ABSTRACT
The gene encoding copper-dependent laccase from Bacillus subtilis strain R5 was cloned and expressed in Escherichia coli. Initially the recombinant protein was produced in insoluble form as inclusion bodies. Successful attempts were made to produce the recombinant protein in soluble and active form. The laccase activity of the recombinant protein was highly dependent on the presence of copper ions in the growth medium and microaerobic conditions during protein production. The purified enzyme exhibited highest activity at 55 °C and pH 7.0. The recombinant protein was highly thermostable, albeit from a mesophilic source, with a half-life of 150 min at 80 °C. Similar to temperature, the recombinant protein was stable in the presence of organic solvents and protein denaturants such as urea. Furthermore, the recombinant protein was successfully utilized for the degradation of various synthetic dyes reflecting its potential use in treatment of wastewater in textile industry.
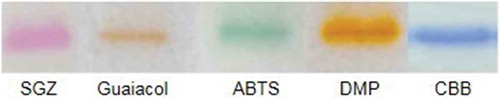
Abbreviations: ABTS,2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; CBB, Coomassie brilliant blue; SGZ, syringaldazine; DMP, 2,2-dimethoxy phenol.
Graphical Abstract

Decolourization of various synthetic dyes by recombinant Bsu-Lac. Laccase activity was measured without the addition of any metal ion in the reaction mixture.
Laccases are multinuclear copper-containing oxidoreductases which possess four redox-active copper atoms attached to the conserved cysteine-histidine residues by coordinate binding [Citation1,Citation2]. They catalyze the oxidation of wide variety of phenolic substrates with the subsequent reduction of oxygen to water [Citation3]. In addition to the phenolic compounds, laccases can utilize non-phenolic substrates with the help of mediator compounds [Citation2,Citation4]. They are industrially relevant enzymes performing diverse oxidative functions. They are being used for dye decolorization in textile industry [Citation5], for pulp biobleaching in paper industry [Citation6], for the removal of unwanted phenolics in food processing [Citation7], for the synthesis of functional organic compounds [Citation8,Citation9], in drugs and antibiotics synthesis, in pharmaceutical field [Citation10], and for environmental bio-remediation [Citation11]. Laccases are reported from insects, plants, fungi, bacteria and archaea. Among them fungal laccases are extensively characterized. However, they become inactivated at elevated temperature [Citation12] which make their use limited in industrial applications requiring higher temperature. In contrast, bacterial laccases are more stable at high temperatures [Citation13]. Among bacteria, the laccases have been mainly reported from genus Bacillus. They have been characterized from Bacillus subtilis [Citation14‒Citation15], Bacillus halodurans [Citation16], Bacillus amyloliquefaciens [Citation17], Bacillus clausii [Citation18], Bacillus licheniformis [Citation19], Bacillus vallismortis [Citation20], Bacillus tequilensis [Citation21], Bacillus pumilus [Citation22], Bacillus altitudinis [Citation23], and Bacillus thuringiensis [Citation24].
We have characterized a novel laccase from Geobacillus thermopakistaniensis, the first laccase characterized from genus Geobacillus, and identified the encoding gene which is different than the previously annotated laccase in the genomes of various members of this group [Citation25]. This laccase can efficiently decolorize several synthetic dyes, however, no decolorization of bromophenol blue and reactive orange was observed. In order to explore a thermostable laccase which can be used to decolorize these dyes alone or with the combination of the other enzymes, we analyzed a laccase homologue (Bsu-Lac) from Bacillus subtilis R5, a strain isolated from Osaka which produces several extracellular enzymes [Citation26]. Although Bacillus species grow at moderate temperatures, a few enzymes characterized from them are heat stable [Citation13]. In the present study, we have cloned the gene encoding Bsu-Lac and characterized the purified gene product produced in Escherichia coli. Application of this enzyme in decolorization of synthetic dyes has also been explored.
Materials and methods
Bacterial strains and growth conditions
Bacillus subtilis strain R5 was cultivated in LB (tryptone 1%, yeast extract 0.5%, NaCl 0.5%, pH 7.0) medium at 30 °C. Commercial grade general chemicals and materials were purchased either from Sigma Aldrich or Fluka Chemical Corp. DNA polymerases, restriction enzymes, cloning vectors, and DNA purification kits were all purchased from Thermo-Fisher Scientific. Gene specific primers were commercially synthesized by Macrogen. The vector pTZ57R/T (Thermo-Fisher) was used for cloning while pET-28a(+) (Novagen) was used as an overexpression vector for the target gene. Escherichia coli cells DH5α and BL21-CodonPlus(DE3)-RIL (Stratagene) were used as host organisms for gene cloning and heterologous expression, respectively.
