ABSTRACT
4-Hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF) is a key aroma compound in Fragaria × ananassa (strawberry). A considerable amount of HDMF is converted into HDMF β-D-glucoside and accumulated in mature strawberry fruits. Here we isolated a novel UDP-glucose: HDMF glucosyltransferase, UGT85K16 from Fragaria × ananassa. UGT85K16 preferentially glucosylated the hydroxyl group of HDMF and its structural analogs. Although UGT85K16 also catalyzed the glucosylation of vanillin, its affinity and efficiency toward HDMF was higher. The expression of UGT85K16 mRNA correlated with the accumulation of HDMF and its glucoside in Fragaria × ananassa plants. These results suggest that UGT85K16 might be UDP-glucose: HDMF glucosyltransferase in strawberries.
Abbreviations: DMMF: 2,5-dimethyl-4-methoxy-3(2H)-furanone; EHMF: 2(5)-ethyl-4-hydroxy-5(2)-methyl-3(2H)-furanone; GBV: glycosidically bound volatile; HDMF: 4-hydroxy-2,5-dimethyl-3(2H)-furanone; HMF: 4-hydroxy-5-methyl-3(2H)-furanone; HMMF: 4-hydroxy-5-methyl-2-methylene-3(2H)-furanone; PSPG: Plant secondary product glycosyltransferase; RT-PCR: reverse transcription-PCR; OMT: O-methyltransferase; UGT: UDP-glycosyltransferase
Graphical Abstract
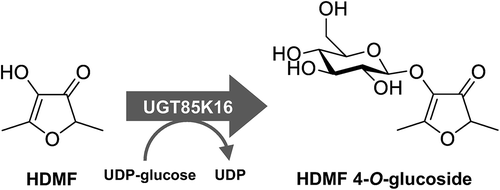
Characterization of HDMF glucosyltransferase (UGT85K16) from strawberry.
KEYWORDS:
Plant volatile compounds play important roles in biological interactions between plants and a wide range of organisms, such as bacteria, fungi, plants, and animals. They act as communication tools including attractants or defense compounds and also contribute as floral and fruit aromas [Citation1]. Volatile compounds are often stored as glycosides (glycosidically bound volatiles, GBVs), which are less volatile and more chemically stable than their aglycones [Citation2,Citation3]. The glycosylation of the volatiles by glycosyltransferases and hydrolysis of GBVs by glycosidases may be important steps in the regulation of the amounts and characteristics of plant aromas.
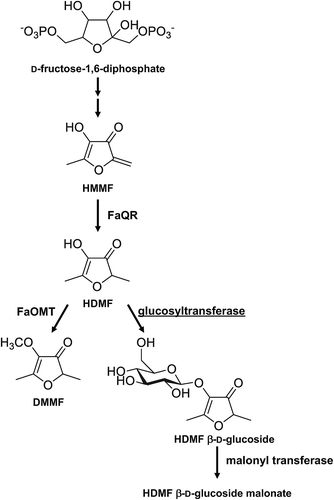
4-Hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF) is one of the most important aroma compounds contributing to strawberry flavor among a large number of volatile compounds present in the fruits [Citation4–Citation7]. HDMF is also a prominent aroma compounds in other fruits, such as pineapple and grape [Citation8,Citation9]. In strawberries, HDMF is biosynthesized by the transformation of 4-hydroxy-5-methyl-2-methylene-3(2H)-furanone (HMMF) derived from 6-deoxy-D-fructose-1-phosphate () [Citation10,Citation11]. HDMF is further converted into a methyl derivative (DMMF, 2,5-dimethyl-4-methoxy-3(2H)-furanone) [Citation4,Citation5], a glucosylated derivative (HDMF 4-O-glucoside) [Citation12], and a malonylated derivative (HDMF 4-O-glucoside malonate) [Citation13]. Quinone oxidoreductase (FaQR) and methyltransferase (FaOMT) have been identified in the biosynthetic pathway of HDMF and its derivatives in strawberries () [Citation10,Citation14–Citation16]. HDMF was reported to be rapidly converted to HDMF glucoside during fruit maturation. Maximum levels of the glucoside corresponded with late stages of fruit maturation [Citation5].
