ABSTRACT
The present study determines whether antler extract (AE) possesses inhibitory effects in a prostate cancer (PC) xenograft model and explores the underlying mechanism. After therapeutic intervention for two weeks, AE significantly inhibited prostate cancer xenograft tumor growth by 65.08%, and prostate-specific antigen (PSA) and serum dihydrotestosterone (DHT) levels. However, AE increased the serum testosterone level compared to the vehicle control group. Furthermore, our investigation of the inhibitory effects on angiogenesis and epithelial-to-mesenchymal transition (EMT)-related genes revealed that AE downregulated matrix metalloproteinase 2 (MMP)-2, (MMP)-9, vascular endothelial growth factor (VEGF), zinc finger protein (SNAIL1), twist-related protein 1 (TWIST1), and zinc-finger E-box-binding homeobox 1 (ZEB1) in vivo. In contrast, AE increased tissue inhibitor of MMP (TIMP)-1, (TIMP)-2, and E-cadherin. The results suggest that AE possesses potent anti-PC activity, and this is the first report on the anti-PC effect of AE in vivo.
Graphical Abstract
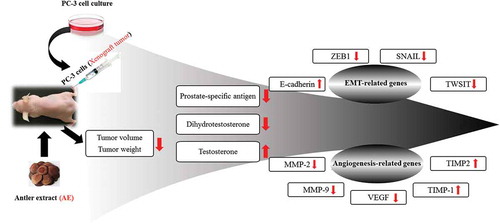
It demonstrated the potent anti-prostate cancer effects of the antler extract including anti-angiogenesis activity, which all appeared to be mediated by the alteration of levels of relevant epithelial to mesenchymal transition-related genes.
Worldwide, prostate cancer (PC) is the second most commonly diagnosed cancer and the fifth leading cause of cancer-related death among men [Citation1]. Although it can be cured at the early stage by radical prostatectomy or radiation therapy, most patients later experience local recurrence and distant metastasis [Citation2]. Generally, after short-term remission (18–24 months), surviving tumor cells recur, causing castrate-resistant PC (CRPC) with inevitable progression and death within 2–3 years in most men [Citation3,Citation4]. In CRPC progression, tumor cells acquire the ability to both survive in the absence of androgens and proliferate using non-androgenic stimuli for mitogenesis [Citation5]. CRPC has characteristics such as elevated prostate-specific antigen (PSA), a higher metastasis rate, and increased aggressiveness [Citation6].
Moreover, according to a previous survey, chemical medicines cause severe side effects in patients [Citation7]. Therefore, there is an urgent need to develop novel drug therapies for single or combination administration to overcome the shortcomings of current chemotherapeutic drugs – including their side effects – in the treatment of CRPC. Sika deer velvet antler is one of the most popular traditional medicines in China and Korea. Velvet antler was recorded in Chinese medical classic literature such as the ShenNong Ben Cao Jing 2,000 years ago. The book indicates that the velvet antler nourishes the Yin, possesses body strengthening, immunomodulatory, and anti-aging effects; it also tonifies the kidney, invigorates the spleen, strengthens the bones and muscles, and promotes blood flow [Citation8].
Numerous studies have demonstrated that velvet antler possesses other properties such as anticancer, anti-cardiovascular disease, antioxidant, and immunity-enhancement [Citation9–Citation12]. Our previous research study revealed that antler extract (AE) inhibited PC cell migration by downregulating the expression of PSA, matrix metalloproteinase-9 (MMP-9), and vascular endothelial growth factor (VEGF), and increasing tissue inhibitor of MMP (TIMP)-1 and TIMP-2 [Citation9]. However, the in vivo anti-PC effect of AE has not yet been investigated. Therefore, the present study aims to investigate the inhibitory effect of AE in a PC xenograft tumor model and the underlying mechanism for the first time.
Materials and methods
Materials
We purchased cisplatin from Sigma-Aldrich Chemical Corp. (St. Louis, MO, USA) and we obtained PC-3 human PC cell line from the Korean Cell Line Bank (Seoul, Korea, KCLB numbers: 21,435). The Roswell Park Memorial Institute (RPMI) 1,640 medium, fetal bovine serum (FBS), penicillin/streptomycin, 0.5% trypsin-ethylenediaminetetraacetic acid (EDTA), and phosphate-buffered saline (PBS) for the cell culture were from Invitrogen (Carlsbad, CA, USA).
