ABSTRACT
In insect hemolymph, many factors are present that can influence feeding motivation, such as lipids, carbohydrates, and other metabolites. Levels of these hemolymph factors fluctuate according to metabolic, nutrient and feeding states, eventually affecting feeding motivation and consequent regularly occurring feeding cycles. Such fluctuations contribute to energy homeostasis and innate feeding behavior in insects possibly by endocrine systems. Ultimately, orchestration of bioactive factors in the hemolymph modulate feeding motivation and nutrient selective behavior in insects.
Graphical Abstract
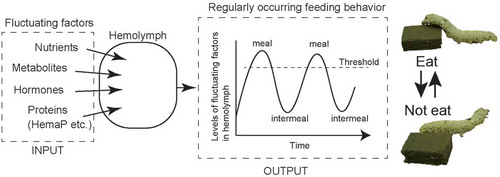
Fluctuation of the levels of hemolymph factors can modulate feeding motivation in insects possibly via endocrine control.
KEYWORDS:
Biologically active compounds play critical roles in a number of biological processes that allow for normal growth and development in all living creatures. Such regulatory compounds are known to control behavioral events, including feeding and foraging behaviors [Citation1,Citation2]. Many studies have demonstrated that feeding behavior is modulated by endogenous mechanisms which are orchestrated by metabolic and endocrine controls. To address the regulatory mechanisms of such instinctive behavior, in particular, feeding behavior, we used an insect as the experimental species. Herein, I discuss the biologically active components, which are associated with metabolic and endocrine controls of insect feeding behavior including our recent progress.
Factors modulating feeding behavior
A number of physiological studies have shown that motivation to feed in insects is thought to be controlled by numerous factors [Citation2]. For example, the presence of food, the appearance of a natural enemy, extreme or unusual weather, and temperature changes are critical factors that will modify feeding motivation in insects. In addition, the nutritional state of the body is essential in the determination of the motivation to feed, being known as satiety and hunger basically to maintain energy homeostasis [Citation3]. Practically, factors related to nutritional aspects are classified into two predominant factors capable of modulating feeding motivation: “volumetric” and “nutritional” causal factors. Because arthropods including insect species have rigid exoskeletons, there is little change in their body size within the intermolting periods during growth [Citation4]. Therefore, as the volumetric effects on feeding motivation are likely minimal in arthropods, feeding motivation in insects can be regarded mainly by “nutritional control”. Because insects have an open circulatory system, the released or secreted factors are present in “hemolymph”, the circulating media in insects for which the designation means “blood (hem) plus lymph.” Also, ingested exogenous nutrients and their metabolites are present in the hemolymph. Concerning the facts that tissues are constantly exposed to the dynamics of components in the hemolymph, feeding motivation might be resulted from the contents of hemolymph which are temporally transiting due to the nutritional and feeding conditions [Citation4].
Feeding as a regularly occurring patterned behavior
Insect species can be classified in several groups by feeding strategies, such as sucking and chewing insects, for example. Most chewing insects including lepidopteran species exhibit regularly occurring feeding patterns under experimental conditions of the unlimited diet [Citation5,Citation6]. Then, few exogenous factors including natural enemy attack prevent the feeding motivation, eventually causing the regularly occurring feeding cycles. It indicates that the experimentally conditioned insects are good models for investigations on the endogenous regulation of feeding motivation.
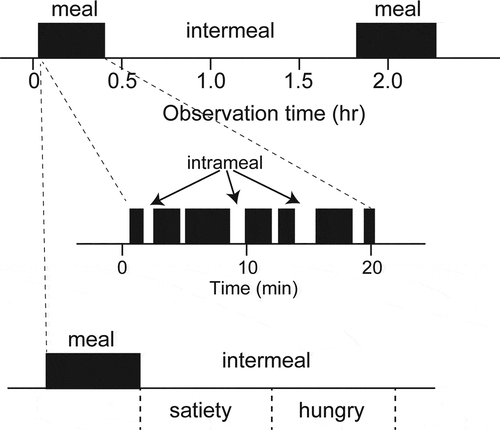
In the case of the silkworm, Bombyx mori, observations revealed an approximately two-hour feeding cycle, which is independent of circadian rhythms [Citation5]. As the schematic diagram of the feeding cycle of B. mori larvae indicates, the quiescent periods between feeding durations can be categorized as a long and a relatively short period within their feeding cycles (). Because these two different intervals of feeding periods are observed as intermeal or intrameal quiescent periods, which are separable by the time of a bout criterion, duration of meal or intermeal can be easily observed [Citation5]. The substantial behavioral repetition between meal and intermeal indicates that regularly occurring feeding motivation is generated by endogenous factors, and not by dietary factors, such as odorants or nutrients that attract the silkworms. It also indicates that the time from the end of the previous meal is a good index which allows us to evaluate the differences between the states of satiety and hunger in this species ().