Figure 1. Reactions catalyzed by glucosyltransferase and the metabolism of HDMF in strawberries.
Enzymatic conversion of 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF) to HDMF 4-O-β-D-glucoside. FaOMT, Fragaria × ananassa O-methyltransferase; FaQR, Fragaria × ananassa quinone oxidoreductase.
Several glucosyltransferase cDNAs that catalyze the transformation of HDMF to its glucoside were isolated and characterized from strawberries including FaGT2 [Citation17] and UGT71K3 [Citation18–Citation20]. However, these UDP-glucosyltransferases exhibit broad substrate specificity. Recently a UDP-glucosyltransferase UGT85K14, isolated from grape was shown to be highly specific for HDMF glucosylation [Citation21]. This report prompted us to mine public databases in search of a novel strawberry UDP-glucosyltransferase. Our efforts resulted in the identification of UGT85K16, a highly specific glucosyltransferase for HDMF that is present in strawberry receptacles. Our findings not only shed light on the process of HDMF glucosylation process in strawberry, but may also lead to future metabolic engineering of fruit aromas through manipulation of GBV levels.
Materials and methods
Plant materials and chemicals
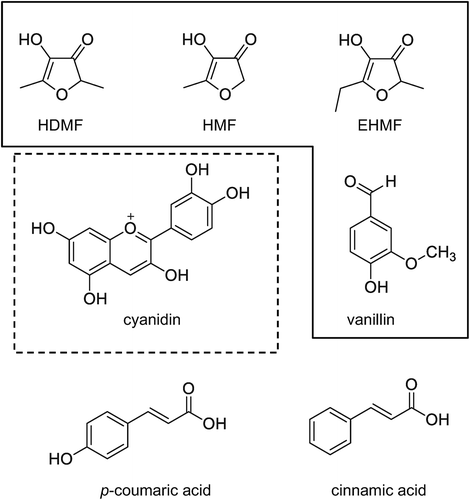
For cDNA cloning of glucosyltransferases, ripe strawberry receptacles (Fragaria × ananassa var. kaorino) were purchased from a supermarket in Japan. For gene expression analysis, strawberries (Fragaria × ananassa cv. Garden Strawberry) were grown in the Medicinal Plant Garden of Nagoya City University. HDMF, 2(5)-ethyl-4-hydroxy-5(2)-methyl-3(2H)-furanone (EHMF), 4-hydroxy-5-methyl-3-(2H)-furanone (HMF), and p-coumaric acid were purchased from Sigma-Aldrich Japan (Osaka, Japan). UDP-glucose and vanillin were purchased from Wako Pure Chemical Industries (Osaka, Japan). Cyanidin chloride was purchased from EXTRASYNTHESE (Lyon, France). Vanillin 4-O-β-D-glucoside was purchased from Toronto Research Chemicals, Inc. (Downsview, Canada). All other chemicals were of commercial reagent-grade quality.
Isolation of putative HDMF glucosyltransferase cDNAs from strawberry
Total RNA was prepared from strawberries using the Fruit-mait for RNA purification kit (TaKaRa Bio, Shiga, Japan) and Plant Total RNA Extraction Miniprep System (Viogene BioTek Corp., Taipei, Taiwan). cDNAs were synthesized using the CapFishingTM Full-length cDNA Premix Kit (Seegene, Rockville, MD) and SMARTer® RACE 5ʹ/3ʹ kit (TaKaRa Bio). PCR was carried out with GoTaq® Green Master Mix (Promega, Fitchburg, WI, USA) and KOD-Plus-Neo (Toyobo, Osaka, Japan), with cDNAs as templates. PCR primers were designed based on sequence data available from public databases (Supplementary Table S1). PCR products of approximately 1.5 kb were subcloned into a pMD20 T-vector (TaKaRa Bio) using a DNA Ligation Kit (TaKaRa Bio); they were confirmed by sequencing.