Cloning of the Bsu-Lac gene
The primers, for amplification of Bsu-Lac gene by polymerase chain reaction (PCR), were designed based on the nucleotide sequence of the corresponding gene in Bacillus subtilis. Bsu-Lac gene was amplified from genomic DNA of strain R5 using a set of forward (5ʹ-CATATGACACTTGAAAAATTTGTGGATGCTCTCC, and reverse (5ʹ- CTTGCCTGCTAGAGGCAAGTTTGTCG) primers. NdeI site, introduced in the forward primer, is underlined. The PCR amplified Bsu-Lac gene was ligated in pTZ57R/T and the resulting plasmid was named pTZ-Bsu-Lac. The recombinant plasmid, pTZ-Bsu-Lac, was cut with NdeI (introduced in the forward primer) and EcoRI (present in the gene) and the liberated part of Bsu-Lac gene was cloned in pET-28a(+) expression vector. The resulting construct (containing partial Bsu-Lac gene) was named pET-Bsu-LacP. For cloning the second part of the gene, recombinant plasmid pTZ-Bsu-Lac was cut with EcoRI and HindIII and the liberated gene fragment was ligated in pET-Bsu-LacP. The resulting plasmid (containing the full length gene) was named as pET-Bsu-LacF.
Expression of Bsu-Lac gene in E. coli
Expression strain, BL21-CodonPlus (DE3)-RIL cells were transformed using pET-Bsu-LacF recombinant plasmid and expression was induced by 0.2 mM IPTG in the presence of 0.5 mM copper sulfate. After 4 h of post-induction at 37 °C and 120 rpm, shaking was stopped in order to provide microaerobic conditions. Cells were harvested and centrifugation was done at 6000 × g. The pellet was resuspended in 50 mM Tris-HCl (pH 8.0) and sonication was done to lyse the cells. The lysed cells were centrifuged at 12,000 × g to separate the soluble and insoluble fractions. Analysis of proteins was done by 12% SDS-PAGE. Production of recombinant Bsu-Lac (rBsu-Lac) was also examined at low temperature (17 °C) with or without a heat shock of 30 min at 45 °C (prior to induction).
Purification of rBsu-Lac
Soluble fraction, containing recombinant rBsu-Lac, was partially purified by heat treatment at 60 °C for 45 min. Heat-labile proteins of the host, E. coli, were denatured and removed by centrifugation. The soluble fraction, after centrifugation, was dialyzed against 0.5 M NaCl and 25 mM imidazole, made in 50 mM Tris-HCl buffer (pH 8.0), for overnight and then applied to high capacity nickel chelate affinity matrix Ni-CAM HC Resin (Sigma) column which was equilibrated with the same buffer. The bound proteins were eluted with a step-wise gradient of 25, 50, 75, 100, 150, 200, 250 and 300 mM imidazole. The eluted proteins were dialyzed and applied to anion exchange, HiTrap QFF, column. The bound proteins were eluted with a linear gradient of 0–100% of 1 M NaCl. The eluted fractions were analyzed by 12% SDS-PAGE.
Laccase activity assay and zymography
Laccase activity was examined by measuring the rate of oxidation of syringaldazine (SGZ) at 530 nm. The assay mixture contained 30 μL SGZ (stock solution 0.2 mM), 950 μL of 50 mM sodium phosphate buffer (pH 7.0) and 20 μL (10–20 μg) rBsu-Lac in a reaction volume of 1 mL. Final concentration of SGZ in the reaction mixture was 6 μM. For calculating the laccase activity molar extinction coefficient of the oxidized SGZ, ε = 65,000 M−1 cm−1, was used. Laccase activity was measured by continuous rate determination using a Shimadzu UV-1601 spectrophotometer equipped with a thermoelectric cell.
For zymography, the protein samples were prepared in 1× loading dye (without reducing agent) and analyzed by 12% SDS-PAGE. After electrophoresis, the gel was washed for 10 min with distilled water and then soaked with SGZ solution (with gentle agitation) to observe the color change.
Effect of pH, temperature and metal ions
To determine the effect of pH on laccase activity of rBsu-Lac, assays were performed at various pH in different buffers but fixed temperature at 55 °C. The pH of all the buffers used was adjusted at room temperature. The buffers used were: 50 mM MES (5.5‒6.0), 50 mM sodium phosphate (6.0‒7.5) and 50 mM Tris-HCl (7.5‒8.5).
