ABSTRACT
In this review, I present the main highlights of my works in the development of bioelectrocatalysis, which can be used in widespread applications, particularly for the design of biosensor and biofuel cells. In particular, I focus on research progress made in two key bioelectrocatalytic reactions: glucose oxidation by flavin adenine dinucleotide-dependent glucose dehydrogenase and oxygen reduction by bilirubin oxidase. I demonstrate the fundamental principles of bioelectrocatalysis and the requirements for enhancing the catalytic performance, including the choice of a mediator of redox reactions, immobilization, and electrode materials. These methods can allow for achieving control of the bioelectrocatalytic reaction, thereby overcoming obstacles toward their industrial applications.
Graphical Abstract
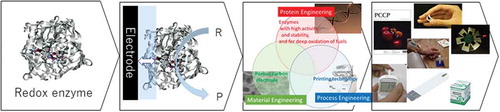
From fundamentals to applications of bioelectrocatalysis.
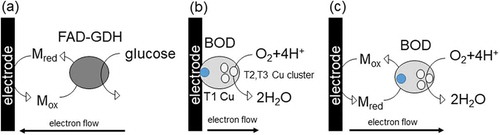
Redox reactions play an important role in energy conversion and substance metabolism in living organisms. Enzyme-based bioelectrocatalysis integrates the catalytic function of oxidoreductase or the metabolic function of microorganisms containing enzymatic redox reactions with the electrode reaction for proper electron transfer [Citation1]. The electrode reactions can be categorized into mediated electron transfer (MET) or direct electron transfer (DET) between the enzyme’s active site and the electrode (). MET requires redox mediators for most redox enzymes because the active site of oxidoreductase is usually deeply buried in the insulated protein shell and is not electrochemically active. In contrast, DET reactions occur when the electroactive sites of enzymes lie close to the enzyme surface, thereby exhibiting electrocatalytic activity without requiring redox mediators. Such enzymes can be used for the development of continuous monitoring sensors and biofuel cell (BFC) devices without concern about leaching of the redox mediator from the electrode surface. However, the main drawback of DET-based systems is their low current output efficiency, which is limited by the number of electroactive enzymes on the electrode surface. This limiting factor is determined not only by the molecular size of the enzyme but also by the orientation of the enzyme on the electrode surface; the electron transfer rate between the enzyme and electrode exponentially depends on the inverse of the electron transfer distance. Thus, most of these enzymes will show no electrocatalytic activity without proper control of the orientation such as positioned on a flat electrode, because of the long electron transfer distance.
Figure 1. Schematic representation of bioelectrocatalysis (a) mediated electron transfer of FAD-GDH, (b) direct electron transfer to BOD adsorbed on an electrode, (c) mediated electron transfer to BOD.
The creation of new biodevices based on bioelectrocatalysis has attracted considerable attention in the fields of environment, energy, information communication, and healthcare, among others. However, methods to gain good control of the bioelectrocatalytic reaction have not been established, which has largely hindered their industrial applications. To resolve this issue, the author has been working on the basic characterization of oxidoreductase as an electrode catalyst, and development of technologies for enzyme modification on the electrode, the redox mediator, and porous carbon electrode materials. Based on these works, rational design guidelines are proposed for the development of a new bioelectrocatalysis-based system and associated materials. This review focuses on two key bioelectrocatalytic reactions; electrochemical oxidation of glucose catalyzed by flavin adenine dinucleotide (FAD)-dependent glucose dehydrogenase (FAD-GDH) and electrochemical oxygen reduction reaction by bilirubin oxidase (BOD), and their application to the development of biosensors and energy conversion devices (i.e., BFCs).
Enzymatic BFCs are fuel cells that employ enzymes instead of conventional noble metal catalysts ((a)) [Citation2]. The working principle is the same as in conventional polymer electrolyte membrane fuel cells, namely the fuel is oxidized at the anode side and the electrons reach the cathode where they combine with an oxygen molecule to water. BFCs are promising devices for sustainable green energy applications; however, they are still at an early stage of development, with many yet-to-be-resolved fundamental scientific and engineering problems. Two critical problems that need to be overcome are the short lifetime and poor power density, both of which are related to enzyme stability, electron transfer rate, and enzyme loading.
Figure 2. Enzymatic biofuel cells. (a) Illustration of a membraneless enzymatic BFC in which the fuel is oxidized at the anode by an oxidative enzyme. The electrons released during the oxidation move through the external wire to the cathode at which O2 is reduced to water by the enzyme. (b) H2(hydrogenase)-O2(BOD) BFC operating at neutral pH [Citation22], (c) one compartment-type glucose (PQQ-GDH)-O2 (BOD) BFC based on hydrogel technology [Citation24], (d) DET-based fructose (fructose dehydrogenase)-O2 (laccase) BFC using porous carbons [Citation45], (e) glucose (NAD-GDH)-O2 (BOD) BFC [Citation50], (f) printed glucose(GOx)-O2(BOD) BFC [Citation49], (g) printed glucose(GOx)-O2(BOD) BFC array [Citation58].