Extract preparation
The antlers were extracted according to a previous method [Citation11]. Briefly, the antlers were harvested at approximately growing day 50 and then divided into three segments: top, middle, and base. In this study, we used the top antler segment, which was lyophilized, homogenized using a grinder, and then a 100 g sample was added to 1,000 mL distilled water (DW) and extracted in boiling DW for 1 h. The AE was subsequently filtered (0.25-μm pore size) and lyophilized in a freeze dryer for five days.
Animal experiments
The BALB/c nude male mice (15–17 g, eight-week-old) used in the present study were provided by Samtako Bio Co., (Osan, Korea). The mice were maintained in an air-conditioned room (20–25°C) on a 12-h light/dark cycle with free access to food and water. They were allowed to acclimatize to the new environment for a week before experiments commenced (). The mice were inoculated subcutaneously with 6.5 × 105 PC-3 cells suspended in 100 μL PBS three times a week. When the xenograft tumors reached approximately 100 mm3, the mice were randomly assigned to four groups (n = 6 per group). Group 1 served as the sham control and was administered PBS, Group 2 received 10 mg/kg body weight cisplatin (Cis, standard drug); and Groups 3 and 4 received 200 and 400 mg/kg body weight AE (AEL and AEH), respectively by gavage.
Figure 1. Study scheme of xenograft tumor model.
Normal (phosphate-buffered saline [PBS] 200 μL), control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. From day 0, we administered an injection three times a week to Con, Cis, AEL, and AEH groups and waited for 1 week. When xenograft tumor volume was approximately 100 mm3, mice were randomly assigned to four groups and treated accordingly.
![Figure 1. Study scheme of xenograft tumor model.Normal (phosphate-buffered saline [PBS] 200 μL), control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. From day 0, we administered an injection three times a week to Con, Cis, AEL, and AEH groups and waited for 1 week. When xenograft tumor volume was approximately 100 mm3, mice were randomly assigned to four groups and treated accordingly.](/cms/asset/fa92646f-9361-4f53-a039-91758bb707f8/tbbb_a_1537775_f0001_b.gif)
After the treatment, the gavage tube was left in place for several seconds to avoid regurgitation and ensure that the total calculated dose was administered. Tumor sizes were monitored every two days using digital calipers, and the tumor volumes were calculated according to the formula L × S2 × 0.5, where L and S represent the tumor’s longest and shortest diameter, respectively [Citation13]. The mice were also weighed and the extract was administered once daily at a fixed time over the four-week experimental period.
At the end of the experiment, all the mice fasted overnight, and the xenograft tumors were rapidly excised and weighed. The tumor was placed in liquid nitrogen immediately for western blot analysis and blood samples were collected from the heart. All animal care procedures and experiments were approved by the Institutional Animal Care and Use Committee of Konkuk University (KU15114).
Serum biochemical parameters
We obtained serum from the mice by centrifuging blood samples at 3,500 × g for 15 min at 4°C. The PSA (Cusabio Biotech, Wuhan, China), testosterone (Abcam, Cambridge, UK), and DHT (MyBiosource, San Diego, CA, USA) were determined using an enzyme-linked immunosorbent assay (ELISA) kit.
Western blotting
Cell extracts were prepared using the detergent lysis procedure as described previously. Protein samples (40 μg) were electrophoresed using Novex 4–12% Bis-Tris gels (Life Technologies, Carlsbad, CA, USA) and then transferred them to nitrocellulose membranes for 7 min using the iBlot dry blotting system (Life Technologies, Carlsbad, CA, USA). The membranes were blocked overnight at 4°C with clear milk (Thermo Scientific, IL, USA) and then subsequently incubated for 1 h with the primary antibodies (1:2,000 in 1× Tris-buffered saline plus 0.1% Tween 20 [TBST]). MMP-2, −9, VEGF, TIMP-1, −2, and actin antibodies were purchased from Santa Cruz Biotechnology. E-cadherin, TWIST1, zinc finger protein 1 (SNAIL1), and zinc finger E-box-binding homeobox 1 (ZEB1) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). We used goat anti-rabbit and goat anti-mouse horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) at 1:2,000 dilution in 1× TBST. We ascertained equal protein loading using Ponceau S staining of blotted membranes and western blotting with a β-actin antibody and we performed Immunodetection using an enhanced chemiluminescence detection kit (Amersham Pharmacia, Piscataway, NJ, USA).
