ABSTRACT
The present study describes the hair growth-promoting effects of sodium thiosulfate (STS), a widely used compound, in mice. STS accelerated hair growth in the “telogen model”, suggesting that it stimulates telogen hair follicles to reenter the anagen phase of hair growth. In the same model, STS potentiated hair growth in an additive manner with minoxidil (MXD), a drug used for the treatment of androgenic alopecia. Furthermore, in the “anagen model”, STS promoted hair growth, probably by promoting hair follicle proliferation. Since STS elevated the skin surface temperature, its hair growth-promoting activity may be partly due to vasorelaxation, similar to MXD. In addition, STS is known to generate a gaseous mediator, H2S, which has vasorelaxation and anti-inflammatory/anti-oxidative stress activities. Therefore, STS and/or provisionally its metabolite, H2S, may aid the hair growth process. Collectively, these results suggest that salts of thiosulfate may represent a novel and beneficial remedy for hair loss.
Graphical Abstract
We found the hair growth-promoting effects of sodium thiosulfate (STS) in mice. STS may represent a beneficial remedy for hair loss.
The dermal papilla induces new hair follicle formation and maintains hair growth. The hair follicle cycle comprises anagen (growth phase), catagen (regression phase), and telogen (resting phase) to regenerate hairs (). The blood vessels in the dermal papillae nourish the hair follicles, promoting cell division and maintaining growth of the hair follicle cells. However, when nutrient supply is insufficient, follicles remain in the telogen phase[Citation1].
Figure 1. Schematic representation of the hair cycle in C3H/He mice. (a) Phases of the hair follicle cycle. The hair cycle of mouse is determined in a strict time-dependent manner[Citation1,Citation13]. (b) Time scale of the hair cycle in normal conditions. (c) Time scale of the hair cycle after noninvasive mild clipping (arrow). (d) Time scale of the hair cycle after stimulus depilation (arrow). All the hair follicles simultaneously enter the anagen stage after depilation in a synchronized manner[Citation1,Citation13].
![Figure 1. Schematic representation of the hair cycle in C3H/He mice. (a) Phases of the hair follicle cycle. The hair cycle of mouse is determined in a strict time-dependent manner[Citation1,Citation13]. (b) Time scale of the hair cycle in normal conditions. (c) Time scale of the hair cycle after noninvasive mild clipping (arrow). (d) Time scale of the hair cycle after stimulus depilation (arrow). All the hair follicles simultaneously enter the anagen stage after depilation in a synchronized manner[Citation1,Citation13].](/cms/asset/58c182d7-4264-481f-a600-ceb8e72bf60a/tbbb_a_1518705_f0001_b.gif)
It is known that hair loss is partly caused by vasoconstriction. Minoxidil (MXD) promotes hair growth via its activity as a vasodilator, and increases the cutaneous blood flow to the scalp[Citation2], thereby delivering sufficient oxygen and nutrients to the hair follicle[Citation3]. Hair loss is associated with a number of risk factors, including elevated oxidative stress, metabolic syndrome, alcohol consumption, smoking, and ultraviolet radiation[Citation4–Citation7]. In general, antioxidants can prevent, to varying degrees, damage of healthy tissues by free radicals.
Sodium thiosulfate (STS, Na2S2O3) has been clinically used for decades for the treatment of cyanide intoxication[Citation8]. It is also known to be an effective reducing and antioxidant agent[Citation9,Citation10]. In addition, STS is known to produce a gaseous mediator, H2S, in an enzymatic reaction involving mercaptopyruvate sulfurtransferase (MPST)[Citation11]. Importantly, H2S has vasorelaxation-inducing and anti-inflammatory/antioxidative stress activities[Citation12].
Based on this, we hypothesized that STS may have beneficial effects on hair growth via its antioxidant, anti-inflammation, and/or vasorelaxation-inducing activities. In the present study, we tested this hypothesis using two mouse models, a “telogen model” and an “anagen model”, which have been widely used to evaluate the hair growth-promoting activities of various compounds.