Figure 1. Schematic diagram of feeding cycle of lepidopteran species. Black bars indicate feeding periods during observation. Insects repeat meal and intermeal periods. Meal is generated by eating time and intrameal quiescent period. The time from the previous meal is a useful index to evaluate feeding states between satiety and hungry.
Evaluation of feeding motivation using the silkworm larvae
Evaluation of differences in the feeding condition between satiety and hunger is very important in the investigation of feeding behavior. To date, feeding motivation has been analyzed by measuring food consumption or food intake for several days after factor-treatment. However, such assays do not determine the activities within the regularly occurring feeding cycles of several hours. In contrast to the experimental convenience of long-term assays for feeding activities, accompanying problems of memories and learning must influence feeding activities in addition to the dietary information. In the silkworm, as larvae take meals in a regular pattern of approximately two hours, the motivation to eat meals can be analyzed by measuring the time from the previous meal’s end (). Therefore, the use of B. mori larvae is convenient for evaluation of feeding motivation. In fact, under the usage of an artificial diet and the laboratory conditions, insects tend to eat regularly timed meals [Citation5–Citation7]. Thereafter, we evaluated feeding motivation in most insects by observations and by measuring the time until the first bite after sample administration [Citation8].
Endocrine control in feeding behavior
As mentioned above, many biological processes are controlled and modulated by endogenous factors. Among those endocrinal factors, peptide hormones and bioactive peptides are crucial for controlling feeding behavior because of their effects on metabolic and behavioral regulation, even in insects [Citation9,Citation10]. The endocrine controls are exerted in many tissues; the nervous system, including the brain, intestinal tissues, and adipose tissues in both vertebrate and invertebrate species. Such tissues generally include endocrine cells to produce the biologically active factors including peptide hormones. In the case of vertebrates, the feeding and satiety centers in the pituitary glands produce many feeding modulating factors including a feeding-activating neuropeptide, neuropeptide Y (NPY). Similarly, the factors modulating feeding motivation in insects are distributed in the tissues of the brain and central nervous system (CNS), and the fat body and gut, as discussed later.
The presence of these factors was ensured by conducting assays to measure the first bite after injection of the crude extracts from tissues of B. mori larvae [Citation8]. Data from this assay indicates that almost all extracts showed inhibitory effects on feeding initiation in this species. In particular, the extract from the midgut showed the strongest inhibitory activity [Citation8]. This result agreed with the fact that the midgut in insects is an essential site possessing endocrine cells, as well as nervous tissues [Citation11]. Moreover, when the several peptidyl factors predicted from genomic and transcriptomic information were subjected to this assay, some of those peptidyl factors exhibited stimulatory and inhibitory effects on feeding initiation [Citation12]. For example, myosuppressin and allatotropin exhibited strong inhibitory activities, indicating that these peptides are candidates for the inhibitory factors modulating feeding motivation in B. mori larvae [Citation12,Citation13]. Inversely, short neuropeptide F (sNPF) and tachykinin were the factors that enhanced feeding initiation, possibly motivating feeding behavior of this species [Citation12,Citation13].
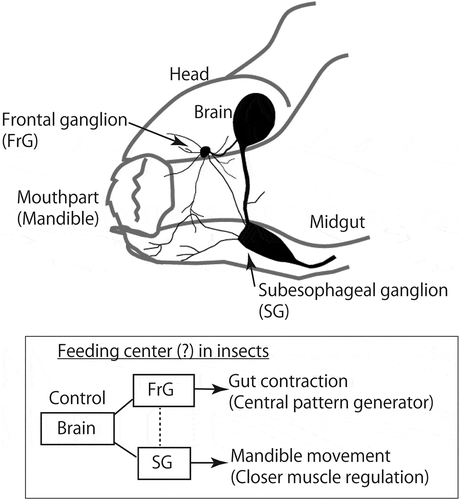
Interestingly, most of these factors are commonly expressed in the frontal ganglion (FrG) in addition to the brain and other ganglia [Citation10,Citation13,Citation14]. Along with the fact that the FrG is innervated by the brain via frontal connectives, the function of FrG is a central pattern generator to control the contraction of the foregut in insect species [Citation15]. In addition, the other predominant endocrine area of the feeding regulatory factors is concentrated within the subesophageal ganglion (SG), which is an important site that controls mandible movements via closer muscle for biting behavior [Citation16]. Therefore, the local short circuit generated by the brain, FrG, and SG might be the feeding center in insects ().