Goat anti-rabbit and goat anti-mouse horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) were used at 1:2,000 dilution in 1× TBST.
The results of the experiments are expressed as summaries of data for at least three experiments. All data are presented as mean ± standard error (SE), and statistical analyses were performed using the statistical analysis software (SAS) program (SAS Institute, Cray, NC, USA). Treatment effects were analyzed using a one-way analysis of variance (ANOVA), followed by Dunnett’s multiple range test. Results with p < 0.05 were considered significant.
Results
Effect of AE on PC xenograft tumor growth
Male BALB/c nude mice with PC-3 cells xenograft tumors were treated with the vehicle, cisplatin, or AE for four weeks (). At the start of treatment, the mean food intake, body weight, and tumor volumes of the groups were similar; however, there was a significant change after four weeks (). The AE and Cis groups showed a significant reduction in food intake compared to the normal group ()). Moreover, there were no significant changes in body weight for the normal and AE groups. However, compared to the other groups, the Cis group showed the least body weight change ()). The results showed that treatment with AE and Cis attenuated the xenograft tumor growth. Tumor growth in the Cis, AEL, and AEH groups was inhibited by 68.55%, 61.25%, and 65.08%, respectively, compared to that of the sham control group () and ()). The volumes of the excised tumors in the Cis, AEL, and AEH groups were significantly smaller than those of the control group () and ()).
Figure 2. Effect of antler extract (AE) on food intake, body weight, and tumor volume.
Normal (phosphate-buffered saline [PBS] 200 μL), control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. (a) Food weight, (b) body weight, (c) tumor volume, (d) AE treatment of prostate cancer (PC) xenograft model image, (e and f) Tumor image and weight. a, b, c and d Means with different superscript in the same row are different (p < 0.05).
![Figure 2. Effect of antler extract (AE) on food intake, body weight, and tumor volume.Normal (phosphate-buffered saline [PBS] 200 μL), control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. (a) Food weight, (b) body weight, (c) tumor volume, (d) AE treatment of prostate cancer (PC) xenograft model image, (e and f) Tumor image and weight. a, b, c and d Means with different superscript in the same row are different (p < 0.05).](/cms/asset/d367cd61-ec46-4eaa-90c0-cc5b08ac371a/tbbb_a_1537775_f0002_oc.jpg)
Effect of AE on PSA, DHT, and testosterone levels in the PC xenograft model
The analysis of the serum PSA and DHT levels showed they were respectively 227.32 ± 25.69 ng/mL and 12.56 ± 0.44 nmol/L in the control group (), ()) while the AE groups showed significantly reduced values of up to 154.22 ± 20.79 ng/mL and 7.88 ± 1.46 nmol/L, respectively. The testosterone levels were highest in the AEH group at 8.17 ± 0.40 ng/mL ()).
Figure 3. Effect of antler extract (AE) on prostate-specific antigen (PSA), dihydrotestosterone (DHT), and testosterone levels in prostate cancer (PC) xenograft model.
(a) Serum PSA, (b) DHT, and (c) testosterone changes in treated and untreated nude mice. Normal (phosphate-buffered saline [PBS] 200 μL), control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. a, b, c and d Means with different superscript in the same row are different (p < 0.05).