Heterologous expression of recombinant proteins
The heterologous expression and purification of candidate glucosyltransferases has been previously described [Citation22,Citation23]. Gene ORFs were amplified by PCR using specific primers (Supplementary Table S2). The expression vector pQE30 (Qiagen) was used in all cases, except for UGT85K16, which was cloned into pET45b (Merck, Darmstadt, Germany). All expression vectors were transformed into E. coli NiCo21 (DE3) (New England BioLabs, Inc.). Protein content in the enzyme preparations was estimated using the Bradford method [Citation24]. Enzyme purity was confirmed by SDS-PAGE on 10% (w/v) slab gels.
Recombinant enzyme assay
The method for standard enzyme assay has been previously described [Citation23]. The reaction products were analyzed by HPLC as described in the Supplementary Methods. To determine kinetic parameters, enzyme assays were performed at 30°C, for 2 min, in triplicate at each substrate concentration with 15 µg of purified enzyme as previously reported [Citation22,Citation23].
Quantitative determination of HDMF glucosides
Methanol extracts from plant tissues of Fragaria × ananassa were prepared as previously described [Citation23]. The methanol extracts were analyzed by HPLC as stated above. The amounts of HDMF glucosides were calculated on the basis of a calibration curve prepared using their respective standards.
Analysis of gene expression by semi-quantitative RT-PCR
Total RNA was prepared from organs of Fragaria × ananassa as described above. First-strand cDNAs for semi-quantitative RT-PCR were synthesized from 0.5 µg of total RNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). The specific primers used were: UGT85K16_Fw (GGCTCAAAGACATCCCTAGCTTCA); UGT85K16_Rv (TGCTAGCACTTGCTCTTGTGGA) UGT78A16/17_Fw (CCACTTGCAACCGCAGTTTTCA); and UGT78A16/17_Rv (AATCAAAGGCACTCCACCTGCTAC) for UGT85K16 and UGT78A16/17 amplification using GoTaq® Green Master Mix. A β-actin primer pair was used as an internal control. PCR was performed under the following conditions: precycling at 94°C for 5 min; followed by 35 cycles of 30 s at 94°C; 30 s at 50°C; and 30 s at 72°C; and then 5 min at 72°C. The PCR products were separated on a 1.5% agarose gel and stained with SAFELOOKTM Post-Green Nucleic Acid Stain (Wako), followed by fluorescent signal detection. Signal intensity analysis was performed using Image J software (NIH).
Results
Analysis of plant secondary product glycosyltransferase candidates from Fragaria × ananassa
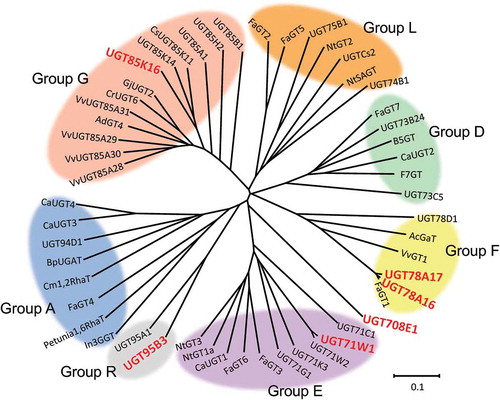
The extraction of uncharacterized genes from a genome or EST database is a useful method for comprehensive analysis of unknown enzymes [Citation22,Citation25]. In this study, the EST database PlantGDB database (Resources for Plant Comparative Genomics, http://www.plantgdb.org/) was used to survey Fragaria × ananassa plant secondary product glycosyltransferases (PSPGs). Using keywords related to PSPGs a total of 157 putative glycosyltransferase cDNA contigs were retrieved. This initial list of PSPG candidates was manually cleaned to include only contigs that are identical or nearly identical to PSPGs, resulting in 28 candidates. In