![Figure 2. Enzymatic biofuel cells. (a) Illustration of a membraneless enzymatic BFC in which the fuel is oxidized at the anode by an oxidative enzyme. The electrons released during the oxidation move through the external wire to the cathode at which O2 is reduced to water by the enzyme. (b) H2(hydrogenase)-O2(BOD) BFC operating at neutral pH [Citation22], (c) one compartment-type glucose (PQQ-GDH)-O2 (BOD) BFC based on hydrogel technology [Citation24], (d) DET-based fructose (fructose dehydrogenase)-O2 (laccase) BFC using porous carbons [Citation45], (e) glucose (NAD-GDH)-O2 (BOD) BFC [Citation50], (f) printed glucose(GOx)-O2(BOD) BFC [Citation49], (g) printed glucose(GOx)-O2(BOD) BFC array [Citation58].](/cms/asset/21ef6df3-15f6-4534-b767-4043d78704d4/tbbb_a_1527209_f0002_oc.jpg)
FAD-GDH from Aspergillus terreus
Introduction
FAD-GDHs have recently garnered considerable attention as bioelectrocatalysts for glucose biosensors and BFCs because of their unique characteristics, including oxygen insensitivity, high biocatalytic activity, and substrate specificity [Citation3]. In fact, FAD-GDHs isolated from Aspergillus species have already been employed as catalysts in disposable blood glucose sensor strips, indicating that they may be promising alternatives to glucose oxidases (GOxs). GOx was the enzyme most commonly used for the electrocatalytic oxidation of glucose, and can utilize not only a redox mediator but also oxygen as a natural electron acceptor, reducing it to hydrogen peroxide. However, the reduction of oxygen and consequent production of hydrogen peroxide are undesired reactions that can affect the sensitivity of mediator-assisted biosensors, inducing a loss of coulombic efficiency in BFCs, and ultimately resulting in damage to the electrodes. FAD-GDHs from Aspergillus species require redox mediators to shuttle electrons from the FAD site to the electrode. Hexacyanoferrate ([Fe(CN)6]3−; ferricyanide) was first tested as a redox mediator and is now commonly applied in commercial FAD-GDH-based blood glucose sensor strips because of its high solubility in water, low cost, and high stability [Citation3]. The first amperometric glucose sensor that was developed based on FAD-GDH from Aspergillus terreus shows current responses with good linearity as a function of glucose concentrations in the presence of a relatively high concentration of ferricyanide as a redox mediator. The response current is not sensitive to the oxygen dissolved in the analyte and the sensor is not responsive to other saccharides such as maltose and mannose. The author also devised a batch-type coulometric d-glucose sensor using FAD-GDH that showed high precision even in the presence of interferents, and does not require calibration curves that take advantage of the properties of this newly discovered enzyme [Citation4]. A potential-step coulometry was demonstrated to electrolyze respective interferents such as ascorbate at low potential and d-glucose at high potential using FAD-GDH along with potassium octacyanomolybdate (IV) as a mediator possessing an appropriate formal potential of 0.6 V vs. Ag|AgCl(sat. KCl) In this review, all potentials are referred to the Ag|AgCl (sat. KCl) electrode. Although both the amperometric and coulometric sensors worked successfully, selection of a suitable mediator is critical for the further development and performance improvement of bioelectrochemical devices. Indeed, the reactivity between FAD-GDH and negatively charged molecules is quite low, and therefore the next step of this research involved development of an improved redox mediator for enhancing the performance of the device.
Redox hydrogel
Toward this end, we focused on osmium (Os) complexes [Citation5], a group of metal complex-type redox mediators used with GOx. Os complex-type mediators are useful because they possess a positive charge that opposes the negative charge of ferricyanide, display high kinetic constants through their efficient self-exchange electron transfer capabilities, and it is possible to tune the potential simply by replacing the ligand [Citation6]. Moreover, Os complexes can be immobilized jointly with an enzyme on the electrode surface based on a redox “wiring” technique pioneered by A. Heller [Citation7]. Poly(1-vinylimidazole) was used to tether an Os complex (Os(2,2’-bipyridine)2Cl) as a redox mediator, and FAD-GDH was cross-linked by a diepoxy-type cross-linking agent to form a redox hydrogel on the carbonaceous electrode surface [Citation5]. The electrons generated during enzymatic glucose oxidation are then transferred from the reduced FAD to the electrode via the Os complexes. This “wiring” technique allows the electrode to generate a high catalytic current density because the enzyme and mediator are co-immobilized on the electrode surface at high concentrations. The steady-state catalytic current for glucose oxidation was determined to be 2.6 mA cm−2 at pH 7 and 25°C, which increased by 1.6-fold (4.1 mA cm−2) under the same conditions after oxidative deglycosylation of the FAD-GDH. This current magnitude represents the highest value reported to date for a non-porous glassy carbon electrode-based glucose anode [Citation5].