The effect of temperature on the rBsu-Lac activity was determined by examining the laccase activity at various temperatures ranging from 30 to 70 °C and a fixed pH of 7.0 in 50 mM sodium phosphate. The reaction mixture was kept at respective temperature for 3 min before the addition of the substrate SGZ.
Thermostability of rBsu-Lac was examined at 60 and 80 °C. The rBsu-Lac was heated at respective temperature for 0–200 min and residual activity was measured at 55 °C and pH 7.0 using SGZ as substrate.
The effect of various metal ions on laccase activity of rBsu-Lac was examined by adding the chloride salts of Fe2+, Co2+, Cu2+, Ni2+, Mg2+, Mn2+, Hg2+, and Zn2+ in the reaction mixture at a final concentration of 100 μM. Activity without the addition of any metal ion was considered as 100%.
Effect of inhibitors, organic solvents and various chemicals/detergents on laccase activity
The effect of different inhibitors including NaN3, L-cysteine, EDTA, DTT and SDS was examined on laccase activity of rBsu-Lac. All inhibitors were used at a final concentration of 0.1, 1 and 5 mM. The activity assay was performed in the same way as described above using SGZ as substrate.
Effect of organic solvents including methanol, ethanol, acetone, propanol, 2-propanol and DMSO at a final concentration of 10 and 20% was examined on the laccase activity of Bsu-Lac. The activity was measured at 55 °C and pH 7.0 in 50 mM sodium phosphate using SGZ as a substrate.
Laccase activity of rBsu-Lac was also checked in the presence of various chemicals/detergents including Triton X-100, Tween 20, Tween 80, ß-mercaptoethanol, glycerol, and imidazole at a final concentration of 1 or 10%. The activity assay was performed in the same way as described above using SGZ as substrate. The activity without the addition of these reagents was considered as 100%.
Kinetic studies
Steady state enzyme kinetics for oxidation of SGZ was measured in triplicates in 50 mM sodium phosphate buffer (pH 7.0) by varying the concentrations of substrate from 0 to 40 μM. The kinetic parameters, Km, Vmax and kcat were calculated from the Michaelis-Menten plot.
Circular dichorism analysis
For stability studies the far-UV CD spectrum of purified rBsu-Lac was analyzed at 50, 60, 70, 80 and 90 °C at a wavelength range of 200–280 nm. The protein used for thermostability studies was in 50 mM Tris-HCl buffer (pH 8.0) with a concentration of 0.7 mg/mL.
Stability against urea
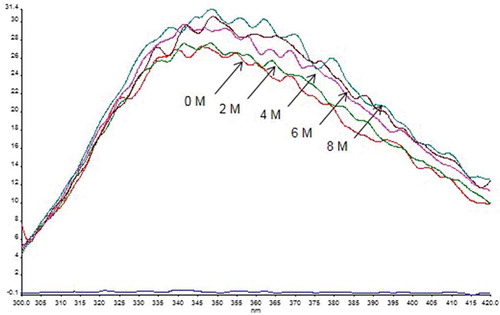
For denaturation studies, purified protein samples at a final concentration of 0.2 mg/mL, prepared in different concentrations of urea (0–8 M final concentration) were incubated at room temperature for 4 h or overnight. Fluorescence of the samples was recorded as an emission scan from 300–400 nm with excitation monochromator at 280 nm in a 1 cm quartz cuvette using PerkinElmer LS45 fluorescence spectrometer. The data were analyzed using PerkinElmer FL WinLab v4.00.03 software.
Application of rBsu-Lac in dye decolorization
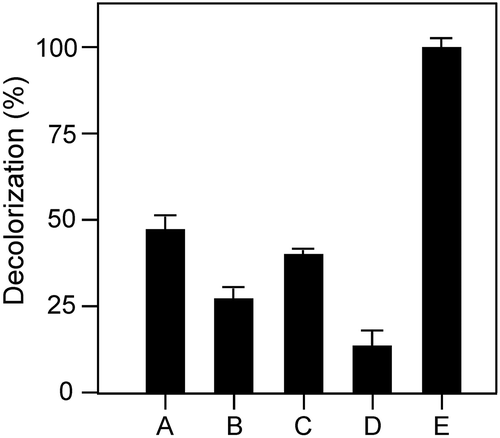
Decolorization of synthetic dyes including methyl red, crystal violet, bromophenol blue, orange G, and congo red was performed in 2 mL eppendorf tubes containing the appropriate buffer, respective dye and rBsu-Lac in 50 mM sodium phosphate (pH 7.0). Dye solution at a concentration of 200 mg/L was prepared in distilled water. The reaction mixture was incubated at 55 °C for various intervals of time. Decolorization percentage was determined by monitoring absorbance of the sample using a UV-vis spectrophotometer at a wavelength where the dye exhibits maximum absorbance (λ maximum). Absorption of methyl red was measured at 410 nm, crystal violet at 590 nm, bromophenol blue at 595 nm, orange G at 485 nm, and congo red at 495 nm. The negative control was prepared by adding the heat-inactivated enzyme to the dye solution. All experiments were performed in triplicate.