general, plant PSPGs are clustered into 18 distinct groups designated A to R by phylogenetic analysis [Citation26,Citation27]. Phylogenetic analysis categorized these candidates into distinct groups based on PSPG amino acid sequence homology. Among these candidates, 10 contigs were removed because they belonged to group L, whose members catalyze the regioselective glycosylation of carboxylic acids [Citation26]. In addition, one putative PSPG was identified as a homologue of an HDMF-specific glucosyltransferase sequence from grape (UGT85K14) from the new genome database of strawberries (Strawberry GARDEN [Genome And Resource Database ENtry]) [Citation28]. Attempts to isolate 19 PSPG cDNAs by RACE, using RNA prepared from ripe strawberries, based on the sequences of these 19 contigs, resulted in the production of six full-length cDNA clones. The amino acid sequences encoding these cDNAs included a PSPG-box (Supplementary Figure S1). These clones were subsequently assigned to UGT71W1, UGT78A16, UGT78A17, UGT85K16, UGT95B3, and UGT708E1, by the UGT Nomenclature Committee; and their DDBJ accession numbers are LC312708, LC312709, LC312710, LC312711, LC312707, and LC312706, respectively. Phylogenetic analyses based on the amino acid sequences further indicated that, of the six PSPGs, two (UGT78A16 and UGT78A17) belonged to group F, one (UGT71W1) to group E, one (UGT85K16) to group G, and one (UGT95B3) to group R (). The remaining PSPG (UGT708E1) was distinctly separate from these.
Figure 2. Phylogenetic tree of family 1 PSPGs.
Isolated PSPG clones are shown in boldface. The tree was generated by the neighbor-joining method following multiple alignments using the ClustalW algorithm. The bar indicates a 0.1 amino acid substitution/site. For the names and DDBJ/GenBankTM/EBI accession numbers of the other PSPGs, see Supplementary Table S3.
Characterization of HDMF glucosyltransferase
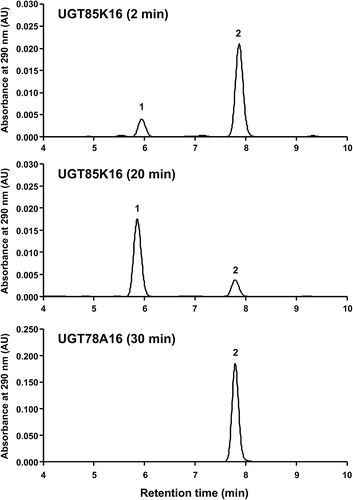
The ORFs of the six cDNAs were expressed in E. coli as previously described [Citation22,Citation23,Citation29]. SDS-PAGE analysis confirmed that four of the recombinant proteins (UGT78A16, UGT78A17, UGT85K16, UGT708E1) were detected in the soluble fractions of E. coli, although with somewhat differing expression levels. HDMF glucosylation assays were performed using the crude enzyme fractions in the presence of UDP-glucose. The crude fraction containing recombinant UGT85K16 converted HDMF to its glucoside (). This product was purified by preparative HPLC and identified as HDMF 4-O-glucoside on the basis of 1H-NMR, 13C-NMR, and ESI-TOF MS analyses (Supplementary Fig. S2). No other products were detected upon prolonged incubation. The UGT85K16 cDNA comprises an ORF corresponding to a protein of 480 amino acid residues with a predicted molecular mass of 54.0 kDa. It shares 68.8% amino acid identity with UGT85K14 isolated from grape.
Figure 3. Glucosylation of HDMF by UGT85K16.
HDMF was incubated with UGT85K16 or UGT78A16 in the presence of UDP-glucose for 5 min, 20 min, and 30 min. The assay mixture was subsequently subjected to HPLC analysis. Peak 1 was identified as HDMF 4-O-β-D-glucoside. Peak 2 was identified as HDMF.