Materials and methods
Animals
Six-week-old male C3H/HeNCrl (C3H/He) mice were obtained from the Charles River Laboratories (Kanagawa, Japan). Mice were housed in groups of five or six in standard cages in a temperature and humidity-controlled room with a 12-h light/dark cycle (lights on at 08:00), with free access to standard laboratory chow and tap water. Our experimental procedures were approved by the RIKEN Animal Ethics Committee. Some of the experiments and analyses were also conducted at Nihon Bioresearch Inc. (Gifu, Japan) (https://www.nbr.co.jp/).
Mouse models
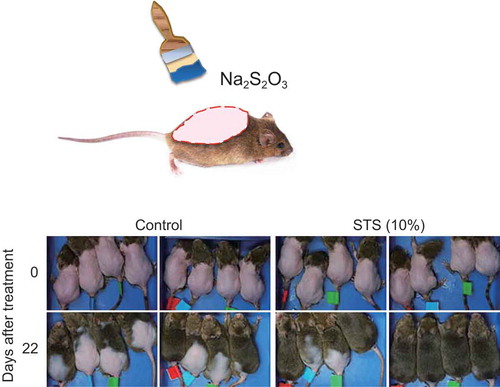
Six-week-old male C3H/He mice were allowed to adapt to their environment for one week. After entry into the second telogen phase at late six weeks of age (), we generated two mouse models at seven weeks of age. To examine the effects of reagents in the spontaneous “telogen phase”, we created a “telogen model” by performing noninvasive mild clipping (Ref). The coat hair on the dorsal skin was gently and carefully clipped using an electric shaver to avoid injury and mechanical stimulation (). The experiment was started one day after clipping.
To examine the effect of reagents in the synchronized “anagen phase”, we created an “anagen model” using stimulus depilation[Citation13]. The coat hair on the dorsal skin was shaved using razors to trigger the anagen phase of the hair cycle. The experiment was started three days after depilation ().
Reagents
STS was purchased from Sigma (St. Louis, MO, USA) and MXD was purchased from Wakojunyaku (Tokyo, Japan). For the telogen model, the reagents were dissolved in 50% ethanol [STS (1%, 2%, or 4%), MXD (1%), a mixture of MXD (1%)/STS (1%, 2%, or 4%)] or 40% ethanol [STS (10%), a mixture of MXD (1%)/STS (10%)]. For the anagen model, the reagents were diluted in 40% ethanol for STS (4% or 10%) or 60% ethanol for MXD (5%) (Supplemental Table 1).
Application of reagents to the skin
For the telogen model, the animals were randomized into the following groups: [setting-1] (1) control (50% ethanol in water), (2) MXD (1%), and (3)–(6) STS (1%, 2%, 4%, and 10%, respectively); [setting-2] (1) control (50% ethanol in water), (2) MXD (1%), (3)–(6) a mixture of MXD (1%)/STS (1%, 2%, 4%, and 10%, respectively), and (7) STS (10%). Each solution (200 μL) was applied topically to the shaved dorsal area once a day for 13–14 weeks.
For the anagen model, the animals were randomized into the following groups: (1) control (40% ethanol in water), (2) MXD (5%), (3)–(4) STS (4% and 10%, respectively), and (5) MXD (5%)/STS (10%), where STS (10%) was first applied to the skin, immediately followed by MXD (5%). Each solution was applied topically to the shaved areas once a day for 24 days (Supplemental Table 1).
Evaluation of hair growth speed
To assess the hair growth speed in each group, we observed and photographed the dorsal skin of animals once a week for the telogen model or at specific time intervals (days 1, 5, 7, 10, 13, 16, 19, 22, and 25) for the anagen model. All experiments were performed between 14:00 and 16:00. Photographic data were analyzed by using ImageJ software to measure the objective area of skin. To evaluate hair growth in the telogen model, we used the following scores: 0, no hair growth; 1, hair growth started (hair growth area is >10% of the shaved area). In the anagen model, we assigned a score of 0 (hair growth area is within 50%) or 1 (hair growth area is >50%).