Results
Gene cloning, expression optimization and purification of rBsu-Lac
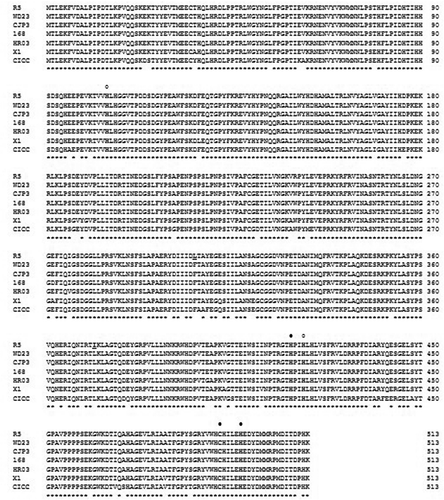
The PCR, using gene specific primers, resulted in the amplification of 1.5 kb DNA fragment matching the size of the Bsu-Lac gene. Restriction digestion analysis of pTZ-Bsu-Lac using NdeI and HindIII resulted in liberation of 1.5 kb DNA fragment confirming the presence of Bsu-Lac gene. Similarly restriction analysis of pET-Bsu-LacF, using NdeI and HindIII, confirmed the presence of Bsu-Lac gene in the expression vector (data not shown). The complete sequence of the cloned gene was determined by DNA sequencing using M13 forward and reverse primers. DNA sequence analysis showed that laccase gene consisted of 1,542 nucleotides, which encodes a protein of 513 amino acids with a calculated molecular weight of 58,499 Da and a theoretical pI of 5.91. The amino acid sequence showed 99% identity with copper-dependent laccases of various strains of Bacillus subtilis ().
Figure 1. Alignment of amino acid sequence of characterized laccases from various strains of B. subtilis. Amino acids conserved in all the sequences are shown by asterisks below the sequence. The amino acid residues distinct in Bsu-Lac are bold underlined. The residues involved in the binding of copper at the T1 and T2 sites are shown by filled and unfilled circles, respectively, at the top of the sequence. Following are the accession numbers of the sequences used for alignment: WD23, AID81987; cjp3, ADZ57279; 168, CAB12449; HR03, FJ663050; X1, AGK12417; CICC20613, JN043511.
Expression of the Bsu-Lac gene, in E. coli BL21-CodonPlus(DE3)-RIL at 37 °C, resulted in production of the recombinant protein mainly in insoluble form (data not shown). Therefore, the expression of Bsu-Lac gene was examined at 17 °C and found that about 30% of the total recombinant protein was produced in the soluble fraction. A comparison of Bsu-Lac production at 37 and 17 °C is shown in . Furthermore, expression of Bsu-Lac gene was optimized using different concentrations of copper in the growth medium. Expression level was almost same in cells grown in the presence of 250 μM to 1 mM of copper (), however, the activity was higher in cells grown in the presence of 500 μM to 1 mM ().
Figure 2. Coomassie brilliant blue stained 12% SDS-PAGE showing the expression of Bsu-Lac gene at 37 and 17 °C. Lanes 1–3 represent expression at 37 °C and lanes 4–6 show expression at 17 °C. Lanes 1 and 4, total cell lysate; lanes 2 and 5, soluble fractions; lanes 3 and 6, insoluble fractions.
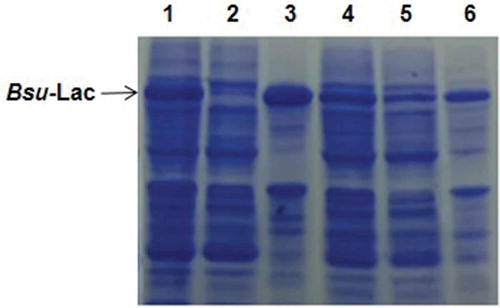
Figure 3. Expression levels and laccase activity comparisons in E. coli cells grown in the presence of various concentrations of copper. (a) Coomassie brilliant blue stained 12% SDS-PAGE showing expression levels at 17 °C. Lanes 1–5, soluble lysates of the cells grown in the presence of 0, 0.1, 0.25, 0.5 and 1 mM copper, respectively; lane 6, purified rBsu-Lac. (b) Comparison of relative activity of rBsu-Lac produced in E. coli cells grown in the presence of different concentrations of copper. Values are the averages from three measurements, and bars indicate the SD values.