The glucosyl acceptor specificity of UGT85K16 was analyzed using a variety of natural compounds. The chemical structures of the compounds used are shown in . HDMF, its structural analog EHMF, HMF, and vanillin were substrates that could be glucosylated, whereas the remaining compounds (p-coumaric acid, cinnamic acid, and cyanidin) did not, suggesting that UGT85K16 is a likely candidate for UDP-glucose: HDMF glucosyltransferase. In addition, the glucosyl acceptor specificities of UGT78A16 and UGT78A17 were evaluated using compounds that have previously been shown to be recognized by FaGT1 () [Citation30]. UGT78A16 and UGT78A17 exhibited glucosylation activity toward cyanidin, which is present in mature receptacles [Citation30]. UGT708E1 was unable to glucosylate any of the compounds in this study.
Figure 4. Substrate specificity of recombinant UGT78A16, UGT78A17 and UGT85K16.
The compounds within the frame were glucosylated by UGT85K16. Cyanidin within the dotted frame was glucosylated by UGT78A16 and UGT78A17.
The kinetic parameters of UGT85K16 for HDMF, using UDP-glucose as the sugar donor substrate, were determined using the affinity-purified protein (). The apparent Km values for HDMF, vanillin, and UDP-glucose were 0.32 ± 0.06, 1.5 ± 0.2, and 0.92 ± 0.17 mM, respectively (mean ± standard deviation, n = 3). The kcat/Km ratio for HDMF was 55-fold higher than that for vanillin. The kinetic parameters for EHMF and HMF could not be determined due to weak activities detected in the kinetic assays.
Table 1. Kinetic parameters of recombinant UGT85K16 for HDMF, vanillin and UDP-glucose.
Relationship between UGT85K16 expression and concentration of HDMF and its glucoside in strawberry
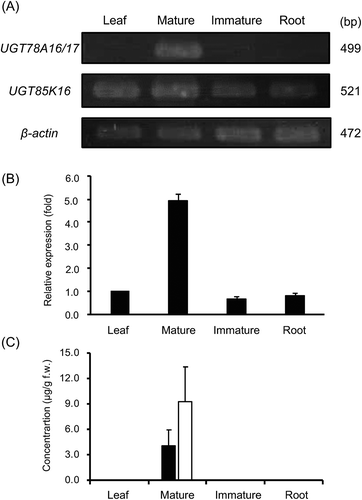
The expression levels of UGT85K16, UGT78A16 and UGT78A17 mRNA were quantified by semi-quantitative RT-PCR using two specific primer sets. As shown in ), UGT85K16 expression was detected in all organs, whereas UGT78A16 and UGT78A17 expression was only detected in mature receptacles. UGT85K16 expression was detected at levels five-fold higher in mature receptacles compared with other organs ()). The concentration of HDMF and its glucoside was measured by HPLC, and both compounds were found only in mature receptacles and at similar concentrations ()). There was significant correlation between the mRNA levels of UGT85K16 and the concentration of HDMF and its glucosides during fruit maturation.
Figure 5. Tissue distribution of UGT78A16, UGT78A17 and UGT85K16 mRNA, HDMF and its glucosides in Fragaria × ananassa.
All organs were collected from an individual of Fragaria × ananassa on the same day, powdered in liquid nitrogen, and used for mRNA preparation and HPLC analysis of HDMF and its glucosides. (A) Semi-quantitative RT-PCR analyses of mRNA levels of UGT78A16, UGT78A17 and UGT85K16. (B) Relative mRNA levels of UGT85K16 in different organs. (C) HDMF and HDMF glucoside contents in different organs. Closed and open bars represent contents of HDMF and HDMF glucoside, respectively. Each box and bar represent an average value with standard deviation from triplicate measurements.