Porous carbon scaffold
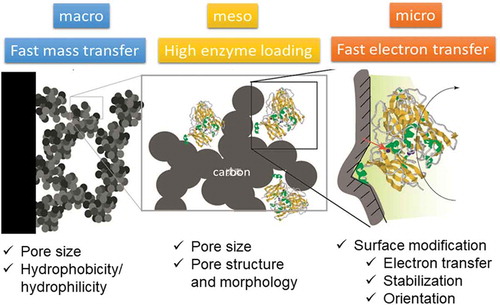
The use of porous carbon materials with a specific pore structure enable increasing enzyme loading and the electron transfer efficiency. A commercially available carbon black Ketjen Black (KB) was first used as an electrode material for enzyme electrode with high surface area. An electrode on which KB was modified by drop casting, modified with a hydrogel composed of a redox polymer and GOx showed a current density of up to 5.1 mA cm−2, which was only 60% higher than that on a GC electrode [Citation8]. KB features a high specific surface area of 800–1200 m2 g−1 and a primary particle diameter of 40 nm, which provides a good platform for encaging enzymes by the carbon walls, but the pore-size distribution of the KB-modified electrode ranges from 1 to 100 nm, suggesting that the parts that were smaller than the enzymes were inefficiently used for enzyme loading and electrochemical reactions (molecular weights of GOx and FAD-GDH are 160 and 135 kDa, respectively). Moreover, the hydrogel that formed on the surface of the KB layer could readily prevent mass transfer of glucose, GOx, and the redox polymer. Thus, as shown in , the hierarchical structure is important for realizing a high catalytic current. In particular, a macropore structure enables the rapid mass transfer of enzymes into the porous carbon layer during enzyme electrode fabrication and the smooth mass transfer of fuel during the bioelectrocatalytic reaction. An electrode made from MgO-templated carbon (MgOC [Citation9,Citation10]) with average pore diameter of 40-nm was further coated with a biocatalytic hydrogel composed of a conductive redox polymer, deglycosylated FAD-GDH (d-FAD-GDH), and a cross-linker () [Citation11]. In this case, the glucose-oxidation current density was 100 mA cm–2 at 25°C and pH 7 with a hydrogel load of 1.0 mg cm–2. For the same hydrogel composition and loading, the current density on the MgOC-modified GC electrode was more than 30 times higher than that detected on the flat carbon electrode. In the studies described above, the MgOC was collected on the carbon disc by electrophoretic deposition to form macropores [Citation11,Citation12]. By contrast, when MgOC was modified by drop-casting, the electrode lacked macropores larger than 10 μm, and the maximal current was less than 40 mA cm−2 even at an electrode rotation rate of 9000 rpm [Citation11]. The maximal current was ascribed to the limitations of the biocatalyst and glucose transport in the carbon-layer structure on the GC support. Furthermore, the stability of the hydrogel electrode was enhanced using mesoporous carbon materials: > 95% of the initial catalytic current remained after storage for 220 days in 4°C phosphate buffer, and 80% of the activity was observed after 7 days of continuous operation at 25°C. The jump-up was achieved not only by controlling the pore structure and optimizing the hydrogel composition, but also by the electrolyte composition. The properties and concentration of the electrolyte strongly affect the hydrogel structure and interaction between the redox molecules and enzyme [Citation13]. The electrolyte also affects the stability of the electrochemical response of FAD-GDH; the response of the hydrogel on the GC electrode was diminished at 37°C after 2 days of continuous operation in 0.1 M phosphate buffer at pH 7, but the response stability was improved by immobilizing the hydrogel on the porous carbon structure via encapsulating the hydrogel within the mesopores under 1 M phosphate buffer [Citation11,Citation14].
Figure 4. One example of bioelectrocatalysis on hierarchical structure controlled carbon electrode [Citation11]. Mesoporous carbon (average pore diameter of 40 nm) was coated on GC electrode with a surface roughness of several tens of micrometers. In the mesopore, FAD-DGH was immobilized with a crosslinker within a hydrogel formed from poly(vinylimidazole) complexed with [Os(2,2’-bipyridine)2Cl]+/2+.
![Figure 4. One example of bioelectrocatalysis on hierarchical structure controlled carbon electrode [Citation11]. Mesoporous carbon (average pore diameter of 40 nm) was coated on GC electrode with a surface roughness of several tens of micrometers. In the mesopore, FAD-DGH was immobilized with a crosslinker within a hydrogel formed from poly(vinylimidazole) complexed with [Os(2,2’-bipyridine)2Cl]+/2+.](/cms/asset/3a2c179f-3a6c-4abf-aa2f-f9dfe650317a/tbbb_a_1527209_f0004_oc.jpg)
Lowering the potential of redox mediator
Based on this background, a mediator with low redox potential that nevertheless exhibits high electron transfer activity is desired for the design of high-power BFC and highly sensitive biosensors, which will not be affected by interfering substances such as uric acid or ascorbic acid. However, a modified electrode constructed by assembling FAD-GDH with an Os-based redox polymer with a low formal potential (−0.03 V), in which Os complexes with imidazole-based ligands are tethered to the polymer backbone via a 13-atom alkyl chain and a crosslinker, delivered 5 mA cm−2 at 37°C and pH 7, which only accounts for 10% of the glucose oxidation current densities obtained with the GOx electrode [Citation15]. This low catalytic current density was ascribed to the low affinity of the Os complex polymer toward FAD-GDH; however, the detailed mechanism is still under investigation.
Therefore, there is a need to select or identify other compounds that can efficiently mediate redox reactions with FAD-GDH. The choice of mediator is very important as it determines the potential at which the reaction will proceed, as well as the reaction rate. However, it is not easy to obtain information on the optimum properties of mediators for FAD-GDHs, even when structural and chemical information of the active center pocket is available. The author’s research group recently proposed a strategy for determining the optimum mediator for FAD-GDHs by screening a set of organic redox mediators, including quinones and phenothiazines, with a variety of structures and redox potentials that could be easily tuned by changing the substituents, in view of their bimolecular rate constants (k2) for the enzyme reaction [Citation16]. At pH 7.0, the logarithm of the k2 value appeared to depend on the specific redox potentials. Notably, the rate constant of each molecule for FAD-GDH was approximately 2.5 orders of magnitude higher than that for GOx from Aspergillus sp. These results suggested that the electron transfer kinetics are mainly determined by the potential difference between the mediator and FAD in the enzyme molecule (i.e., the driving force for the electron transfer), and the electron transfer distance between the redox-active sites of the mediator and the FAD that is affected by the steric or chemical interactions (electrostatic, pi-pi, or hydrophobic interactions). k2 values for ortho-quinones were higher than for para-quinones in the reactions with FAD-GDH and GOx, which was likely due to the relatively lower steric hindrance in the active site in the case of the ortho-quinones. Thus, a reasonable strategy to improve the BFC performance is to develop a 1,2- naphthoquinone-based redox polymer hydrogel for an FAD-GDH-based electrode [Citation17]. The findings of this study should also be useful as a starting point for the retrosynthetic analysis of new mediators [Citation18].