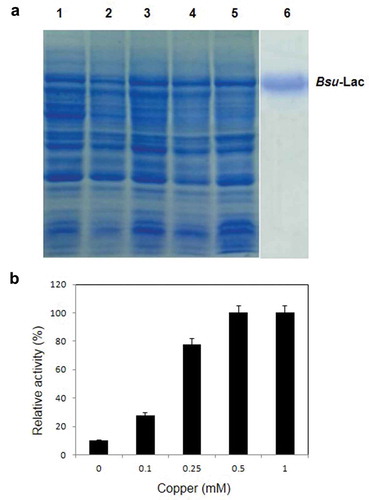
First step for purification of rBsu-Lac was heat treatment of the soluble fraction of E. coli cell lysate at 60 °C for 30 min which resulted in precipitation of several proteins of the host. The precipitated proteins were removed by centrifugation and the soluble fraction was purified to homogeneity using nickel affinity chromatography where it eluted between 75 to 100 mM imidazole. The purified protein, to apparent homogeneity, is shown in .
Purified rBsu-Lac was analyzed for in-gel activity assay using SGZ, Guaiacol, ABTS and DMP as substrates. The results demonstrated that rBsu-Lac was active against all the four substrates ().
Effect of temperature and pH
For measurement of effect of temperature, laccase activity of rBsu-Lac was examined at various temperatures ranging from 30 to 70 °C, keeping the pH constant. The enzyme mixture, without the substrate, was incubated at respective temperature for 5 min and then the reaction was started with the addition of SGZ. Highest activity was observed at 55 °C ().
Figure 5. Effect of temperature and pH on enzyme activity of Bsu-Lac. (a) Effect of temperature. Activity assays were performed in 50 mM sodium phosphate (pH 7.0) buffer at 30–70 °C. (b) Effect of pH. Activity assays were conducted at 55 °C using MES buffer (pH 5.5–6.0, circles); sodium phosphate buffer (pH 6.0–7.5, squares) and Tris-HCl buffer (pH 7.5–8.5, triangles). Values are the averages from three measurements, and bars indicate the SD values.
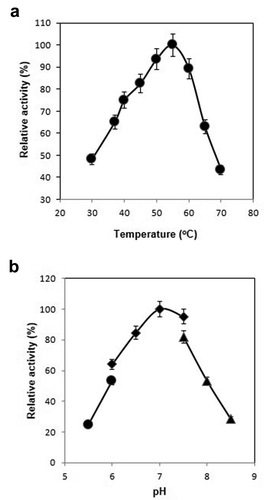
To determine the optimum pH, enzyme activity was examined in buffers of various pH at a fixed temperature. Highest activity was found at pH 7.0 in sodium phosphate buffer ().
Effect of inhibitors on laccase activity
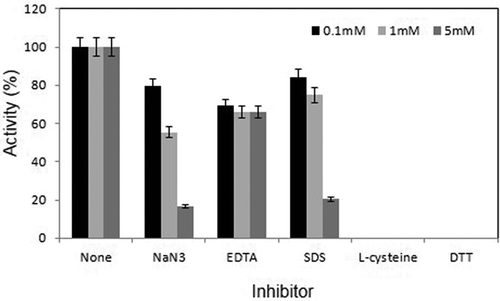
Effect of various chemical inhibitors such as EDTA, SDS, DTT, L-cysteine and sodium azide was examined at 55 °C and pH 7.0. All inhibitors were used at 0.1, 1 and 5 mM concentrations in the assay mixture. The results showed that L-cysteine and DTT completely inactivated the enzymatic activity even at 0.1 mM concentration. There was a slight but equivalent inhibition of activity in the presence of 0.1, 1 and 5 mM EDTA. SDS and NaN3 inhibited activity only at higher concentrations (). Furthermore, effect of various water-miscible organic solvents including methanol, ethanol, propanol, isopropanol, acetone and dimethyl sulfoxide was examined at a final concentration of 10 or 20% (v/v) in the assay mixture. There was no significant effect on the enzyme activity in the presence of these solvents at a concentration of 10%. However, at 20%, the laccase activity was reduced to half by all the solvents examined, except for methanol where no inhibition was detected (data not shown). In addition to organic solvent, effect of Triton X-100, Tween 20, Tween 80, Glycerol and ß-mercaptoethanol was examined on the laccase activity of rBsu-Lac. The activity was reduced to almost 20% in the presence of Triton X-100, Tween 20 and Tween 80. Glycerol did not show a significant effect. No activity could be detected in the presence of ß-mercaptoethanol (data not shown).