Histological analysis
In the anagen model, skin was surgically removed from the mice after euthanizing using isoflurane anesthesia to examine the histological features at the end of the treatment period. The skin samples were fixed in phosphate-buffered 10% formalin for 24 h. The samples were embedded in paraffin blocks. Paraffin sections (4-μm thick) were processed for histological analysis. General histology was examined by hematoxylin and eosin staining. We also counted the number of hair follicles and measured the subcutis thickness.
Blood biochemical analysis
Blood samples were collected using heparin-coated syringes between 14:00 and 16:00 via intracardiac puncture from the telogen model mice after the hair growth experiment, and kept on ice for 30 min. After centrifugation, plasma was collected and divided into 200-μL aliquots and stored at −80°C until used. The plasma samples were analyzed using a FUJI DRI-CHEM 3500V (FUJIFILM, Tokyo, Japan) automated clinical chemistry analyzer and FUJI DRI-CHEM slides.
Body surface and rectal temperatures
To measure the body surface temperature, thermal images using near infrared light irradiation were captured by thermography (Thermoshot F30S, NEC/Avio, Tokyo, Japan). The thermal distribution was analyzed using commercial software (InfRec Analyzer NS9500 Standard, NEC/Avio, Tokyo, Japan). Rectal temperature was monitored over a period of 1 min using a thermometer (KN-91, Natsume Seisakusho, Tokyo, Japan).
Sampling and fixation of human scalp hair follicles
Hair follicles were plucked from the scalp of one participant using forceps. The hairs were checked for the presence of a sheath. After a brief rinse in phosphate-buffered saline (PBS), the hairs were trimmed to approximately 1.5 cm in length, containing the bulb region, and placed in a 1.5-mL microfuge tube (BM Equipment, Tokyo, Japan) containing 1 mL of 10% neutral-buffered formalin (4°C, 1 h). The fixed hairs were preembedded in 4% agarose (Sigma-Aldrich, MO, USA) in PBS, pH 7.4. At this point, it was possible to orientate the hairs into their desired position for sectioning (longitudinally or transversely). The blocks were embedded in capsules filled with O.C.T. compound (Sakura Finetek, Tokyo, Japan) and stored at −80 °C until used for immunohistochemistry[Citation14]. The human study conformed to the principles set out in the World Medical Association’s Declaration of Helsinki and the National Institutes of Health Belmont Report, and was approved by the RIKEN ethics committees. The participant gave informed written consent to participate in the study.
Immunohistochemistry of human hair follicles
After rinsing in PBS, 8-μm thick cryostat sections of the plucked hairs were processed for immunohistochemistry. The sections were blocked using 10% goat serum in 0.05 M Tris-buffered saline plus 0.05% Tween 20 (TBS-T), followed by three rinses in TBS-T (20 min each). The sections were incubated in primary antibodies overnight at 4°C. After three washes in TBS-T (20 min each), the samples were incubated in secondary antibodies for 1 h at room temperature. Nuclei were counterstained using 4ʹ,6-diamidino-2-phenylindole (DAPI), added to the secondary antibodies. After washing in TBS-T, slides were mounted in PermaFluor Aqueous Mounting Medium (Thermo Fisher Scientific, MA, USA). Fluorescent signals were detected using a confocal laser-scanning microscope FV1000 (Olympus, Tokyo, Japan)[Citation14].
Antibodies
Primary polyclonal guinea pig antibodies were used for K14 (PROGEN Biotechnik GmbH, Heidelberg, Germany; 1:100 dilution), K71 (PROGEN; 1:100 dilution), and K85 (hHb5) (PROGEN; 1:200 dilution). Primary polyclonal rabbit antibody was used for MPST detection (Santa Cruz Biotechnology, TX, USA; 1:100 dilution). For the secondary antibody, Alexa Fluor 488 or 594-goat anti-rabbit IgG or goat anti-guinea pig IgG (Thermo Fisher Scientific; 1:400 dilution) was used.