BOD as a cathodic electrocatalyst
Introduction
The multi-copper oxidases have four types of copper atoms in their active sites, which can be classified into type 1 (T1), type 2 (T2), and type 3 (T3) Cu centers according to their spectroscopic and magnetic properties ((b, c)). The T1 Cu center accepts electrons from both reducing substrates and from a solid electrode. Therefore, MCOs have received substantial attention as oxygen reduction reaction (ORR) catalysts in enzymatic BFCs [Citation19]. Laccases are widely used in the majority of reported bioelectrocatalytic O2 reductions; however, the laccase-based bioelectrocatalytic currents appear only in acidic solutions (less than pH 5.0) owing to the very low activity of laccases above pH 5.0. Alternatively, BOD from Myrothecium verrucaria, first reported in 1983 [Citation20,Citation21], has now become one of the most widely used bioelectrocatalysts for the four-electron ORR under neutral pH conditions, which has enabled the demonstration of a variety of BFCs operating at neutral pHs () [Citation22–Citation24].
MET reaction of BOD: characteristics and immobilization
In 2001, the author’s group first reported a bioelectrocatalytic ORR developed under neutral pH using BOD as an electrocatalyst and 2,2’-azinobis (3-ethylbenzothiazolin-6-sulfonate) (ABTS) as a redox mediator ((c)) [Citation25]. ABTS transports electrons from the glassy carbon electrode to the T1 Cu, and has a formal potential of 0.505 V, which is very close to the formal potential of O2|H2O at pH 7.0. Therefore, the overpotential for ORR was very small, as low as 50 mV at 5 μA cm−2. Moreover, ABTS exhibited the ideal characteristics of a BOD mediator in view of its excellent kinetics (high kcat value, 820 s−1) and affinity (low Km value, 11 μM). The particularly unique characteristic of BOD as an electrocatalyst is likely derived from its relatively high formal potential under neutral pH of 0.45 V [Citation26]. This was evaluated by one-compartment bulk electrolysis to control the solution potential with simultaneous spectroscopic measurements, in which a conventional spectroscopic cuvette was applied to mediate the titration of the redox proteins and enzyme for determining their redox potentials [Citation26,Citation27]. This new analytical method provides the opportunity to evaluate even low-absorbance protein samples with low solubility or small absorption coefficients. In fact, this method further allows for the evaluation of redox potentials of a variety of oxidoreductases, including copper-containing laccases and heme-iron containing fructose dehydrogenase. Since the discovery of BOD as an efficient electrocatalyst at neutral pH, several more MCOs with high ORR activity have been widely explored. For example, copper efflux oxidase (CueO) from Escherichia coli and metagenome laccases were found to exhibit high ORR activity under near-neutral pH, although the potential was lower than that of the BOD from M. verrucaria [Citation28–Citation31]. Despite attempts to shift the potential of CueO to positive, it remained lower than that of the BOD [Citation32,Citation33].
To further extend the application for BFC development, BOD and the redox mediator ABTS should be stably co-immobilized on the electrode surface without their desorption from the electrode to the electrolyte; the desorption would lead to a decrease in the bioelectrocatalytic O2 reduction current and in the performance of the anode. However, the ABTS molecule is not easy to immobilize with BOD stably on the electrode surface without activity loss. To address this challenge, alternative mediators for the BOD cathode were tested using cyano–metal complexes, including hexacyanoferrate [Fe(CN)6]3/4−, which showed a high catalytic rate constant and very low Michaelis constant for BOD, likely owing to their negative surroundings in comparison with bilirubin and ABTS [Citation34]. In addition, hexacyanoferrate can be easily electrostatically co-immobilized with negatively charged BOD (the isoelectric point of BOD was around 4.1 [Citation21]) on the electrode surface using a positively charged polymer such as poly-l-lysine. The resultant modified cathode showed a high catalytic ORR current controlled by O2 diffusion operating under neutral pH. However, because the enzyme and mediator are both immobilized on the electrode surface as a result of electrostatic interaction, the hexacyanoferrate is readily eluted into the solution under conditions of high ionic strength. To overcome this problem, pentacyanoferrate-coordinated poly(vinyl imidazole) was synthesized and covalently cross-linked with BOD [Citation35]. Consequently, high catalytic currents were generated on the electrodes on which the cyanoferrate complex was modified, but the onset potential was lower than that of the ABTS-mediated system because of the low formal potential of hexacyanoferrate, at 0.22 V. Octacyano-tungstate, with a formal potential of 0.31 V, was identified as a suitable alternative mediator to the cyanoferrate complex through a screening process; unfortunately, the stability of the complex was not sufficient for the long-term operation [Citation36]. However, use of carbon felt with a large surface area resulted in a high catalytic current density per geometric surface area. A polymer tethering Os-complex with desired ligands emerged as another option for a BOD mediator. BOD and an Os complex tethered to a polymer can be modified on the electrode surface, and the modified electrode shows a clear O2 reduction current [Citation24]. The formal potential and surface charges of the Os complex can then be tuned by varying its ligands; for example, an Os complex tethered to poly(vinyl imidazole) coordinated with two 4,4’-dichloro-2,2’-bipyridine ligands and one chloride ligand was optimized for design of a BOD-hydrogel electrode [Citation37]. The biocathode with thermophilic BOD from Bacillus pumilus showed bioelectrocatalytic activity even at 70°C. The disadvantages of the hydrogel technique based on the Os polymer include the relatively high cost and associated difficulties presented by applying an electrode with a large surface area.