Kinetic studies
Kinetic parameters were determined at 55 °C and pH 7.0 using various concentrations of SGZ. The enzyme activity with various concentration of the substrate was determined and Michaelis-Menten plot was made to determine Km and Vmax values. Km and Vmax values calculated from the plot were 12.72 μM and 11.59 μmol min−1 mg−1, respectively. From the data, a kcat value of 302 min−1 was calculated. Catalytic efficiency (kcat/Km) was found to be 393 mM−1s−1.
Thermostability analysis of Bsu-Lac
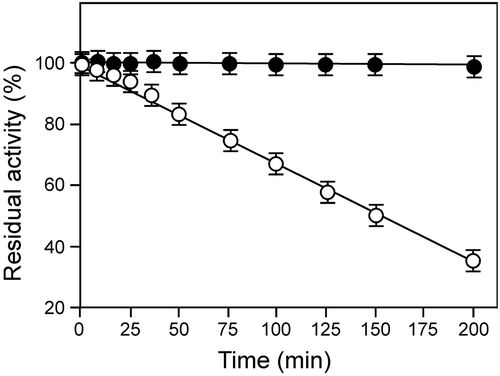
Thermostability experiments were performed by incubating the protein at 60 or 80 °C in sodium phosphate buffer at pH 7.0 for various intervals of time. The enzyme activity was examined at 55 °C. rBsu-Lac was thermally stable, with no significant loss in activity, upto 200 min at 60 °C while it exhibited a half-life of 150 min at 80 °C ().
Circular dichroism analysis
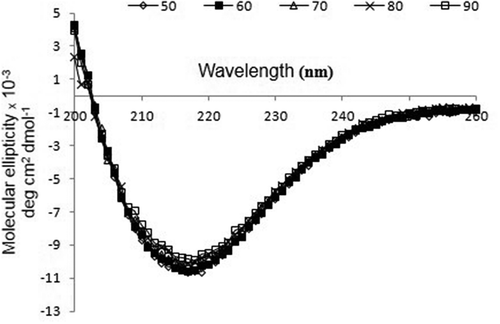
CD spectrum of rBsu-Lac was analyzed at temperatures ranging from 50 to 90 °C with every 10 degree difference and 1 min heating at each temperature using 0.5 mg/mL protein. No significant change in the spectrum was observed up to 90 °C which indicated that the rBsu-Lac maintained its secondary structure at elevated temperatures ().
Urea-based stability studies of rBsu-Lac
In order to examine the stability studies in the presence of urea, rBsu-Lac, 0.1 mg/mL, was incubated in 2 mL mixture containing 0–8 M urea (made in 50 mM Tris-HCl pH 8.0) and incubated at room temperature for 4 h or overnight. There was no significant change in spectra after 4 h or overnight incubation with urea till 8 M, suggesting that rBsu-Lac is structurally stable even in 8 M urea (). Similar to spectra, there was no significant difference in the laccase activity of these samples.
Dye decolorizartion
Usually laccase needs copper ions for decolorization of dyes. Addition of copper contaminates the environment. To examine the effect of rBsu-Lac on the decolorization of pollutant dyes, decoloization assay was performed in the absence of any additional copper ions. rBsu-Lac successfully decolourized bromphenol blue, though at a slower rate, without the addition of copper. Highest rate of color removal was found for orange G ().
Discussion
Most of the bacterial laccases are produced in insoluble form when the corresponding genes are expressed in E. coli [Citation13,Citation27,Citation28]. Several attempts have been reported for the solubilization and refolding of laccase from various species of genus Bacillus. However, most of them were ineffective. In order to obtain the active enzyme, correct positioning of copper ions is necessary. rBsu-Lac was produced in high amount when the corresponding gene was expressed in E. coli. However, most of the protein was produced in insoluble and inactive form. We attempted production of rBsu-Lac in soluble and active form by lowering the expression temperature, giving heat shock to the host cells before induction and incubating the cells without shaking to limit the aeration. This resulted in a slight, but not significant, increase in production of recombinant protein in soluble form. The enzyme activity of rBsu-Lac was highly dependent on the presence of copper and limited oxygen conditions during protein production. Purified rBsu-Lac was not dependent on addition of copper ions in assay mixture for activity, it needed copper ions only during the production stage which reflects that copper ions have a role in proper folding of rBsu-Lac similar to other laccases [Citation29].