Statistical analyses
All statistical analyses were carried out using Prism 6 software (GraphPad Software, San Diego, CA, USA). The hair growth area and the body surface temperature were evaluated by two-way repeated measures ANOVA followed by Tukey’s multiple comparison post hoc test. The time taken for hair growth to start or the hair growth area to reach more than 50% of the shaved area, was evaluated using Kaplan–Meier survival analysis and log-rank test. Differences in continuous variables (histological parameters, rectal temperature, and blood biochemical data) were evaluated by one-way ANOVA followed by Dunnett’s multiple comparison test as a post hoc test. P < 0.05 was considered significant.
Results
Effect of STS treatment in the telogen model
To examine the efficacy of STS in the telogen phase of the hair follicle cell cycle, mice were treated with one of following solutions: control (vehicle: 50% ethanol in water), MXD (1%), and STS (1%, 2%, 4%, or 10%). We calculated the percentage hair growth area per shaved area, and found a significant effect of treatment [two-way (treatment × time) repeated measures ANOVA, F(5, 25) = 5.552, P = 0.001] (). Notable hair growth promotion was seen in the STS (10%) group following 11-week treatment [P < 0.0007 compared with the control or MXD (1%) group] and in the STS (4%) group following 12-week treatment [P < 0.0290 compared with the control or MXD (1%) group] (, Supplemental Table 2, Supplemental Figure 1).
Figure 2. Effects of STS alone or combined MXD/STS treatments in the telogen model. (a) Time course of hair growth in control, MXD (1%), and STS (1%, 2%, 4%, or 10%)-treated groups. The values represent mean ± SE (n = 5–6/group). (b) Assessment of hair growth in control, MXD (1%), and STS (1%, 2%, 4%, or 10%)-treated groups at week 14 of treatment. The values represent mean ± S.E. (n = 5–6/group). (c) Kaplan–Meier estimates from no hair growth to visible hair growth in the control, MXD (1%), STS (1%, 2%, 4%, or 10%)-treated groups. (d) Time course of hair growth in control, MXD (1%), and MXD (1%)/STS (1%, 2%, 4%, or 10%)-treated groups. The values represent mean ± S.E. (n = 5–12). (e) Assessment of hair growth in control, MXD (1%), and MXD (1%)/STS (1%, 2%, 4%, or 10%)-treated groups at week 13 of treatment. The values represent mean ± S.E. (n = 5–12). (f) Kaplan–Meier estimates from no hair growth to visible hair growth in control, MXD (1%), and MXD (1%)/STS (1%, 2%, 4%, or 10%)-treated groups. For detailed statistical information, see Supplemental Tables 1–4.
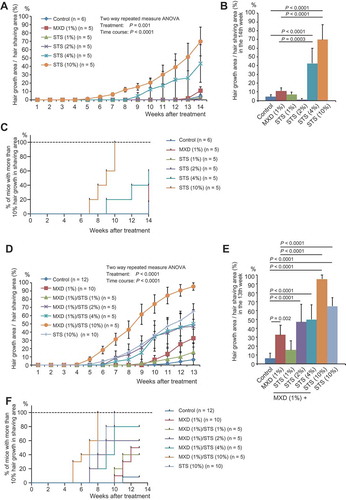
The time taken for hair growth to become visible (>10% hair growth area per shaved area) was significantly shorter in the STS (10%) group compared with both the control (P = 0.0088) and the MXD (1%) (P = 0.0216) groups (, Supplemental Table 3).
These results suggest that STS may stimulate telogen hair follicles to reenter the anagen phase.
Additive effect of STS and MXD in the telogen model
To evaluate the additive effects of MXD and STS, mice were treated with one of following solutions: control (vehicle: 50% ethanol in water), MXD (1%), STS (10%), or MXD (1%)/STS (1%, 2%, 4%, or 10%). We observed a significant treatment effect [two-way (treatment × time) repeated measures ANOVA, F(6, 45) = 11.90, P < 0.0001] () in the percentage hair growth area per shaved area. From the seventh week of treatment, the MXD (1%)/STS (10%) group showed marked hair growth promotion (P < 0.0044) compared with the control or MXD (1%) group. The MXD (1%)/STS (2% or 4%) groups also showed remarkable hair growth from weeks 10 to 13 of treatment [P < 0.0398 compared with the control or MXD (1%) group]. In addition, the MXD (1%)/STS (10%) group showed significantly increased hair growth compared with the STS (10%) group from the eighth week of treatment (P < 0.0234) (, Supplemental Table 4, Supplemental Figure 2).