Discussion
Many aromatic plants accumulate lipophilic volatile compounds as glycoside conjugates, which are water-soluble and odorless [Citation2,Citation3]. Therefore, not only the biosynthetic potential, but also the glycosylation/deglycosylation activities of volatile compounds are important factors to regulate the aroma level of particular plants.
In strawberries, HDMF is the one of the most important volatile compounds, contributing to the characteristics of strawberry flavor and aroma. HDMF glucoside and malonyl glucoside were found as water-soluble conjugates [Citation7]. Several glucosyltransferases were cloned from strawberries and functionally characterized. FaGT2, isolated as a ripening-related and oxidative stress-induced glucosyltransferase was isolated and shown to exhibit broad substrate specificity for different phenolic compounds and to be involved in detoxification of xenobiotics [Citation17]. Recently, Song et al. isolated nine ripening related glucosyltransferases [Citation18]. Among them allelic UGT71K3a/b catalyzed glucosylation of HDMF [Citation19]. However, the enzyme showed higher glucosylation activity toward vanillin than toward HDMF although kinetic parameters were not compared between these two substrates. In addition to vanillin, UGT71K3 catalyzed glucosylation of a diverse group of phenolic compounds including acetyl phologlucinol, quercetin, 1-naphthol, and pelargonidin [Citation20]. Furthermore, the concentration of HDMF glucoside and malonyl glucoside were modestly decreased in the UGT71K3-silenced strawberry fruits [Citation19]. These results suggest that some other glucosyltransferase(s) may be involved in HDMF glucosylation; although UGT71K3 is likely at least partially involved in this process.
In this study, PSPGs from the public databases were surveyed and six cDNAs expressed in the receptacles of strawberries were isolated. Recombinant enzyme assays revealed that UGT85K16 exhibits specific glucosylation activity toward HDMF. The recombinant protein also glucosylated vanillin, but catalytic efficiency of the enzyme for HDMF was about 55-fold greater than that of vanillin. The enzyme showed no glucosylation activity toward the other phenolic substrates examined. Furthermore, mRNA expression of UGT85K16 was highest in the receptacles coinciding with the distribution of HDMF glucoside. These results suggest that UGT85K16 efficiently catalyzes the transformation of HDMF to HDMF glucoside and potentially the glucosylation of other GBVs such as vanillin, in the receptacles of Fragaria × ananassa. However, UGT78A16 and UGT78A17 were not involved in HDMF glucosylation. These PSPGs may glucosylate pigments, such as cyanidin, in mature receptacles.
It is interesting to note that several members of PSPGs belonging to the UGT85K subfamily have been shown to be involved in glucosylation of volatile compounds. For example, UGT85K14, a PSPG most similar to UGT85K16 (68.8% identity) was shown to be responsible for the glucosylation of HDMF in grapes [Citation21]. UGT85K11 of Camellia sinensis was shown to glucosylate volatile compounds with a variety of chemical structures such as geraniol, eugenol, (Z)-3-hexenol, and benzyl alcohol [Citation2]. Some members belonging to UGT85A subfamily, including UGT85A3 from Arabidopsis thaliana, UGT85A28, A30 and A33 from grapevines and UGT85A32 from sweet potatoes, were shown to catalyze the transformation of monoterpene alcohols to their glucosides [Citation2]. In contrast, UGT85K2 and UGT85K3 of Lotus japonicus exhibits glucosylation activity toward cyanohydrin [Citation31], and UGT85K4 and UGT85K5 from cassava was apparently involved in the biosynthesis of cyanogenic glucoside in spite of its broad substrate specificity toward the cyanohydrins, flavonoids, and simple alcohols [Citation32]. It is possible that some of the PSPGs belonging to UGT85 family have evolved as key molecules to produce GBVs.