DET reactions of BOD: quantitative analysis
In contrast, DET-type reactions for BOD have been explored since 2004, using a variety of carbon forms (such as highly oriented graphite) as the electrode [Citation38]. The current-potential curves were interpreted by the following equations:
where kc is the enzymatic catalytic constant, k° is surface electron transfer kinetic parameter, E° is the formal potential, E is electrode potential, and ΓE is the amount of electroactive enzyme on the electrode surface. This study also revealed that the surface functional group and nanostructure of the electrode influence the electrocatalytic activity of the enzyme. The amount of electrochemical activity can be evaluated by quartz crystal microbalance, allowing for evaluation of the ratio of electrochemically active enzyme to the total amount of enzyme loaded on the electrode [Citation39]. The theoretical equations also allow for quantitative comparison of the catalytic functions and redox potentials of newly developed enzymes, including protein-engineered enzymes [Citation40]. Furthermore, based on the estimated mechanism of electron transfer at the interface between the enzyme and the electrode at the electrode interface (i.e., the interaction between the molecule and the surface, orientation control, etc.), various modifications can be made on the electrode surface to improve the catalytic efficiency of the enzyme reaction at the electrode [Citation30,Citation41].
To increase the catalytic current density, the author’s group attempted to modify BOD with a cationic polymer to increase the amount of enzyme on the electrode surface [Citation42]. The modified electrode successfully produced a diffusion-controlled voltammogram for the O2 reduction in a quiescent solution. Under an O2 saturated condition, a steady-state voltammogram was obtained with a limiting current density of approximately 1 mA cm−2. The steady-state voltammogram could be explained by an equation derived on the basis of a reaction layer theory, in which BOD was considered to be diffusible in the immobilized layer.
Porous carbon electrode and air-diffusion biocathode
The magnitude of the maximum catalytic current depends on the effective surface area available for the electrochemical reaction involving BOD. Therefore, when a mesoporous carbon electrode such as a carbon aerogel or an MgO-templated carbon with average pore diameter of 40-nm was used for BOD with molecular weight of 52,000 [Citation21] to increase the specific surface area and surface interaction between the enzyme and the electrode, the BOD-modified electrode showed a catalytic current density as high as 5–8 mA cm−2 per geometric surface area, without a mediator [Citation43,Citation44]. Furthermore, because the interfacial electron-transfer rate depends on the electron-transfer distance between the electrode surface and the electrochemically active site of the enzyme, the cathode performance could be improved by either exploiting surface modification to enhance interfacial electron transfer from the electrode surface to the active site of BOD, or by loading BOD onto the surface and thereby providing suitable mutually attractive interactions. Accordingly, the author examined the interaction of the enzyme with its most favorable known mediator ABTS that is adsorbed on the surface of the pore size-controlled MgO-templated carbon (average pore diameter of 40 nm) electrode to enhance the electrocatalytic activity of the BOD [Citation44]. Indeed, the maximum catalytic current increased by two-fold in the presence of the adsorbed ABTS across a wide pH range.
Significant efforts toward the development of biocathode systems using highly active enzymes, redox mediators, and porous electrode materials have shown that cathode performance is dependent on the oxygen supply, because oxygen has low solubility in water. In fact, the performance of a biocathode fully dipped in the electrolyte solution could be improved simply by solution stirring and bubbling oxygen gas into the system [Citation45]; however, such active-type biofuel cell systems are not suited for practical use. Thus, to increase the oxygen supply and current density, the control over hydrophobicity (for oxygen transport) and hydrophilicity (for enzyme reactivity and ion transport) is crucial. The author’s group reported the first air-breathing biocathode that uses polytetrafluoroethylene (PTFE) as a binder for holding carbon materials and also serves as an effective water repellent [Citation46,Citation47]. A mixture of KB and PTFE was applied on the current collector such as Toray carbon paper, followed by modification of the enzyme on the resulting water-repellent carbon electrode. The steady-state current density catalyzed by CueO from E. coli reached as high as 20 mA cm−2 at 0 V in a 1.0 M citrate buffer solution at pH 5.0 in a passive cell system. However, such high catalytic current could not be obtained on the CueO electrode at neutral pH. The air-breathing BOD electrode without a redox mediator also produced 5 mA cm−2 of catalytic current at pH 7 [Citation48]. The technology was successfully adapted to a printed BFC in which an air-cathode printed by a screen printer on a paper substrate generated 1.5 mA cm−2 of catalytic O2 reduction current at neutral pH ((f)) [Citation49]. For further development of an air-based biocathode, BOD requires a redox mediator such as that described above. To increase the hydrophilic BOD and ferricyanide loadings on hydrophobic carbon felt electrodes while maintaining its water-repellent surface after immobilization, a semi-permeable membrane such as cellophane was initially applied as a separator between the anode and cathode to prevent the cathode from flooding ((e)) [Citation50]. Although the cell power was consequently increased to 1.5 mW cm−2 under passive conditions, it was difficult to control the wettability of the biocathode exposed to air and the continuous supply of oxygen and protons to the electrode surface. A carbon felt electrode immobilized with BOD and ferricyanide/poly-L-lysine was modified with a water repellent from Scotchgard™ dissolved in methyl isobutyl ketone [Citation51]. This method allowed for gaining greater control of the enzyme-modified electrode hydrophobicity without damaging the enzyme, and a catalytic current density of 25 mA cm−2 at pH 7 could be achieved under passive conditions.