Enzyme activity of rBsu-Lac was inhibited by the known inhibitors of laccases such as sodium azide and EDTA. The inhibition by sodium azide can be a result of the binding of N3− to the trinuclear copper center, disturbing the internal electron transfer, which ultimately affect the overall oxidation process catalyzed by laccases [Citation21,Citation30]. EDTA, probably deprived the copper ions present at type 1 copper center and inhibited the enzyme activity [Citation31]. No enzyme activity in the presence of reducing agents, such as DTT and L-cysteine, can be attributed to reduction of the oxidized substrate by the sulfhydryl groups of the redox reagents [Citation32].
Presence of higher levels of denaturants, such as urea, may negatively modulate the enzyme structure which in turn results in reduced enzyme activity. In the present study it was observed that there was no significant change in fluorescence spectra as well as the enzyme activity even in the presence of 8 M urea which reflects that urea did not induce conformational changes in the rBsu-Lac structure. Similarly, rBsu-Lac was stable at higher temperatures, as determined by activity assays and CD spectroscopy, though it was originated from a mesophilic source. The high thermostability may be attributed to the presence of large number of proline residues (44, 8.8%, highest among all the amino acids) and Glu (36, 7.2%) which provide compactness to the protein structure. Although the secondary structure elements of rBsu-Lac were stable even at 90 °C, the laccase activity was remarkably reduced at or above 70 °C. A possible justification could be the flexibility of the structure similar to CotA laccase from B. subtilis which contains a flexible region close to the substrate binding pocket near the T1 copper center [Citation33]. Moreover, the T2 copper is reported as the labile copper atom in these multicopper oxidases [Citation13]. The amino acid sequences of Bsu-Lac is highly similar to the laccases from Bacillus species as shown in . Therefore, there is a possibility that at higher temperature, although the secondary structure elements were maintained, the coordinating copper ions were not intact in the protein structure which resulted in a drastic decrease in activity.
When the kinetic parameters of rBsu-Lac for SGZ oxidation were estimated under optimal conditions and compared with the available data as shown in , it was found that the kcat, against SGZ, was not much different than the corresponding bacterial enzymes including B. subtilis HR03, B. licheniformis, B. pumilus, and G. thermopakistanienis. The Km values of rBsu-Lac and the laccases from B. licheniformis and G. thermopakistanienis were also similar. However, they were quite different from the values for the laccases from B. subtilis HR03 and B. pumilus (). The low Km of these laccases demonstrate their high affinity to this substrate.
Table 1. Comparison of various parameters of bacterial laccases using SGZ as substrate.
In conclusion, rBsu-Lac is highly stable against organic solvents, denaturants and temperature and can be a potential candidate for applications in removing colors of synthetic dyes of waste water of various industries.
Author Contribution
N.R. conceived of the presented idea and wrote the manuscript with the help of S.B. S.B. carried out the experiments. M.S.A. performed the computations and sequence alignments. M.A. helped to supervise the project. All authors discussed the results and contributed to the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Reiss R, Ihssen J, Richter M, et al Laccase versus laccase-like multi-copper oxidase: a comparative study of similar enzymes with diverse substrate spectra. PLoS One. 2013;8:e65633.
- Sharma P, Goel R, Capalash N. Bacterial laccases. World J Microbiol Biotechnol. 2007;23:823–832.
- Santhanam N, Vivanco JM, Decker SR, et al Expression of industrially relevant laccases: prokaryotic style. Trends Biotechnol. 2011;29:480–489.
- Bourbonnais R, Paice MG, Freiermuth B, et al Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol. 1997;63:4627–4632.
- Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226.
- Camarero S, García O, Vidal T, et al Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb Technol. 2004;35:113–120.
- Osma JF, Toca-Herrera JL, Rodríguez-Couto S. Uses of laccases in the food industry. Enzyme Res. 2010;2010:918761.
- Burton S. Laccases and phenol oxidases in organic synthesis. Curr Org Chem. 2003;7:1317–1331.
- Kunamneni A, Camarero S, García-Burgos C, et al Engineering and applications of fungal laccases for organic synthesis. Microb Cell Fact. 2008;7:32.
- Ossiadacz J, Al-Adhami AJH, Bajraszewska D, et al On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol. 1999;72:141–149.
