ABSTRACT
Contact hypersensitivity (CHS) is frequently used as an animal model for human allergic contact dermatitis (ACD). Diets of pomegranate polyphenols (PPs) or soy isoflavones (SIs) each alleviated CHS symptoms; however, the effect of diets containing a mixture of PPs and SIs on CHS is unclear. We investigated the CHS-inhibitory effects of diets supplemented with a mixture of PPs and SIs at human physiologically relevant doses. Consuming the mixture of PPs and SIs attenuated ear swelling and reduced infiltration of Gr-1-positive cells. Ear swelling decreased in the PP and SI-treated mice compared to the SI-treated mice. The auricle tissues of the PP and SI-fed mice exhibited decreased production of CXCL2 and MCP-5 compared to the SI- and PP-treated mice, respectively. These results suggest that dietary supplementation with a mixture of PPs and SIs may have ACD-preventive effects and may prove more beneficial than supplementation with PPs or SIs alone.
Graphical Abstract
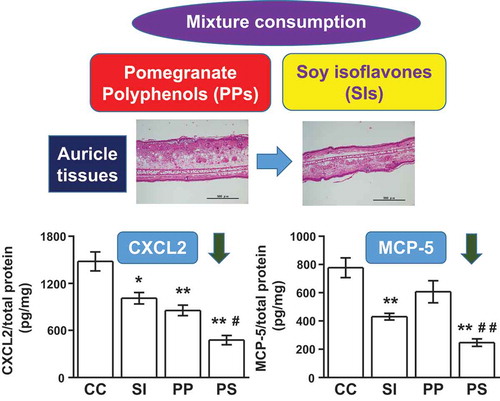
Consumption of PPs and SIs at doses physiologically relevant to humans attenuated contact hypersensitivity and reduced CXCL2 and MCP-5.
Pomegranates (Punica granatum L.) are frequently consumed as juice, which is rich in polyphenols and exhibits higher antioxidant activity than do juices of other fruits such as grape, orange, and apple [Citation1,Citation2]. Ellagitannins (ETs) are the predominant polyphenol components in pomegranate juice (PJ), and their high antioxidant activities are thought to provide a range of health benefits for those with medical conditions including cardiovascular disease, diabetes, inflammatory bowel diseases, and cancer [Citation3–Citation6].
Soy isoflavone (SI) is a major phytochemical in soybeans and has been associated with beneficial effects against chronic conditions such as cardiovascular disease, certain types of cancers, menopausal symptoms, and osteoporosis [Citation7–Citation11].
Contact hypersensitivity (CHS) is an animal model often used to study allergic contact dermatitis (ACD), a common occupational and environmental skin disorder induced by low molecular weight chemicals [Citation12–Citation15]. In a prior investigation, we studied the CHS-suppressive effect of dietary pomegranate polyphenols (PPs), and found that low-dose PP diets reduced the severity of CHS symptoms. The low PP dose administered to the mice corresponds to approximately 1 g polyphenols for a human per day [Citation16]. The polyphenol content in PJ varies between 0.2–1%, which amounts to 0.4–2 g of polyphenols per 200 mL of juice [Citation1]. We also previously studied the CHS-inhibitory effects of SI-enriched diets and observed that low-dose SI diets attenuated CHS symptoms. This low SI dose administered orally to mice was equivalent to approximately 75 mg SI for a human per day [Citation17]. Following Japanese nutritional guidelines, 70–75 mg/day is the maximum safe intake level for dietary SI as aglycone. In Japan and East Asian countries, the intake of SI varies from 25 to 50 mg per day [Citation18]. Therefore, our findings suggest that dietary PPs or SIs provide beneficial effects for patients with ACD when included in their regular diets. In addition, analyses of DNA microarray and antibody array indicated that different cytokines were suppressed by dietary PPs and SIs, suggesting that the beneficial effects on CHS were exerted by different mechanisms [Citation16,Citation17]. However, the CHS-suppressive effects mediated by diets containing a mixture of PPs and SIs are unclear.
In the present study, we investigated the CHS-inhibitory effects of diets containing a mixture of PPs and SIs at doses physiologically relevant to humans. Moreover, we utilized antibiotic arrays to elucidate the underlying mechanisms of the CHS-suppressive effects of consuming a mixture of PPs and SIs.
Materials and methods
Mice and materials
Female BALB/c mice (six weeks old) and the soybean-free diet F2PLD1 were purchased from Japan SLC (Hamamatsu, Japan) and Oriental Yeast (Tokyo, Japan), respectively. PPs were prepared as previously described [Citation19]. The main polyphenolic components were ETs, as detailed in previous studies [Citation20,Citation21]. Soyaflavone HG (HG), an SI-enriched formulation, was obtained from Fuji Oil (Izumisano, Japan). Daidzein, glycitein, and genistein accounted for 61%, 30%, and 9%, respectively, of the total SI content in HG as considered aglycones.