![Figure 3. Effect of antler extract (AE) on prostate-specific antigen (PSA), dihydrotestosterone (DHT), and testosterone levels in prostate cancer (PC) xenograft model.(a) Serum PSA, (b) DHT, and (c) testosterone changes in treated and untreated nude mice. Normal (phosphate-buffered saline [PBS] 200 μL), control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. a, b, c and d Means with different superscript in the same row are different (p < 0.05).](/cms/asset/2a126e0b-3c43-41da-b049-011f95ad3aff/tbbb_a_1537775_f0003_b.gif)
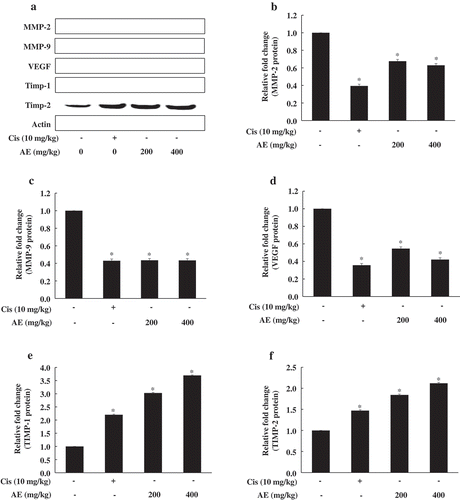
Effect of AE treatment on angiogenesis-related protein expression in tumor tissue
We investigated the expression of angiogenesis-related genes in mouse tumor tissue (). The AE groups showed a dose-dependent decrease in MMP-2, MMP-9, and VEGF levels, while those of TIMP-1 and TIMP-2 increased significantly and dose-dependently in the AE groups; these results show that the AE groups had adjusted angiogenesis-related gene levels.
Figure 4. Effect of antler extract (AE) on angiogenesis-related gene expression in tumor tissue.
Control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. β-actin was loading control. Blot represents mean of three individual experiments performed in triplicate. *Significant difference from the Con group shown at p < 0.05.
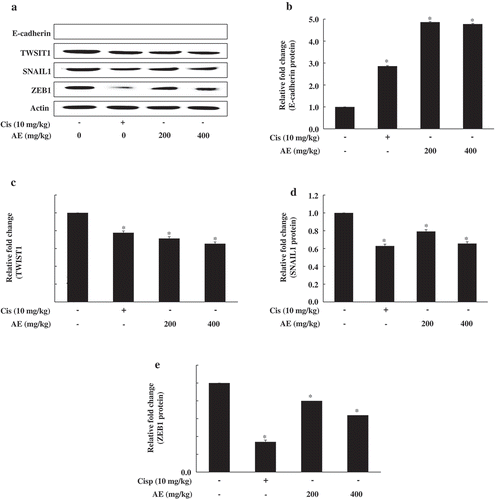
Effect of AE on EMT-related protein expression in tumor tissue
EMT is a key physiological process that plays an essential role in tumor metastasis. To characterize EMT, we detected E-cadherin, TWIST1, SNAIL1, and ZEB1 expression. As shown in , we found increased expression of the epithelial marker E-cadherin and decreased mesenchymal markers including TWIST1, SNAIL1, and ZEB1 in the tumor tissue of the AE group. These observations supported the potential inhibitory effect of AE on tumor tissue EMT-related genes.
Figure 5. Effect of antler extract (AE) on epithelial to mesenchymal transition (EMT)-related gene expression in tumor tissue.
Control (Con, PBS 200 μL + tumor cells); cisplatin (Cis, 10 mg/kg + tumor cells); antler extract low- and high-dose (AEL and AEH, 200 and 400 mg/kg, respectively plus tumor cells. β-Actin was a loading control. Blot represents mean of three individual experiments performed in triplicate. *Significant difference from the Con group shown at p < 0.05.
Discussion
In recent years, the incidence of prostate cancer has increased each year, seriously jeopardizing the health of older men. Prostate cancer can be treated with surgery and hormone therapy in its early stage and chemotherapy is mainly used in its advanced stage. There are no obvious clinical symptoms in the early stage of prostate cancer, so it is often late when it is found. Chemotherapy drugs for prostate cancer are expensive and have large side effects, while traditional drugs can help treat tumors without harming the body.