Hierarchically structured electrode for BOD
Further, the author has previously reported a DET reaction of BOD using MgOC with a pore diameter of 40 nm, which was comparable to the molecular size of the enzyme, and consequently improved the stability of the enzyme on the electrode with respect to both enzyme structure and attachment by the surrounding carbon mesopores [Citation52,Citation53]. However, the catalytic current was limited by the amount of enzyme adsorbed in the mesopores at the surface of the carbon particles. In contrast, a high current density was obtained when the MgOC pore size was much larger than the enzyme size since a sufficient amount of the enzyme could be adsorbed in the mesopores on the surface of, and inside, the MgOC materials [Citation52,Citation53]. The presence of a macropore significantly improved the electrode performance. The DET catalytic current based on an MgOC electrode with a 40-nm mesopore was improved by introducing macropores for smooth mass transfer using 200-nm MgO particles as a template [Citation54]. Another approach to successfully satisfy the requirements of both a large specific surface area along with stable entrapment for enzyme loading and rapid mass transport of fuel is to design a three-dimensional (3D) hierarchical pore structure to improve the current production efficiency and stability of direct electron transfer-type biocathodes. Such a 3D hierarchical electrode structure was fabricated using an MgO-templated porous carbon framework produced from a mixture of MgO templates with different crystalline sizes of 40 nm and 150 nm. The maximum current was almost double that detected on the electrode containing MgOC with pore sizes of 40 nm or 150 nm. Thus, the macropores improved mass transfer inside the carbon material, and the mesopores improved the electron transfer efficiency of the enzyme by surrounding the enzyme with carbon [Citation55].
Conclusions
Building a rational bioelectronics system requires gaining a detailed understanding of the properties of the enzyme, which can inform the appropriate design of reaction materials and material development based on the bioelectrochemical theory to consequently dramatically increase the activity of the enzyme electrode. This in turn leads to improvements in the sensitivity and accuracy of biosensor systems along with improvement in the output power of the BFCs, allowing for functional integration with technologies such as electronics. Such applications are expected to lead to innovations such as wearable type healthcare devices powered by BFC to which printing technology can be applied ((f, g)) [Citation49,Citation56–Citation58], and further enabling in vivo operations. Nevertheless, bioelectrocatalysis in an artificial reaction field differs from the function in a natural biological environment; thus, it is possible that the enzyme would exhibit different functions, especially at the nanometer scale confined space or in high salt concentration solutions. The design of a novel reaction field that differs from living systems opens up new opportunities for the drastic improvement of enzyme activity or stability. In the future, the author plans to clarify the theory of the specific influence of such environmental factors, while elucidating the enzymatic function and providing a new methodology for the development of biomimetic catalysts.
Acknowledgments
I thank all the coworkers of this study. I am especially grateful to Professor Kenji Kano (Kyoto University) and Emeritus Professor Tokuji Ikeda (Kyoto University) for their continuous encouragement and valuable discussion.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Ikeda T, Kano K. An electrochemical approach to the studies of biological redox reactions and their applications to biosensors, bioreactors, and biofuel cells. J Biosci and Bioeng. 2001;92:9–18.
- Barton SC, Gallaway J, Atanassov P. Enzymatic biofuel cells for implantable and microscale devices. Chem Rev. 2004;104:4867–4886.
- Tsujimura S, Kojima S, Kano K, et al Novel FAD-dependent glucose dehydrogenase to construct dioxygen-insensitive glucose biosensor. Biosci Biotech Biochem. 2006;70:654–659.
- Tsujimura S, Kojima S, Ikeda T, et al Potential-step coulometry of D-glucose using novel FAD-dependent glucose dehydrogenase. Anal Bioanal Chem. 2006;386:645–651.
- Murata K, Akatsuka W, Sadakane T, et al Glucose oxidation catalyzed by FAD-dependent glucose dehydrogenase within Os complex-tethered redox polymer hydrogel. Electrochim Acta. 2014;136:537–541.
- Tsujimura S, Kano K, Ikeda T. Electrochemical oxidation of NADH catalyzed by diaphorase conjugated with poly-1-vinylimidazle complexed with Os(2,2’-dipyridylamine)2Cl. Chem Lett. 2002;31:1022–1023.
- Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008;108:2482–2505.
- Suraniti E, Vives S, Tsujimura S, et al Designing thin films of redox hydrogel for highly efficient enzymatic anodes. J Electrochem Soc. 2013;160:G79–G82.
- Inagaki M, Toyoda M, Soneda Y, Tsujimura S, Morishita T. Templated mesoporous carbons: synthesis and applications. Carbon. 2016;107:448–473.
- Murata K, Akatsuka W, Tsujimura S. Bioelectrocatalytic oxidation of glucose on a MgO-templated mesoporous carbon modified electrode. Chem Lett. 2014;43:928–930.
- Tsujimura S, Murata K, Akatsuka W. Exceptionally high glucose current on a hierarchically structured porous carbon electrode with “wired” flavin adenine dinucleotide-dependent glucose dehydrogenase. J Am Chem Soc. 2014;136:14432–14437.