Endocrine factors related to feeding behavior in insects
Using the feeding initiation assay [Citation8], we characterized several peptidyl factors that positively or negatively controlled feeding behavior in insects including B. mori [Citation10,Citation13,Citation14]. Some of them were analogs of mammalian peptidyl factors. For example, Neuropeptide F (NPF) and sNPF are structural analogs of vertebrate NPY [Citation9,Citation17]. Insulin is structurally and functionally similar to that in vertebrates [Citation18]. In addition, functional analogs were also observed, such as adipokinetic hormone (AKH), which is a functionally opposing factor to insulin in terms of energy homeostasis, and corresponds to mammalian glucagon in insects.
The assays were also conducted in the omnivorous orthopteran species, the two-spotted cricket, Gryllus bimaculatus. The transcriptomic analyses using next generation sequencer and observation of the crickets after sample injection revealed that similar peptidyl factors contributed to feeding behavioral control. The RNA interference (RNAi) is the significant experimental advantageous availability of G. bimaculatus supporting the functional analyses by knockdown targeting on the peptidyl factors at the transcriptional level. Using this species, other additional bioactive peptides AKH, AKH/Corazonin-related peptide (ACP), and allatoregulatory peptide (Allatostatin-B, AST-B) have been identified as the factors regulating insect feeding behavior and energy homeostasis [Citation19–Citation21].
Many bioactive peptides have been reported as feeding-regulatory factors from various insect species, so far. Together, we now understand that myo-suppressive and myo-activating peptides and metabolism-related peptides can contribute strongly to mechanisms of feeding behavior in insects. Those factors seem to influence directly on the myoactivity of gut contraction and mandible movements via central pattern generator.
Not only those peptidyl factors, but also biogenic amines are functional in the feeding regulation of both insects and mammals. Several pharmacological studies have indicated that insect feeding is accelerated by dopamine produced in the brain and subesophageal ganglion [Citation22,Citation23].
Additional factors influencing the feeding processes
Hemolymph components are constituted of many factors, including proteins and small chemical compounds, such as metabolites, amino acids, lipids, carbohydrates, and signal mediators. The components of hemolymph are, therefore, determined by the nutritional and metabolic states of the body. The feeding motivation are generated by those hemolymph factors. For example, carbohydrate and lipid levels affect critically on feeding regulation via insulin signaling even in insects [Citation18]. In G. bimaculatus, for example, high levels of hemolymph lipids and carbohydrates result in reduction of feeding motivation [Citation24]. Those factors are maintained by endocrine control according to nutrient and metabolic conditions and by the hemolymph major proteins such as an insect lipoprotein, lipophorin and its related peptide, a hemolymph major anionic peptide (HemaP) [Citation23].
A novel peptide, hemolymph major anionic peptide (HemaP)
From the bioassay, several peptidyl factors were identified, as mentioned above. Through the careful observations on feeding behaviors, we noticed that B. mori larvae exhibit several characteristic behaviors before and after meals; surveying, nibbling, biting, and head-swaying [Citation23]. By further observations of the head region, we found that B. mori larvae exhibited the micromovement of head-shaking immediately before a meal [Citation23]. Because the probability of the initiation of a meal after that head-shaking movement was extremely high, we then used this head micromovement as an index for the motivated condition for feeding of B. mori larvae. According to the assay, the factor in the hemolymph driving the feeding initiation was purified from 200 mL of B. mori larval hemolymph via several steps of reversed-phase high-performance liquid chromatography (HPLC). The structural analyses provided the entire peptide sequence of the biologically active compound composed of 62 amino acids [Citation23]. We then designated the peptide as HemaP after a hemolymph major anionic peptide. Although the sequence of HemaP has shown no similarity to other peptides thus far, a homologous peptide in other lepidopteran species was present in the hemolymph of the sweet potato hornworm Agrius convolvuli with similar biological activity [Citation25], indicating conservation of this peptide among other lepidopteran species. Similar approach and in silico searches revealed that general lepidopteran species such as the noctuid moth Spodoptera litura, the cabbage white Pieris rapae and the wax moth Galleria mellonella [Citation26] had HemaP-like peptides (). Interestingly, antimicrobial activity was observed in G. mellonella HemaP-like peptide, although B. mori HemaP did not show any antimicrobial activity [Citation23,Citation26]. It indicates that the HemaP-like peptide seems to exert distinctive function according to species. However, no homologous genes or peptides to HemaP have not been observed in other insect orders including Drosophila melanogaster and Gryllus bimaculatus.