The time taken for visible hair growth (>10% hair growth area per shaved area) in the MXD (1%)/STS (2%, 4%, or 10%) groups was significantly shorter or tended to be shorter than that of the control [P = 0.0824 for MXD (1%)/STS (2%), P = 0.0056 for MXD (1%)/STS (4%), P = 0.000016 for MXD (1%)/STS (10%)]. In particular, the time taken in the MXD (1%)/STS (10%) group was shorter than that of the MXD (1%) group (P = 0.00008) (, Supplemental Table 5). These results support an additive effect of MXD and STS on hair growth in the telogen model.
Effect of STS treatment in the anagen model
To investigate the hair growth-promoting effect of STS in the anagen phase, we shaved mice in order to promote the transition from telogen to anagen phase, then treated the mice with one of the following solutions: control (vehicle: 40% ethanol in water), MXD (5%), STS (4% or 10%), and MXD (5%)/STS (10%). A marked treatment effect [two-way (treatment × time) repeated measures ANOVA, F(4, 35) = 20.94, P < 0.0001] was noted. We observed greater hair growth speed in the MXD (5%)/STS (10%) and the STS (10%) groups compared with the control group. For example, at day 16 of treatment, P < 0.0001 for MXD (5%)/STS (10%) vs. control and P < 0.0001 for STS (10%) vs. control (, Supplemental Table 6, Supplemental Figure 3).
Figure 3. Effects of STS or combined STS/MXD treatment in the anagen model. (a) Time course of hair growth in control, MXD (5%), STS (4% or 10%), and MXD (5%)/STS (10%)-treated groups. The values represent mean ± S.E. (n = 8/group). (b) Kaplan–Meier estimates from no hair growth to 50% hair growth in control, MXD (5%), STS (4% or 10%), and MXD (5%)/STS (10%)-treated groups (n = 8/group). For detailed statistical information, see Supplemental Tables 5–6.
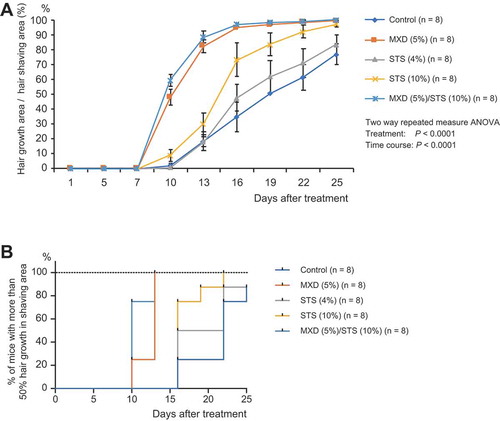
The time taken for 50% hair growth (>50% hair growth area per shaved area) in the STS-treated groups was significantly shorter or tended to be shorter than that of the control [P = 0.0714 for STS (10%), P = 0.0003 for MXD (5%)/STS (4%)] (, Supplemental Table 7).
Our results in the anagen model imply improved hair follicle proliferation using STS.
Histological examination
To elucidate the hair growth-promoting effect of STS in the anagen model, we stained skin sections with hematoxylin and eosin at the end of the treatment period and evaluated the sections using a microscope. First, the anagen ratios in all hair follicles were calculated using the formula: (number of hair follicles in the subcutis/the total number of hair follicles) × 100. As a result, the anagen ratio was significantly decreased in the STS (10%) group (P = 0.0239) and MXD (5%)/STS (10%) group (P = 0.0002) compared with that of the control, suggesting that STS treatment affected the cell cycle of the hair follicles. The thickness of the subcutis was also reduced in the MXD (5%)/STS (10%) group (P = 0.0151) compared with the control group, although there was no statistically significant difference between the STS (10%) and control groups. Follicular density was significantly decreased in the STS (4%) group (P = 0.0219) and tended to be decreased in the STS (10%) (P = 0.0814) compared with that of the control (Supplemental Table 8, Supplemental ). These results suggest that most of hair follicles in the STS-treated groups were in the telogen phase at the end of treatment period. Based on the observation that hair growth effect by STS-treatment was visually evident at day 16 (), we speculate that most of hair follicles of STS-treatment groups might already exit the anagen phase and re-enter to the telogen phase.