- Suzuki A, Murata K, Mano N, Tsujimura, S. Redox hydrogel of glucose oxidase on MgO-templated carbon electrode. Bull Chem Soc Jpn. 2016;89:24–26.
- Suzuki A, Tsujimura S. Hofmeister effects on the glucose oxidase hydrogel-modified electrode. Electrochim Acta. 2016;201:228–232.
- Suzuki A, Tsujimura, S. Long-term continuous operation of FAD-dependent glucose dehydrogenase hydrogel-modified electrode at 37°C. Chem Lett. 2016;45:484–486.
- Suzuki A, Mano N., Tsujimura S. Lowering the potential of electroenzymatic glucose oxidation on redox hydrogel-modified porous carbon electrode. Electrochim Acta. 2017;232:581–585.
- Tsuruoka N. Sadakane T, Hayashi R, Tsujimura S. Bimolecular rate constants for FAD-dependent glucose dehydrogenase from aspergillus terreus and organic electron acceptors. Int J Mol Sci. 2017;18:604.
- Milton RD, Hickey DP, Abdellaoui S, et al Rational design of quinones for high power density biofuel cells. Chem Sci. 2015;6:4867–4875.
- El-Hout SI, Suzuki H, El-Sheikh SM, et al Tuning the redox potential of vitamin K3 derivatives by oxidative functionalization using Ag(I)/GO catalyst. ChemComm. 2017;53:8890–8893.
- Mano N, de Poulpiquet A. O2 reduction in enzymatic biofuel cells. Chem Rev. 2018;118:2392–2468.
- Murao S, Tanaka N. Isolation and identification of a microorganism producing bilirubin oxidase. Agric Biol Chem. 1982;46:2031–2034.
- Tanaka N, Murao S. Purification and some properties of bilirubin oxidase of myrothecium verrucaria MT-1. Agric Biol Chem. 1982;46:2499–2503.
- Tsujimura S, Fujita M, Tatsumi H, Kano K, Ikeda T. Bioelectrocatalysis-based dihydrogen/dioxygen fuel cell operating at physiological pH. Phys Chem Chem Phys. 2001;3:1331–1335.
- Tsujimura S, Wadano A, Kano K. Ikeda T. Photosynthetic bioelectrochemical cell utilizing cyanobacteria and water-generating oxidase. Enz Microb Tech. 2001;29:225–231.
- Tsujimura S, Kano K., Ikeda T. Glucose/O2 biofuel cell operating at physiological conditions. Electrochemistry. 2002;70:940–942.
- Tsujimura S, Tatsumi H, Ogawa J, Shimizu S, Kano K, Ikeda T. Bioelectrocatalytic reduction of dioxygen to water at neutral pH using bilirubin oxidase as an enzyme and 2,2’-azinobis (3-Ethylbenzothiazolin-6-Sulfonate) as an electron transfer mediator. J Electroanal Chem. 2001;496:69–75.
- Tsujimura S, Kuriyama A, Fujieda N, Kano K, Ikeda T. Mediated spectroelectrochemical titration of proteins for redox potential measurements by a separator-less one-compartment bulk electrolysis method. Anal Biochem. 2005;337:325–331.
- Kuriyama A, Arasaki M, Fujieda N, Tsujimura S, Kano K, Ikeda T. Separator-less one-compartment bulk electrolysis with a small auxiliary electrode and its application to spectroelectrochemistry. Electrochemistry. 2004;72:484–486.
- Miura Y, Tsujimura S, Kamitaka Y, Kurose S, Kataoka K, Sakurai T, Kano K. Bioelectrocatalytic reduction of O2 catalyzed by CueO from escherichia coli adsorbed on a highly oriented pyrolytic graphite electrode. Chem Lett. 2007;36:132–133.
- Tsujimura S, Miura Y, Kano K. CueO-immobilized porous carbon electrode exhibiting improved performance of electrochemical reduction of dioxygen to water. Electrochim Acta. 2008;53:5716–5720.
- Tsujimura S, Asahi M, Goda-Tsutsumi M, Shirai O, Kano K, Miyazaki K. Direct electron transfer of a metagenome-derived laccase fused to affinity tags near the electroactive copper site. Phys Chem Chem Phys. 2013;15:20585–20589.
- Murata K, Shigemori Y, Tsujimura S. Electrochemical activation of a novel laccase, MELAC, isolated from compost. Chem Lett. 2015;44:654–655.
- Kataoka K, Kogi H, Tsujimura S, Sakurai T. Modifications of laccase activities of copper efflux oxidase, CueO by synergistic mutations in the first and second coordination spheres of the type I copper center. Biochem Biophys Res Commun. 2013;431:393–397.
- Miura Y, Tsujimura S, Kurose K, Kamitaka Y, Kataoka K, Sakurai T, Kano K. Direct electrochemistry of CueO and its mutants at residues to and near type I Cu for oxygen-reducing biocathode. Fuel Cells. 2009;9:70–78.
- Nakagawa T, Tsujimura S, Kano K, Ikeda T. Bilirubin oxidase and [Fe(CN)6]3-/4- modified electrode allowing diffusion-controlled reduction of O2 to water at pH 7.0. Chem Lett. 2003;32:54–55.
- Ishibashi K, Tsujimura S, Kano K. Pentacyanoferrate and bilirubin oxidase-bound polymer for oxygen reduction bio-cathode. Electrochemistry. 2008;76:594–596.