In strawberry fruit, HDMF is metabolized either to its methyl derivative, DMMF in a reaction catalyzed by O-methyltransferase (FaOMT) [Citation14] or to its glucoside by UGT85K16 and subsequently to the malonylated glucoside [Citation13]. The methylation or glucosylation of HDMF seems to be an important branching point for the development of strawberry flavor/aroma because DMMF is more volatile and HDMF glucosides are less volatile than HDMF [Citation5,Citation7]. The expression of FaOMT rapidly increased during the ripening stage of strawberry fruits; however the expression was not detected in other organs. UGT85K16 transcript levels were also very high in mature fruits although this transcript was also detected in the leaves and roots. It is possible that the regulation of the expression of these two HDMF modifying enzymes is involved in the regulation of strawberry flavor/aroma quantity and quality.
In conclusion, we isolated a novel UDP-glucosyltransferase, UGT85K16, which catalyzed glucosylation of HDMF to HDMF glucoside. Our results provide insights, not only into the glucosylation step of HDMF glucoside biosynthesis in strawberries, but also the identification of a variety of HDMF-specific glucosyltransferases in other HDMF-producing plant species. Furthermore, if manipulating expression of UGT85K16 affects the balance of HDMF sequestration and release, then gene manipulation of volatile compound-specific glycosyltransferases could lead to the engineering of flavors/aromas in fruits.
Author contributions
TM, HM and KT conceived and supervised the study; AY and KT designed the experiments; AY, KI and KT performed the experiments; AY, KI and KT analyzed the data; AY, KI, TM, HM and KT wrote the manuscript.
Supplemental_data.pdf
Download PDF (241 KB)Acknowledgments
We are grateful to Dr. Kanako Sasaki (Kirin Company, Ltd.) for valuable discussion. We would like to thank Dr. Amanda C. Martin (The Kochi Prefectural Makino Botanical Garden) for the English language review.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arimura GI, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 2009;50:911–923.
- Ohgami S, Ono E, Horikawa M, et al. Volatile glycosylation in tea plants: sequential glycosylations for the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015;168:464–477.
- Lücker J, Bouwmeester HJ, Schwab W, et al. Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-β-D-glucopyranoside. Plant J. 2001;27:315–324.
- Pyysalo T, Honkanen E, Hirvi T. Volatiles of wild strawberries, Fragaria vesca L., compared to those of cultivated berries, Fragaria × ananassa cv Senga Sengana. J Agric Food Chem. 1979;27:19–22.
- Pérez AG, Olías R, Sanz C, et al. Furanones in strawberries: evolution during ripening and postharvest shelf life. J Agric Food Chem. 1996;44:3620–3624.
- Li -X-X, Fukuhara K, Hayata Y. Concentrations of character impact odorants in ‘Toyonoka’ strawberries quantified by standard addition method and PQ column extraction with GC-MS analysis. J Japanese Soc Hortic Sci. 2009;78:200–205.
- Schwab W. Natural 4-hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules. 2013;18:6936–6951.
- Rodin JO, Himel CM, Silverstein RM, et al. Volatile flavor and aroma components of pineapple. l. Isolation and tentative identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J Food Sci. 1965;30:280–285.
- Schreier P, Paroschy JH. Volatile constituents from concord, Niagara (Vitis labrusca, L.) and Elvira (V. labrusca, L. × V. riparia, M.) grapes. Can Inst Food Sci Technol J. 1981;14:112–118.
- Raab T, López-Ráez JA, Klein D, et al. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase. Plant Cell. 2006;18:1023–1037.
- Klein D, Fink B, Arold B, et al. Functional characterization of enone oxidoreductases from strawberry and tomato fruit. J Agric Food Chem. 2007;55:6705–6711.
- Mayerl F, Näf R, Thomas AF. 2,5-Dimethyl-4-hydroxy-3(2H)-furanone glucoside: isolation from strawberries and synthesis. Phytochemistry. 1989;28:631–633.
- Roscher R, Herderich M, Steffen J-P, et al. 2,5-Dimethyl-4-hydroxy-3[2H]-furanone 6′O-malonyl-β-D-glucopyranoside in strawberry fruits. Phytochemistry. 1996;43:155–159.