Velvet antlers have several pharmacological properties and have been used clinically to treat numerous diseases [Citation14]. AE has been found to have immunoregulatory, antioxidant, and anticancer activities [Citation15,Citation16]. Our previous study also showed that AE inhibited PC cell growth by the in vitro downregulation of PSA and migration-related genes [Citation9]. The incidence and mortality rate of PC have increased, and currently available CRPC treatment options are associated with numerous adverse effects [Citation7,Citation17]. Therefore, natural medicines have attracted considerable attention due to their advantages of increased anti-cancer effect, smaller drug doses, and reduced side effects [Citation7,Citation17].
Following our previous in vitro research study [Citation9], here we successfully established a human PC xenograft tumor model of male BALB/c nude mice that better simulates the natural growth of human prostate cancer and is a suitable model for observing the biological behavior of prostate cancer. We found that the feed intake and body weight of the Cis group were lower than the AE and control groups, but the AE group values were close to that of the control group, which could have been due to cancer-induced cachexia. Palipoch and Punsawad [Citation18] reported that Cis is an important chemotherapeutic agent for the treatment of various cancers; however, it also has severe side effects. In this present study, AE inhibited the growth of prostate cancer xenograft tumors in a concentration-dependent manner. When the AE concentration was 400 mg/kg, the inhibition rate of tumor growth was as high as 65.08%. Moreover, the AE doses were well tolerated and we observed no obvious toxic reaction during the entire treatment. The results verified that AE inhibited PC growth in vivo with greater effectiveness and safety than the compared treatment.
The serum level of PSA is often elevated in men with PC and so the PSA test is widely used to screen men for PC and monitor its progression. DHT is a sex steroid and androgen hormone [Citation19] that plays a role in the development and exacerbation of PC by enlarging the prostate gland. Therefore, PC growth is highly DHT-dependent [Citation20]. DHT appears to be a key factor that controls the production of PSA. We found that AE significantly decreased the PSA level with a higher inhibitory activity than genistein [Citation21]; in addition, the AE groups exhibited lower DHT levels than the control group. Yegorova [Citation22] reported that AE inhibited 5ɑ-reductase, possibly reducing excessive DHT, which is the primary contributing factor in PC. For 2,000 years, Chinese medicine has recognized that AE can enhance sexual ability [Citation23] and the testosterone levels were highest in the AE groups in our present study.
In practice, numerous studies have found that AE inhibits several cancers [Citation9,Citation15,Citation24]. Fraser et al. [Citation24] reported that the oral administration of AE effectively decreased the severity of colon cancer. AE also prolonged the survival and reduced the tumor weight of mice injected abdominally with sarcoma 180 cells [Citation25,Citation26]. In addition, AE suppressed male hormone-related disease by regulating PSA [Citation9] and inhibited female hormone-related disease by decreasing MMP-2 and MMP-9 [Citation16].
Angiogenesis is the process by which new blood vessels are generated from pre-existing ones. The development of new blood networks is crucial for cancer growth. As cancer cells progress and mature into tumors, they require increased angiogenesis to facilitate their rapid expansion, metastasis, and invasion. Therefore, targeting cancer-associated angiogenesis is a promising strategy for treating cancer or preventing its progression [Citation27–Citation29]. In addition, MMPs act as a trigger mechanism in tumor migration and contribute to the migration of tumor cells into the blood vessels and distant metastasis [Citation30–Citation32]. In this study, our investigation of angiogenesis-related gene expression including MMP-2, MMP-9, VEGF, TIMP-1, and TIMP-2 showed similar results to those of Tang et al. [Citation9] except for MMP-2, which was similar to that of Kim et al. [Citation16].
MMPs are considered potential markers of the migration and metastasis of malignant tumor cells. Elevated MMP expression is associated with increased metastatic potential in numerous tumor cells. Meanwhile, there are natural inhibitors of MMPs called TIMPs, which are a group of peptidases involved in the degradation of the extracellular matrix. As key regulators of MMPs, TIMPs play a pivotal role in determining the effect of the extracellular matrix and cell adhesion molecules. Furthermore, there is evidence that TIMPs have biological activity independent of their inhibition of MMP including in cell growth, differentiation, cell migration, apoptosis, and angiogenesis [Citation33]. VEGF-mediated signaling occurs in tumor cells and contributes to crucial aspects of tumorigenesis. Furthermore, VEGF is considered the main factor that promotes angiogenesis in PC and is related to its metastasis [Citation34].