- Viswanath B, Rajesh B, Janardhan A, et al Fungal laccases and their applications in bioremediation. Enzyme Res. 2014;2014:1–21.
- Baldrian P. Fungal laccases-occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242.
- Martins LO, Soares CM, Pereira MM, et al Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem. 2002;277:18849–18859.
- Guan ZB, Zhang N, Song CM, et al Molecular cloning, characterization, and dye-decolorizing ability of a temperature- and pH-stable laccase from Bacillus subtilis X1. Appl Biochem Biotechnol. 2014;172:1147–1157.
- Qiao W, Chu J, Ding S, et al Characterization of a thermo-alkali-stable laccase from Bacillus subtilis cjp3 and its application in dyes decolorization. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2017;52:710–717.
- Gupta V, Garg S, Capalash N, et al Production of thermo-alkali-stable laccase and xylanase by co-culturing of Bacillus sp. and B. halodurans for biobleaching of kraft pulp and deinking of waste paper. Bioprocess Biosyst Eng. 2015;38:947–956.
- Chen B, Xu WQ, Pan XR, et al A novel non-blue laccase from Bacillus amyloliquefaciens: secretory expression and characterization. Int J Biol Macromol. 2015;76:39–44.
- Brander S, Mikkelsen JD, Kepp KP. Characterization of an alkali- and halide-resistant laccase expressed in E.. Coli: CotA from Bacillus Clausii. PLoS ONE. 2014;9:e99402.
- Lu L, Wang TN, Xu TF, et al Cloning and expression of thermo-alkali-stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Bioresour Technol. 2013;134:81–86.
- Zhang C, Zhang S, Diao H, et al Purification and characterization of a temperature- and pH-stable laccase from the spores of Bacillus vallismortis fmb-103 and its application in the degradation of malachite green. J Agric Food Chem. 2013;61:5468–5473.
- Sondhi S, Sharma P, Saini S, et al Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One. 2014;9:e96951.
- Reiss R, Ihssen J, Thöny-Meyer L. Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011;11:9.
- Zhang Y, Li X, Hao Z, et al Hydrogen peroxide-resistant CotA and YjqC of Bacillus altitudinis spores are a promising biocatalyst for catalyzing reduction of sinapic acid and sinapine in rapeseed meal. PLoS One. 2016;11:e0158351.
- Wan J, Sun X, Liu C, et al Decolorization of textile dye RB19 using volcanic rock matrix immobilized Bacillus thuringiensis cells with surface displayed laccase. World J Microbiol Biotechnol. 2017;33:123.
- Basheer S, Rashid N, Ashraf R, et al Identification of a novel copper-activated and halide-tolerant laccase in Geobacillus thermopakistaniensis. Extremophiles. 2017;21:563–571.
- Jalal A, Rashid N, Rasool N, et al Gene cloning and characterization of a xylanase from a newly isolated Bacillus subtilis strain R5. J Biosci Bioeng. 2009;107:360–365.
- Mollania N, Khajeh K, Ranjbar B, et al An efficient in vitro refolding of recombinant bacterial laccase in Escherichia coli. Enzyme Microb Technol. 2013;52:325–330.
- Fang Z, Zhou P, Chang F, et al Structure-based rational design to enhance the solubility and thermostability of a bacterial laccase Lac15. PLoS One. 2014;9:e102423.
- Hoshida H, Fujita T, Murata K, et al Copper-dependent production of a Pycnoporus coccineus extracellular laccase in Aspergillus oryzae and Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2005;69:1090–1097.
- Ryan S, Schnitzhufer W, Tzanov T, et al An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzyme Microb Technol. 2003;33:766–774.
- Kaushik G, Thakur IS. Purification, characterization and usage of thermotolerant laccase from Bacillus sp. for biodegradation of synthetic dyes. Appl Biochem Microbiol. 2013;49:352–359.
- Johannes C, Majcherczyk A. Laccase activity tests and laccase inhibitors. J Biotechnol. 2000;78:193–199.
- Enguita FJ, Martins LO, Henriques AO, et al Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J Biol Chem. 2003;278:19416–19425.
- Nasoohi N, Khajeh K, Mohammadian M, et al. Enhancement of catalysis and functional expression of a bacterial laccase by single amino acid replacement. Int J Biol Macromol. 2013;60:56–61.
- Luo Q, Chen Y, Xia J, et al Functional expression enhancement of Bacillus pumilus CotA-laccase mutant WLF through site-directed mutagenesis. Enzyme Microb Technol. 2018;109:11–19.