- Wein M, Lavid N, Lunkenbein S, et al. Isolation, cloning and expression of a multifunctional O-methyltransferase capable of forming 2,5-dimethyl-4-methoxy-3(2H)-furanone, one of the key aroma compounds in strawberry fruits. Plant J. 2002;31:755–765.
- Lunkenbein S, Bellido M, Aharoni A, et al. Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiol. 2006;140:1047–1058.
- Zorrilla-Fontanesi Y, Rambla J-L, Cabeza A, et al. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiol. 2012;159:851–870.
- Landmann C, Fink B, Schwab W. FaGT2: a multifunctional enzyme from strawberry (Fragaria × ananassa) fruits involved in the metabolism of natural and xenobiotic compounds. Planta. 2007;226:417–428.
- Song C, Gu L, Liu J, et al. Functional characterization and substrate promiscuity of UGT71 glycosyltransferases from strawberry (Fragaria × ananassa). Plant Cell Physiol. 2015;56:2478–2493.
- Song C, Hong X, Zhao S, et al. Glucosylation of 4-hydroxy-2,5-dimethyl-3(2H)-furanone, the key strawberry flavor compound in strawberry fruit. Plant Physiol. 2016;171:139–151.
- Song C, Zhao S, Hong X, et al. A UDP-glucosyltransferase functions in both acylphloroglucinol glucoside and anthocyanin biosynthesis in strawberry (Fragaria × ananassa). Plant J. 2016;85:730–742.
- Sasaki K, Takase H, Kobayashi H, et al. Molecular cloning and characterization of UDP-glucose: furaneol glucosyltransferase gene from grapevine cultivar Muscat Bailey A (Vitis labrusca × V. vinifera). J Exp Bot. 2015;66:6167–6174.
- Asada K, Salim V, Masada-Atsumi S, et al. A 7-deoxyloganetic acid glucosyltransferase contributes a key step in secologanin biosynthesis in Madagascar Periwinkle. Plant Cell. 2013;25:4123–4134.
- Nagatoshi M, Terasaka K, Nagatsu A, et al. Iridoid-specific glucosyltransferase from Gardenia jasminoides. J Biol Chem. 2011;286:32866–32874.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Liscombe DK, Usera AR, O’Connor SE. Homolog of tocopherol C methyltransferases catalyzes N methylation in anticancer alkaloid biosynthesis. Proc Natl Acad Sci. 2010;107:18793–18798.
- Caputi L, Malnoy M, Goremykin V, et al. A genome-wide phylogenetic reconstruction of family 1 UDP- glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012;69:1030–1042.
- Cui L, Yao S, Dai X, et al. Identification of UDP-glycosyltransferases involved in the biosynthesis of astringent taste compounds in tea (Camellia sinensis). J Exp Bot. 2016;67:2285–2297.
- Hirakawa H, Shirasawa K, Kosugi S, et al. Dissection of the octoploid strawberry genome by deep sequencing of the genomes of Fragaria species. DNA Res. 2014;21:169–181.
- Nagatoshi M, Terasaka K, Owaki M, et al. UGT75L6 and UGT94E5 mediate sequential glucosylation of crocetin to crocin in Gardenia jasminoides. FEBS Lett. 2012;586:1055–1061.
- Griesser M, Hoffmann T, Bellido ML, et al. Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit. Plant Physiol. 2008;146:1528–1539.
- Takos AM, Knudsen C, Lai D, et al. Genomic clustering of cyanogenic glucoside biosynthetic genes aids their identification in Lotus japonicus and suggests the repeated evolution of this chemical defence pathway. Plant J. 2011;68:273–286.
- Kannangara R, Motawia MS, Hansen NKK, et al. Characterization and expression profile of two UDP-glucosyltransferases, UGT85K4 and UGT85K5, catalyzing the last step in cyanogenic glucoside biosynthesis in cassava. Plant J. 2011;68:287–301.