EMT is a basal process in embryogenesis; however, it is also associated with the progression of numerous cancers including PC [Citation35,Citation36], where it is mainly associated with increased invasion and metastasis [Citation37]. Li et al. [Citation38] reported that EMT markers play a key role in the treatment of PC [Citation38]. This study investigated the effect of AE treatment on the EMT-related protein expression of E-cadherin, TWIST1, SNAIL1, and ZEB1. We also observed the expression of mesenchymal markers, TWIST, SNAIL1, and ZEB1, which enhance cell migration and invasion [Citation39]; hence, the EMT-related genes promoted cancer cell metastasis. We found that the AE-induced inhibition of EMT was mediated by E-cadherin, TWIST1, SNAIL1, and ZEB1. Taken together, our findings suggest that AE possesses anti-PC properties that mediate by inhibiting the expression of angiogenesis- and EMT-related genes.
In summary, our study demonstrates that AE can significantly inhibit the growth of transplanted tumors in nude mice. Local tumor metastasis is an important cause of high cancer mortality, and both angiogenesis and EMT are closely related to tumor metastasis. VEGF is currently the most well-studied of all pro-angiogenic factors, and the VEGF signaling pathway is involved in the entire process of angiogenesis. In this experiment, AE adjusted the levels of genes involved in angiogenesis. The AE groups showed a dose-dependent decrease in MMP-2, MMP-9, and VEGF levels, while those of TIMP-1 and TIMP-2 increased significantly and dose-dependently in the AE groups. This study found that the migration and invasion of prostate cancer tumors decreased after AE intervention, which may be related to the down-regulation of E-cadherin, TWIST1, SNAIL1, and ZEB1 protein expression. It is speculated that AE may inhibit angiogenesis and EMT-related genes; therefore, it plays a regulatory role in the growth, migration, and invasion of prostate cancer tumors. Furthermore, AE inhibited tumor growth in PC xenograft model by attenuating PSA and DHT levels; these results indicate that AE possesses anti-metastatic potential for PC treatment and may be a candidate for future clinical studies.
Authors contributions
The authors alone are responsible for the content and writing of the paper. Yujiao Tang, Meiqi Fan and Young-Jin Choi performed the research, Yonghai Yu, Gang Yao and Yongyan Deng collected and analyzed the data, Sang-Ho Moon and Eun-Kyung Kim designed the research study. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):e359–e386.
- Fleshner N. Defining high-risk prostate cancer: current status. Can J Urol. 2005;12(14–17):94–96.
- Gleave ME, Bruchovsky N, Moore MJ, et al. Prostate cancer: 9. Treatment of advanced disease. Cmaj. 1999;160(5):225–232.
- Moreau JP, Delavault P, Blumberg J. Luteinizing hormone-releasing hormone agonists in the treatm1ent of prostate cancer: a review of their discovery, development, and place in therapy. Clin Ther. 2006;28(10):1485–1508.
- Lamoureux F, Thomas C, Yin MJ, et al. A novel HSP90 inhibitor delays castrate-resistant prostate cancer without altering serum PSA levels and inhibits osteoclastogenesis. Clin Cancer Res. 2011;17(8):2301–2313.
- Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17:S72–S79.
- Qu S, Wang K, Xue H, et al. Enhanced anticanceractivity of a combination of docetaxel and Aneustat (OMN54) in a patient-derived, advanced prostate cancer tissue xenograft model. Mol Oncol. 2014;8(2):311–322.
- Wu F, Li H, Jin L, et al. Deer antler base as a traditional Chinese medicine: A review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013;145(2):403–415.
- Tang Y, Jeon BT, Wang Y, et al. First evidence that Sika Deer (Cervus nippon) velvet antler extract suppresses migration of human prostate cancer cells. Korean J Food Sci. 2015;35(4):507–514.
