the treatment. Thus, it was considered that there was need to administrate more amount of allergen above the threshold due to ameliorate the severity of allergy within short term.
Table 1. The allergic reactions after oral food challenge (OFC) and intraperitoneal food challenge (IFC).
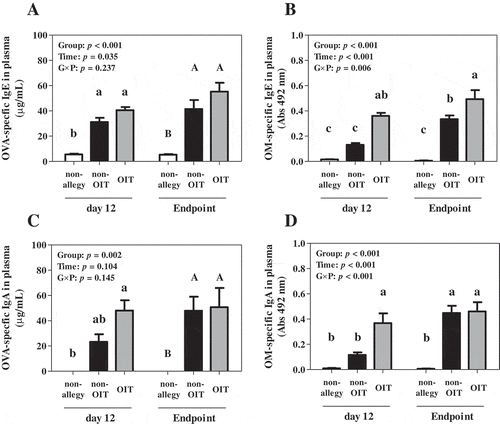
Imbalance in type 1 and type 2 helper T cells (Th1/Th2) triggers allergy [Citation13]. Th1 response drives cell-mediated immunity and releases substance such as interferon-γ (IFN-γ), while Th2 drives humoral immunity and releases substance such as interleukin-4 (IL-4) [Citation13]. The concentration of IFN-γ was lower in the OIT group than in the non-OIT group (). On the other hands, the concentrations of IL-4 were comparable in the non-OIT and OIT groups (). However, the ratio of IFN-γ/IL-4, which reflects Th1/Th2 balance, was not significantly different between the non-OIT and OIT groups (). Moreover, the concentrations of IL-10, an immune suppressor cytokine, also did not exhibit significant difference between the non-OIT and OIT groups (). IgE triggers type I allergic reaction, while IgA promotes allergen tolerance during human infancy [Citation14]. The concentrations of OVA-specific IgE were not significantly different among the non-OIT and OIT groups at day 13 and the study endpoint ()). The levels of OM-specific IgE at day 13 and the study endpoint were higher in the OIT group than in the non-OIT group ()). The concentrations of OVA-specific IgA and levels of OM-specific IgA in the OIT group at day 13 were higher than that in the non-OIT group (,). Leonard et al reported that the levels of serum OVA-specific IgE and IgA in the treated mice were higher than that in the non-treated mice [Citation7]. Moreover, in our previous study, at 2 weeks of OIT, the levels of OM-specific IgE and OVA- and OM-specific IgA in the sensitized mice fed diet supplemented with 1% EW higher than that in the sensitized mice fed control diet [Citation5]. Our hypothesis is that the induction of oral desensitization must stimulate immune cells by allergen because continuous intake of a slight amount of allergen were not effect to protect symptom induction after allergen challenge [Citation5,Citation7]. The allergen stimulation activates B cells thought helper T cells, and promotes the production of antibodies [Citation14]. Therefore, it was concluded that this stepwise OIT for 10 d in the EW allergic mouse model could stimulate the immune cells even encompassing less amount of allergen.
Table 2. The concentrations of interferon-γ (IFN-γ), interleukin-4 (IL-4), and IL-10 following in vitro stimulation of spleen lymphocytes with egg white (EW)†.
Figure 1. Plasma concentrations of ovalbumin (OVA)-specific IgE (a) and IgA (c) and levels of ovomucoid (OM)-specific IgE (b) and IgA (d) at day 12 and the study endpoint.
Note: Values are presented as the means ± SE (n = 5). The quantification limits of OVA- specific IgE and IgA were 25 and 1.0 ng/mL, respectively. The dilution of OM- specific IgE and OM-specific IgA was 1:50. The HRP substrate was used o-phenylenediamine and HRP reaction time for OM-specific IgE and IgA were 15 and 5 min, respectively. Differences were assessed using repeated two-way ANOVA followed by Bonferroni’s multiple comparison test. In the absence of interactions (G × P), different superscript letters (lower-case letters at day 12; upper-case letters at the study endpoint) indicate significant differences. When a group-time interaction was present, one-way ANOVA was conducted followed by Tukey’s multiple-comparison test among all columns, and superscript lower-case letters (a, b, c) indicate significant differences. p < 0.05 was considered significant.