Body surface temperature after STS treatment
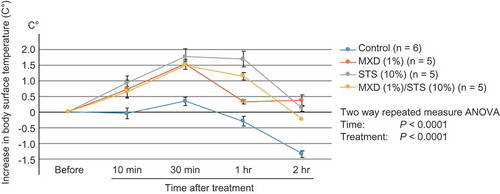
To assess whether STS, like MXD, exerts its biological activity as a vasodilator by increasing cutaneous blood flow, we measured the body surface temperature after treatment in the telogen mouse model. Treatment with STS (10%) and MXD (1%)/STS (10%) significantly increased body surface temperature compared with the control at all the time points examined. In particular, 1 h after treatment, the STS (10%) (P < 0.0001) and MXD (1%)/STS (10%) (P = 0.0025) groups elicited a greater increase in body surface temperature compared with the MXD (1%) group. These results indirectly suggest that STS (10%) treatment induces greater blood flow compared with MXD (1%) (, Supplemental Table 9, Supplemental Figure 5).
Expression pattern of MPST in human hair follicles
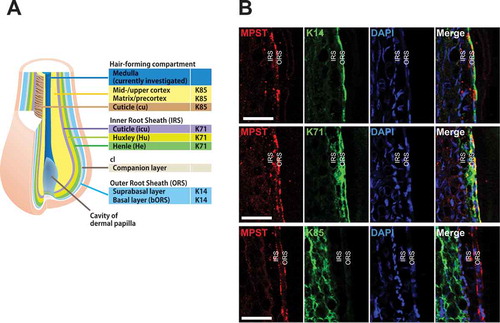
To understand the effects of STS on the mechanism of hair growth, we examined the expression pattern of MPST, an enzyme that produces H2S from thiosulfate[Citation11], in hair follicles. Scalp hair follicles consist of a central hair-forming compartment surrounded by three concentric epithelial structures: the outer root sheath (ORS), the companion layer, and the inner root sheath (IRS). Epithelial keratin, K14, is the main keratin of the ORS, while K71 is specific to the IRS. K85 is a representative marker of the hair-forming compartment ()[Citation14]. The MPST protein was detectable in K71 and K14-expressing areas, although it did not overlap with K85. These data demonstrate that MPST is expressed in the ORS and IRS cuticle (, merged green and red), and suggests that externally administered STS can be converted to H2S by MPST in hair follicles.
Figure 5. Expression patterns of MPST in human hair follicles. (a) Schematic representation of the expression patterns of epithelial/hair keratins. K71 is expressed in the three inner root sheath (IRS) layers and K14 is expressed in the outer root sheath (ORS). K85 is present in the hair-forming compartment. (b) Immunofluorescence labeling of MPST and hair keratins (K14, K71, and K85) in scalp hair follicles. K14 is uniformly expressed throughout all layers of the widely stratified follicular outer root sheath (ORS). K71 is expressed in all compartments of the hair inner root sheath (IRS). Hair keratin K85 expression is detectable from the hair matrix to the upper cortex and hair cuticle. MPST is seen in the ORS and IRS cuticle (merged green and red). Nuclei were stained with DAPI. Scale bars represent 50 μm. We found the hair growth-promoting effects of sodium thiosulfate (STS) in mice. STS may represent a beneficial remedy for hair loss.