- Shao MJ, Wang SR, Zhao MJ, et al. The effects of velvet antler of deer on cardiac functions of rats with heart failure following myocardial infarction. Evid Based Complement Alternat Med. 2012;2012:825056.
- Tang Y, Jeon BT, Wang Y, et al. First evaluation of the biologically active substances and antioxidant potential of regrowth velvet antler by means of multiple biochemical assays. J Chem. 2015;2015.
- Kang SK, Kim KS, Kim SI, et al. Immunosuppressive activity of deer antler extracts of Cervus korean TEMMINCK var. mantchuricus Swinhoe, on type II collagen-induced arthritis. In Vitro Cell Dev Biol Anim. 2006;42(3):100–107.
- Ehteda A, Galettis P, Pillai K, et al. Combination of albendazole and 2-methoxyestradiol significantly improves the survival of HCT-116 tumor-bearing nude mice. BMC Cancer. 2013;13(1):86.
- Zhang Z, Liu X, Duan L, et al. The effects of velvet antler polypeptides on the phenotype and related biological indicators of osteoarthritic rabbit chondrocytes. Acta Biochim Pol. 2011;58:297–302.
- Kim YK, Kim KS, Chung KH, et al. Inhibitory effects of deer antler aqua acupuncture, the pilose antler of Cervus Korean Temminck var. mantchuricus Swinhoe, on type II collagen-induced arthritis in rats. J Int Immunopharmacol. 2003;3(7):1001–1010.
- Kim JH, Yang YI, Ahn JH, et al. Deer (Cervus elaphus) antler extract suppresses adhesion and migration of endometriotic cells and regulates MMP-2 and MMP-9 expression. J Ethnopharmacol. 2012;140(2):391–397.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
- Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by Cisplatin. J Toxicol Pathol. 2013;26(3):293–299.
- Song W, Soni V, Khera M. Combined tests of prostate specific antigen and testosterone will improve diagnosis and monitoring the progression of prostate cancer. Asian J Androl. 2015;17(5):807–810.
- Freedland SJ, Isaacs WB, Platz EA, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23(30):7546–7554.
- Mahmoud AM, Zhu T, Parray A, et al. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS One. 2013;8(10):78479.
- Yegorova I Composition and method for increasing testosterone levels: US 20030198685 A1. United States (US): Europe PMC; 2003.
- Tu G. Pharmacopoeia of the people’s republic of China. Beijing. China (CN): People’s Medical Publishing House; 1988. p. 45–50.
- Fraser A, Haines SR, Stuart EC, et al. Deer velvet supplementation decreases the grade and metastasis of azoxymethane-induced colon cancer in the male rat. Food Chem Toxicol. 2010;48(5):1288–1292.
- Fan Y, Xing Z, Wei Q, et al. A study on the extraction separation and anticancer activity of velvet antler protein. J Econ Anim. 1998;3:27–31.
- Xiong HL Extraction and isolation of activity component from velvet antler and research of its anti-tumor effect [ Master Thesis]. China (CN): Northwest A&F University; 2007.
- Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995;235(26):1757–1763.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257.
- Kalluri R. Basement membranes: structure, assembly and role in tumour Angiogenesis. Nat Rev Cancer. 2003;3(6):422–433.
- McCawlery LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore. Curr Opin Cell Biol. 2001;13(5):534–540.
- Morgan J, Rouche A, Bausero P, et al. MMP-9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve. 2010;42(4):584–595.
- Nishimura T, Nakamura K, Kishioka Y, et al. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J Muscle Res Cell Motil. 2008;29(1):37–44.
- Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012;180(1):12–16.
- Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Phys Heart Circ Physiol. 2001;280(5):h2357–h2363.
- Chuaire MLF, Corredor MCS, Noack LC. Epithelial‑mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin. 2013;54(2):186–205.
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890.
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110.
- Li J, Chong T, Wang Z, et al. A novel anti‑cancer effect of resveratrol: reversal of epithelial‑mesenchymal transition in prostate cancer cells. Mol Med Rep. 2014;10(4):1717–1724.
- Evdokimova V, Tognon CE, Sorensen PH. On translational regulation and EMT. Semin Cancer Biol. 2012;22:437–445.