Figure 3. An alignment of amino acid sequences of HemaP and HemaP-like peptides. Amino acid sequences of HemaP and HemaP-like peptides of B. mori, Agrius convolvuli, Pieris rapae, Spodoptera litura, and Gallaria mellonella, were identified from purified peptides and in silico researches. Highly conserved and similar amino acid residues among aligned sequences are shaded in black and gray, respectively.

Indeed, HemaP is expressed and secreted by the fat body like the other hemolymph major protein as lipophorins. The fact that HemaP functions in the hemolymph by fluctuating its level at relatively higher concentration is similar action to that of lipophorin. In addition, lipophorin level in the hemolymph is increased during the starved condition and under immune challenged conditions [Citation27,Citation28], which is similar mode of action in HemaP.
In the case of lipophorin, five alpha-helixes were distributed in the molecule, which has a high affinity to lipidic compounds [Citation29]. Therefore, HemaP has a possibility to bind some other compounds in the hemolymph. Biochemical analyses of B. mori HemaP showed some structural characteristics of alpha-helix rich protein. The N-terminal and C-terminal portions of HemaP possessed alpha helix-rich domains like lipophorins and antimicrobial peptides which are present in the hemolymph (). This similar characteristic implies somewhat different way to influence on the consequence of physiological events compared with those of bioactive peptides and hormones exerting by low concentration in the hemolymph.
The events in the fat body during changes in feeding motivation
The fat body in insects is a very important tissue, in which energy source is stored for required occasions such as starvation and acute demands. In the case of several insects, this energy source is used for flight muscles for long-flight including migration, as observed in the migratory locust, Locusta migratoria [Citation30].
For the required energy source, a peptidyl factor, AKH, induces the mobilization of lipids and carbohydrates from the fat body into the hemolymph [Citation31]. This mechanism for energy allocation is highly conserved over insect species, including lepidopteran species B. mori and orthopteran species G. bimaculatus [Citation24,Citation32]. The hemolymph lipid is predominantly composed of diacylglycerol (DAG). At the time of a meal, the hemolymph DAG level increases significantly accompanied by increases in blood sugar level. In the crickets, injection of DAG decreased feeding motivation even under starvation conditions [Citation24,Citation32]. The knockdown targeting the mobilization machinery protein, lipophorin III, in crickets did not change the lipids or the feeding levels, even after AKH injection, indicating that there are several pathways that facilitate feeding behavior via hemolymph lipid or carbohydrate levels [Citation24].
Therefore, sensing the hemolymph lipid or carbohydrate levels is crucial for the regulation of feeding motivation in insects. In fact, AKH is produced and secreted by the brain-connected endocrine organ, corpora cardiaca (CC), which is a strong candidate of the sensory organ for detecting changes in hemolymph nutritional states [Citation33]. Recent our study demonstrates that AKH signaling contributes to the nutrient selective behavior in G. bimaculatus [Citation32]. In that study, imbalanced hemolymph lipid contents are complemented by nutrient selective behavior, agreeing with the idea that hemolymph contents are strongly related to the nutrient-dependent behavior.
Summary
The hemolymph is the communication space for the chemicals derived from any tissue in organisms having an open circulatory system, such as insects (). This means that many biologically active compounds, nutrients and metabolic compounds, are present in the hemolymph contributing to biological processes, including maintenance of energy homeostasis. Like those factors, the major constitutive components, proteins and other small molecules, sometimes fluctuate according to exogenous cues such as nutritional and metabolic states after meals or during starvation. Although we do not currently understand the mechanisms by which these factor levels are generated precisely, the underlying mechanism for the maintenance of energy sources is gradually revealed. The part of contributing mechanisms is the endocrine system involving peptidyl factors that possibly are related to feeding motivation. The motivation is also influenced by the major components in hemolymph including lipophorin, HemaP, and lipidic sources such as DAG.