In conclusion, this stepwise OIT with reduced allergen dose could not protect the anaphylaxis symptom after OFC and IFC in the EW allergic mouse model. However, we concluded that this OIT could be influenced by the function of immuno response against the allergen in the EW allergic mouse model. Moreover, it is suggested that the intake of a large amount of allergen is required to ameliorate the severity of allergy within a short term. Recently, it is reported that nutrients and food compounds such as vitamin D3 [Citation14], kakkonto [Citation15] and fructooligosaccharides [Citation16] promotes the therapeutic efficacy of allergen specific immunotherapy. Furthermore, if the short term, it is considered that the supplementation of these compounds is a low risk. Future, it needs to clearly whether the stepwise OIT encompassing less amount of allergen and adding functional ingredients ameliorates the severity of allergy and the allergic biomarkers. Finally, our studies corroborate the previous findings that the initial build-up stage of OIT with large doses of the allergens must be carefully carried out using under intensive management and supervision in a hospital.
Author contributions
AM and KT designed the study. AM, RK, MM, HO, and YN analyzed the samples. AM and KT drafted the manuscript.
Supplemental Material
Download PDF (140.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ebisawa M, Ito K, Fujisawa T, et al. Japanese pediatric guideline for food allergy 2016. Tokyo: Kyowa Kikaku Ltd. Press; 2016.
- Yanagida N, Sato S, Asaumi T, et al Safety and efficacy of low-dose oral immunotherapy for hen’s egg allergy in children. Int Arch Allergy Immunol. 2016;171:265–268.
- Epstein Rigbi N, Katz Y, Goldberg MR, et al Patient quality of life following induction of oral immunotherapy for food allergy. Pediatr Allergy Immunol. 2016;27:263–268.
- Maeta A, Muraki N, Ishibe E, et al. Survey on intake status of low-allergy egg bolo in children with egg allergy. J Jap Soc Pediatr Clin Allergy. 2018; in press (in Japanese).
- Maeta A, Matsushima M, Katahira R, et al Diets supplemented with 1% egg white induce oral desensitization and immune-tolerance in egg white specific allergic mouse model. Int Arch Allergy Immunol. 2018;176:205–214.
- Maeta A, Kaji M, Nagaishi M, et al Rush oral immunotherapy does not reduce allergic response in mice with mild allergy to egg white ovomucoid. J Nutr Sci Vitaminol. 2015;61:400–405.
- Leonard SA, Martos G, Wang W, et al Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129:1579–1587.
- Matsunaga A, Kizu K, Arita M, et al Induction of oral immune tolerance in infant mice via breastfeeding from allergic and non-allergic mothers. J Jap Soc Nutr Food Sci. 2016;69:21–28.
- Francisco CC, Howarth GS, Whittaker AL. Effects on animal wellbeing and sample quality of 2 techniques for collecting blood from the facial vein of mice. J Am Assoc Lab Anim Sci. 2015;54:76–80.
- Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. J Cell Biol. 2007;177:7–11.
- Itoh N, Itagaki Y, Kurihara K, et al Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol Int. 2010;59:43–51.
- García Rodríguez R, Urra JM, Feo-Brito F, et al Oral rush desensitization to egg: efficacy and safety. Clin Exp Allergy. 2011;41:1289–1296.
- Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 7th ed. Philadelphia (PA): Elsevier Saunders; 2011.
- Petrarca C, Clemente E, Amato V, et al Vitamin D3 improves the effects of low dose Der p 2 allergoid treatment in Der p 2 sensitized BALB/c mice. Clin Mol Allergy. 2016;14:7.
- Nagata Y, Yamamoto T, Hayashi M, et al Improvement of therapeutic efficacy of oral immunotherapy in combination with regulatory T cell-inducer kakkonto in a murine food allergy model. PLoS One. 2017;12:e0170577.
- Vonk MM, Diks MAP, Wagenaar L, et al Improved efficacy of oral immunotherapy using non-digestible oligosaccharides in a murine cow’s milk allergy model: A potential role for foxp3+ regulatory T cells. Front Immunol. 2017;8:1230.