- Tsujimura S, Kawaharada M, Nakagawa T, Kano K, Ikeda T. Mediated bioelectrocatalytic O2 reduction to water at highly positive electrode potentials near neutral pH. Electrochem Commun. 2003;5:138–141.
- Suraniti E, Tsujimura S, Durand F, et al Thermophilic biocathode with bilirubin oxidase from bacillus pumilus. Electrochem Commun. 2013;26:41–44.
- Tsujimura S, Nakagawa T, Kano K, Ikeda T. Kinetic study of direct bioelectrocatalysis of dioxygen reduction with bilirubin oxidase at carbon electrodes. Electrochemistry. 2004;72:437–439.
- Kamitaka Y, Tsujimura S, Ikeda T, Kano K. Electrochemical quartz crystal microbalance study on adsorption of bilirubin oxidase as a catalyst in bioelectrocatalytic reduction of dioxygen. Electrochemistry. 2006;74:642–644.
- Kamitaka Y, Tsujimura S, Kataoka K, Sakurai T, Ikeda T and Kano K. Effects of axial ligand mutation of the type I copper site in bilirubin oxidase on direct electron transfer-type bioelectrocatalytic reduction of dioxygen. J Electroanal Chem. 2007;601:119–124.
- Tsujimura S. Abo, T., Matsushita, K., Ano, Y., Kano, K. Direct electron transfer reaction of D-Gluconate 2-dehydrogenase adsorbed on bare and thiol-modified gold electrodes. Electrochemistry. 2008;76:549–551.
- Tsujimura S. Kano, K., Ikeda, T. Bilirubin oxidase in multiple layer catalyzes four-electron reduction of dioxygen to water without redox mediators. J Electroanal Chem. 2005;576:113–120.
- Tsujimura S. Kamitaka, Y., Kano, K. Diffusion-controlled oxygen reduction on multi-copper oxidase-adsorbed carbon aerogel electrodes without mediator. Fuel Cells. 2007;7:463–469.
- Tsujimura S. Murata, K. Electrochemical oxygen reduction catalyzed by bilirubin oxidase with the aid of 2,2’-azinobis(3-ethylbenzothiazolin-6-sulfonate) on a MgO-template carbon electrode. Electrochim Acta. 2015;180:555–559.
- Kamitaka Y, Tsujimura S, Setoyama N, et al Fructose/dioxygen biofuel cell based on direct electron transfer-type bioelectrocatalysis. Phys Chem Chem Phys. 2007;9:1793–1801.
- Kontani R, Tsujimura S, Kano K. Air diffusion biocathode with CueO as electrocatalyst adsorbed on carbon particle modified electrodes. Bioelectrochemistry. 2009;76:10–13.
- Asano I. Hamano, Y., Tsujimura S., Shirai, O., Kano K. Improved performance of gas-diffusion biocathode for oxygen reduction. Electrochemistry. 2012;80:324–326.
- Niiyama A, Tsujimura S. High power glucose/o2 biofuel cell constructed from MgO-temlated carbon modified carbon cloth. ECS Meet Abstr. 2016;41:3261.
- Shitanda I, Kato S, Hoshi Y, et al Flexible and high-performance paper-based biofuel cells using printed porous carbon electrodes. Chem, Comm. 2013;49:11110–11112.
- Sakai H. Nakagawa, T., Sato, A., Tomita, T., Tokita, Y., Hatazawa, T., Ikeda, T., Tsujimura, S., Kano, K. A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ Sci. 2009;2:133–138.
- Nakagawa T. Mita, H., Kumita, H., Sakai, H., Tokita, Y., Tsujimura, S. Water-repellent-treated enzymatic electrode for passive air-breathing biocathodic reduction of oxygen. Electrochem Commun. 2013;36:46–49.
- Funabashi H. Murata, K., Tsujimura, S. Effect of pore size of MgO-templated carbon on the direct electrochemistry of D-fructose dehydrogenase. Electrochemistry. 2015;83:372–375.
- Mazurenko I, Clement R, Byrne-Kodjabachian D, et al Pore size effect of MgO-templated carbon on enzymatic H2 oxidation by the hyperthermophilic hydrogenase from Aquifex aeolicus. J Electroanal Chem. 2018;812:221–226.
- Shitanda I. Nakafuji, H., Tsujimura, S., Hoshi, Y., Itagaki, M. Electrochemical impedance study of screen-printed branch structure porous carbon electrode using MgO-templated carbon and MgO particle and its application for bilirubin oxidase-immobilized biocathode. Electrochemistry. 2015;83:329–331.
- Funabashi H. Takeuchi, S., Tsujimura, S. Hierarchical meso/macro-porous carbon fabricated from dual MgO templates for direct electron transfer enzymatic electrodes. Sci Rep. 2017;7:45147.
- Shitanda I. Kato, S., Tsujimura, S., Hoshi, Y., Itagaki, M. Screen-printed, paper-based, array-type, origami biofuel cell. Chem Lett. 2017;46:726–728.
- Shitanda I. Momiyama, M., Watanabe, N., Tanaka, T., Tsujimura, S., Hoshi, Y., Itagaki, M. Toward wearable energy storage devices: paper-based biofuel cells based on a screen-printing array structure. ChemElectroChem. 2017;4:2460–2463.
- Shitanda I. Nohara, S, Hoshi, Y., Itagaki, M.,Tsujimura, S. A screen-printed circular-type paper-based glucose/O2 biofuel cell. J Power Sources. 2017;360:516–519.