Figure 4. Schematic diagram of feeding regulation via hemolymph components. The levels of factors in the hemolymph fluctuate according to the nutrient and feeding states possibly via endocrine control. Once the levels of those compounds reach at some threshold levels, feeding behavior will occur.
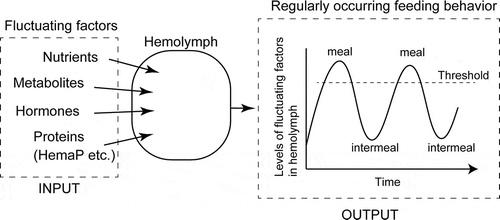
Once those hemolymph factors reached at some threshold levels accompanied by increase of feeding motivation, feeding behavior would take place until these levels will decrease under the threshold (or increase over the threshold). This idea agreed with the simulated pattern of the physiological factors, which regulate feeding motivation and the initiating timing of meal [Citation2,Citation3].
Also, no single critical factors seem to be present for generating feeding regulatory mechanisms from several knockout and knockdown experiments even in insects. Those reports indicate that drastic experimental procedure of investigation such as systemic biological strategies using comprehensive simulation and computation, must be undertaken by virtue of perfectly understanding such innate feeding behavior in the near future.
In the 1930s, a great idea has been proposed known as “self-selection,” which is physiologically and behaviorally observed throughout most of the animal kingdom [Citation34], and results in foraging for the appropriate nutrient that the animal requires or should be complemented. Self-selection is believed to be maintained by the endocrine system because its manipulation can cause dysfunction in the innate “self-selective” behavior. Through investigations on the communication across the feeding center, peripheral organs such as the fat body, and hemolymph, we now believe that the mechanism of the innate nutrient self-selective behavior can be addressed.
Acknowledgments
This study is carried out by supports from Professors Hiromichi Nagasawa, Hiroshi Kataoka, Shohei Sakuda, and from the colleagues, Drs. Hideaki Asazuma-Mabashi, Nobukatsu Morooka, Chiaki Nagai-Okatani, Takahiro Konuma, Yusuke Tsukamoto, and Mrs. and Ms. Keisuke Fukumura, Sumihiro Matsumoto, Zhou Yi-Jun, Ayako Ohara, Yukie Omori, and the members of team N at GSFS the University of Tokyo.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev. 1997;10:151–179.
- Simpson SJ. Why locusts start to feed: A comparison of causal factors. Anim. 1986;34:480–496.
- Simpson SJ, Raubenheimer D. The central role of the hemolymph in the regulation of nutrient intake in insects. Physiol Entomol. 1993;18:395–403.
- Nijhout H. The control of body size in insects. Dev Biol. 2003;261:1–9.
- Nagata S, Nagasawa H. Effects of diet-deprivation and physical stimulation on the feeding behaviour of the larvae of the silkworm, Bombyx mori. J Insect Physiol. 2006;52:807–815.
- Reynolds SE, Yeomans MR, Timmins WA. The feeding behavior of caterpillars (Manduca sexta) on tobacco and on aritificial diet. Physiol Entomol. 1986;11:39–51.
- Simpson SJ. The pattern of feeding. (Biology of grasshoppers.). In: Simpson SJ, Chapman RF, Joern A, editors. In regulatory mechanisms of insect feeding behavior. NY: Oxford press Ltd; 1990. p. 73–103.
- Nagata S, Morooka N, Matsumoto S, et al Characterization of feeding-delaying factors from the silkworm Bombyx mori. Ann N Y Aca Sci. 2009;11632009:481–483.
- Nässel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: any similarities to vertebrate neuropeptide Y signaling?. Peptides. 2011;32:1335–1355.
- Audsley N, Weaver RJ. Neuropeptides associated with the regulation of feeding in insects. Gen Comp Endocrinol. 2009;162:93–104.
- Sehnal F, Zitnan D. Midgut endocrine cells. In: Lehane MJ, Billingsley PF, editors. Biology of the insect midgut: chapman and Hall Ltd. N Y, USA: Chapman and Hall, N Y; 1996. p. 55–85.
- Nagata S, Morooka N, Matsumoto S, et al Effects of neuropeptides on feeding initiation in larvae of the silkworm, Bombyx mori. Gen Comp Endocrinol. 2011;172:90–95.
- Nagata S, Matsumoto S, Mizoguchi A, et al Identification of cDNAs encoding allatotropin and allatotropin-like peptides from the silkworm, Bombyx mori. Peptides. 2012;34:98–105.