Evaluation of adverse effects
To exclude the possibility that the external application of STS to the skin impairs systemic health, we measured the deep body temperature at week 14 in the telogen model by recording rectal temperature twice a day (at 09:00 and 15:00). No significant differences were seen between any of the treatment groups and the control group (Supplemental Table 10), thereby excluding potential noxious events, including inflammation via external STS treatments. Blood biochemistry data suggested no adverse effects of STS (Supplemental Table 11). Moreover, we found no structural abnormalities of the skin in the STS-treated groups of the anagen model by histological examination.
Discussion
In the present study, we explored the potential of STS as a hair growth agent using two mouse models: a “telogen model” and an “anagen model”. In order to develop a hair growth formula, it is preferable for the candidate to show efficacy in both models. STS treatment was able promote hair growth in both models. Importantly, the STS (4% and 10%) groups and the MXD (1%)/STS (2%, 4%, or 10%) groups were more effective in promoting hair growth compared with the MXD (1%) alone group in the telogen model. The results of the combined use of MXD and STS provided evidence for an additive effect of the two reagents.
In relation to the mechanism in which STS exerts its biological activity, STS treatment markedly shortened the time taken for hair growth start in the telogen model. MXD is thought to influence the normal hair cycle by shortening the telogen period, causing premature entry of the resting follicles into the anagen phase, thereby increasing the hair follicle size[Citation15]. In the current study, STS treatment promoted the transition from telogen to anagen phase to the same extent as (or more than) MXD-treatment. Therefore, STS treatment may also influence hair cycle like MXD, although the precise mechanism remains to be elucidated.
Regarding the molecular action, thiosulfate is a known antioxidant[Citation9,Citation10] (https://pubchem.ncbi.nlm.nih.gov/compound/Sodium_thiosulphate#section=Clinical-Trials). Several studies have reported that antioxidants may help to ameliorate hair loss by neutralizing harmful free radicals[Citation16,Citation17]. Therefore, it would be interesting to premise that the hair growth-promoting activity of STS might be mediated by its potential as an antioxidant. To test this hypothesis, further study is needed.
Interestingly, thiosulfate is also known to produce a gaseous mediator, H2S, in an enzymatic reaction involving MPST[Citation11]. In the current study, we revealed that the MPST protein is expressed in the ORS and IRS of hair follicles. H2S exerts multifaceted effects on cellular functions, including induction of vasorelaxation[Citation12], as well as suppression of oxidative stress[Citation18] and inflammation[Citation19]. In the present study, the elevated skin surface temperature in mice treated with STS was maintained longer than in the MXD-treated group. These results might reflect long-lasting H2S generation from thiosulfate via the metabolic pathway in the cells, resulting in prolonged vasorelaxation. In future study, it is important to directly address whether H2S production is involved in the action of STS. As stated earlier, MPST is expressed in the ORS, which locates at a well-known migration route of hair follicle stem cells from the bulge region to hair bulb[Citation20]. Therefore, it would be also an intriguing issue to examine whether STS could affect the conditions of hair follicle stem cells.
In conclusion, the present study provided evidence that thiosulfate may represent a promising and novel remedy to treat human alopecia without potential adverse effects. Thiosulfate can also augment the effects of MXD, which is currently the only medicinal hair tonic approved for the treatment of hair loss. Further research and clinical trials are required to elucidate the precise mechanisms of thiosulfate.
Author contributions
MM, TO and TY: conceived and designed the experiments; MM, YH, YN, HO, CS, CT and YW: performed the experiments; MM: analyzed data; MM, TO, SB and TY: contributed to manuscript writing.
180827_Hair_growth_Supple.pdf
Download PDF (4.3 MB)Acknowledgments
This study was supported by RIKEN Center for Brain Science Funds, RIKEN Industry Partnership Section Funds, and AMED under Grant Numbers JP18dm0107083 (T.Y.) and JP18dm0107129 (M.M.). We thank Dr. Soichi Kojima and Ms. Nozomi Sato of RIKEN Center for Integrative Medical Sciences for fruitful discussion.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data can be accessed here.
References
- Orasan MS, Roman II, Coneac A, et al. Hair loss and regeneration performed on animal models. Clujul Med. 2016;89(3):327–334. PubMed PMID: 27547051; PubMed Central PMCID: PMCPMC4990426. DOI:10.15386/cjmed-583.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004 Apr;50(4):541–553. PubMed PMID: 15034503.
- Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. J Drugs Dermatol. 2010 Nov;9(11):1412–1419. PubMed PMID: 21061765.
- Severi G, Sinclair R, Hopper JL, et al. Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br J Dermatol. 2003 Dec;149(6):1207–1213. PubMed PMID: 14674898.
- Su LH, Chen TH. Association of androgenetic alopecia with metabolic syndrome in men: a community-based survey. Br J Dermatol. 2010 Aug;163(2):371–377. PubMed PMID: 20426781. DOI:10.1111/j.1365-2133.2010.09816.x.
- Trueb RM. Is androgenetic alopecia a photoaggravated dermatosis? Dermatology. 2003;207(4):343–348. PubMed PMID: 14657623.
- Trueb RM. Association between smoking and hair loss: another opportunity for health education against smoking? Dermatology. 2003;206(3):189–191. PubMed PMID: 12673073.
- Singh RP, Derendorf H, Ross EA. Simulation-based sodium thiosulfate dosing strategies for the treatment of calciphylaxis. Clin J Am Soc Nephrol. 2011 May;6(5):1155–1159. PubMed PMID: 21441129; PubMed Central PMCID: PMCPMC3087783. DOI:10.2215/CJN.09671010.
- Bijarnia RK, Bachtler M, Chandak PG, et al. Sodium thiosulfate ameliorates oxidative stress and preserves renal function in hyperoxaluric rats. PLoS One. 2015;10(4):e0124881. PubMed PMID: 25928142; PubMed Central PMCID: PMCPMC4415920. DOI:10.1371/journal.pone.0124881.
- Hayden MR, Tyagi SC, Kolb L, et al. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005 Mar 18;4(4). PubMed PMID: 15777477; PubMed Central PMCID: PMCPMC1079905. DOI:10.1186/1475-2840-4-4.
- Nagahara N, Nagano M, Ito T, et al. Redox regulation of mammalian 3-mercaptopyruvate sulfurtransferase. Methods Enzymol. 2015;554:229–254. PubMed PMID: 25725525. DOI:10.1016/bs.mie.2014.11.017.
- Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002 Aug;283(2):H474–80. PubMed PMID: 12124191. DOI:10.1152/ajpheart.00013.2002.
- Muller-Rover S, Handjiski B, Van Der Veen C, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001 Jul;117(1):3–15. PubMed PMID: 11442744.
- Maekawa M, Yamada K, Toyoshima M, et al. Utility of Scalp Hair Follicles as a Novel Source of Biomarker Genes for Psychiatric Illnesses. Biol Psychiatry. 2015 Jul 15;78(2):116–125. PubMed PMID: 25444170.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004 Feb;150(2):186–194. PubMed PMID: 14996087.
- Trueb RM. Oxidative stress in ageing of hair. Int J Trichology. 2009 Jan;1(1):6–14. PubMed PMID: 20805969; PubMed Central PMCID: PMCPMC2929555. DOI:10.4103/0974-7753.51923.
- Kaya Erdogan H, Bulur I, Kocaturk E, et al. The role of oxidative stress in early-onset androgenetic alopecia. J Cosmet Dermatol. 2017 Dec;16(4):527–530. PubMed PMID: 27987270.
- Bos EM, Wang R, Snijder PM, et al. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013 Apr;24(5):759–770. PubMed PMID: 23449534; PubMed Central PMCID: PMCPMC3636788.
- Snijder PM, De Boer RA, Bos EM, et al. Gaseous hydrogen sulfide protects against myocardial ischemia-reperfusion injury in mice partially independent from hypometabolism. PLoS One. 2013;8(5):e63291. PubMed PMID: 23675473; PubMed Central PMCID: PMCPMC3651205. DOI:10.1371/journal.pone.0063291.
- Lavker RM, Sun TT, Oshima H, et al. Hair follicle stem cells. J Investig Dermatol Symp Proc. 2003 Jun;8(1):28–38. PubMed PMID: 12894992.