- Nagata S, Nagasawa H. Calcitonin-like peptide hormone (CT/DH) in the frontal ganglia as a feeding regulatory peptide of the silkworm, Bombyx mori. Peptides. 2017;98:23–28.
- Penzlin H. Role of frontal ganglion in larvae of American cockroach (Periplaneta americana). J Insect Physiol. 1971;17:559–573.
- Sasaki K, Asaoka K. Different physiological properties in a pool of mandibular closer motor neurons in a caterpillar, Bombyx mori. Neurosci Lett. 2005;374:166–170.
- Nagata S, Matsumoto S, Nakane T, et al Effects of starvation on brain short neuropeptide F-1, −2, and −3 levels and short neuropeptide F receptor expression levels of the silkworm, Bombyx mori. Front Endocrinol. 2012;3:3.
- Dillen S, Chen ZW, Vanden Broeck J. Nutrient-dependent control of short neuropeptide F transcript levels via components of the insulin/IGF signaling pathway in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol. 2016;68:64–70.
- Konuma T, Morooka N, Nagasawa H, et al Knockdown of the adipokinetic hormone receptor increases feeding frequency in the two-spotted cricket Gryllus bimaculatus. Endocrinol. 2012;153:3111–3122.
- Tsukamoto Y, Nagata S. Newly identified allatostatin Bs and their receptor in the two-spotted cricket, Gryllus bimaculatus. Peptides. 2016;80:25–31.
- Zhou YJ, Fukumura K, Nagata S. Effects of adipokinetic hormone and its related peptide on maintaining hemolymph carbohydrate and lipid levels in the two-spotted cricket, Gryllus bimaculatus. Biosci Biotech Biochem. 2018;82(2):274–284.
- Kaufmann L, Schurmann F, Yiallouros M, et al The serotonergic system is involved in feeding inhibition by pymetrozine. Comparative studies on a locust (Locusta migratoria) and an aphid (Myzus persicae). Comp Biochem Physiol C Toxicol Pharmacol. 2004;138:469–483.
- Nagata S, Morooka N, Asaoka K, et al Identification of a novel hemolymph peptide that modulates silkworm feeding motivation. J Biol Chem. 2011;286:7161–7170.
- Konuma T, Tsukamoto Y, Nagasawa H, et al. Imbalanced hemolymph lipid levels affect feeding motivation in the two-spotted cricket, Gryllus bimaculatus. Plos One. 2016;11. DOI:10.1371/journal.pone.0154841.
- Morooka N, Nagata S, Shirai K, et al A hemolymph major anionic peptide, HemaP, motivates feeding behavior in the sweetpotato hornworm, Agrius convolvuli. FEBS J. 2012;279:168–179.
- Brown SE, Howard A, Kasprzak AB, et al A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem Mol Biol. 2009;39:792–800.
- Zdybicka-Barabas A, Cytrynska M. Apolipophorins and insects immune response. Isj-Invertebrate Survival J. 2013;10:58–68.
- Mullen L, Goldsworthy G. Changes in lipophorins are related to the activation of phenoloxidase in the haemolymph of Locusta migratoria in response to injection of immunogens. Insect Biochem Mol Biol. 2003;33:661–670.
- Ryan RO, van der Horst DJ. Lipid transport biochemistry and its role in energy production. Ann Rev Entomol. 2000;45:233–260.
- Van Der Horst DJ, Van Marrewijk WJA, Vullings HGB, et al Metabolic neurohormones: release, signal transduction and physiological responses of adipokinetic hormones in insects. Eur J Entomol. 1999;96:299–308.
- Zandawala M, Tian S, Elphick MR. The evolution and nomenclature of GnRH-type and corazonin-type neuropeptide signaling systems. Gen Comp Endocrinol. 2018;264:64–77.
- Fukumura K, Konuma T, Tsukamoto Y, et al. Adipokinetic hormone signaling determines dietary fatty acid preference through maintenance of hemolymph fatty acid composition in the cricket Gryllus bimaculatus. Sci Rep. 2018;8. DOI:10.1038/s41598-018-22987-2.
- Spring JH, Gӓde G. Factors regulating carbohydrate and lipid metabolism isolated from the corpus cardiacum of the eastern lubber grasshopper, Romalea microptera. J Exp Zool. 1987;241:41–50.
- Richter CP, Holt LE, Barelare B. Nutritional requirements for normal growth and reproduction in rats studied by the self-selection method. Am J Physiol. 1938;122:734–744.