Contact hypersensitivity
The CHS animal experiments were conducted as described previously [Citation22]. Mice were divided into non-CHS control (NC, n = 3), CHS control (CC, n = 6), SI-treated (SI, n = 6), PP-treated (PP, n = 6), and PP and SI-treated (PS, n = 6) groups. The NC and CC groups were provided water, while the SI and PP groups were provided water supplemented with 0.004% HG and 0.01% PP, respectively. The PS group was provided water with both 0.01% PP and 0.004% HG. The solutions of PP and HG were provided to mice by a water bottle 3 weeks before the first 2,4-dinitrofluorobenzene (DNFB; Kanto Chemical, Tokyo, Japan) challenge. On days 0 and 1, the mice were sensitized by the application of 0.5% DNFB to shaved abdominal skin and then challenged on days 5 and 12 by applying 0.2% DNFB to the dorsal side of both ears. Negative control (non-CHS) mice were sensitized and challenged without DNFB. Ear thickness was measured using a soft touch micrometer, and ear swelling was determined as the difference in ear thickness before the first DNFB challenge and 24 h after the second challenge. All experimental procedures were in accordance with the guidelines for animal experimentation and were approved by the Animal Experimental Committee of Kawasaki University of Medical Welfare (authorization number 14–011) and Kawasaki Medical School (authorization number 15–007).
Histological analysis
Ear tissue was collected 24 h after the second DNFB challenge and fixed with IHC Zinc Fixative (BD Biosciences, San Jose, CA, USA) for 24 h, embedded in paraffin, and sectioned. Ear tissue sections were stained with hematoxylin and eosin, and immunohistochemistry was performed by a Ventana DISCOVERY XT instrument (Ventana Medical Systems, Tucson, AZ, USA), as described previously [Citation22]; purified anti-mouse Ly-6G (Gr-1) and mouse anti-rat IgG biotin (eBioscience, San Diego, CA, USA) were utilized as the primary and secondary antibodies, respectively. Images were acquired using a microscope, and the number of Gr-1-positive cells was counted in five randomly selected areas (~210 μm2) of tissue sections.
Cytokine production analysis
Ear tissues were harvested 24 h after the second DNFB challenge and were stored at −80°C until use. The ear tissues were cut, placed into Lysing Matrix D tubes (MP Biomedicals, Santa Ana, CA, USA), and homogenized in PIPA lysis buffer containing protease inhibitors (ATTO, Tokyo, Japan) in a FastPrep-24 instrument (MP Biomedicals). Cytokine expression was detected utilizing the Proteome Profiler Mouse Cytokine Array Kit, Panel A, and Proteome Profile Mouse Chemokine Array Kit (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s instructions. Chemiluminescence was measured with an ImageQuant LAS 4000 Mini instrument (GE Healthcare Biosciences, Piscataway, NJ, USA), and spot intensity was determined with Image Studio Lite software (LI-COR Biotechnology, Lincoln, NE, USA). The production of C-X-C motif chemokine ligand 2 (CXCL2) and monocyte chemoattractant protein-5 (MCP-5) was analyzed using a mouse DuoSet ELISA development system (R&D Systems) as per the manufacturer’s instructions. Protein concentrations were determined using the Pierce BCA protein assay (Thermo Fisher Scientific, Waltham, MA, USA). All assays were performed in duplicate.
Statistical analysis
One-way analysis of variance (ANOVA) was performed, followed by Tukey’s post-hoc tests, to determine statistical significance, using Origin 8.5 software (OriginLab, Northampton, MA, USA). Data are presented as means ± standard errors of the mean (SEM), and p < 0.05 was considered statistically significant.
Results
PP and SI diets attenuate ear swelling and edema
The inhibitory effect of dietary PPs and SIs on CHS was determined by measuring the DNFB-induced ear swelling. There was a significantly greater reduction in ear swelling in the SI (0.115 ± 0.011 mm, 33% decrease, p < 0.01), PP (0.087 ± 0.009 mm, 49% decrease, p < 0.01), and PP and SI (0.072 ± 0.007 mm, 58% decrease, p < 0.01) treated groups than in the CHS controls (0.171 ± 0.011 mm) ()). In addition, the ear swelling significantly decreased in the PP and SI-treated mice compared to the SI-treated mice (p = 0.012). Histological observation of inflamed auricle tissues showed prominent ear swelling and tissue edema in the CHS controls compared to the non-CHS control mice, in which these symptoms were attenuated by the diets enriched with PP and SI ()).
Figure 1. Dietary pomegranate polyphenol (PP) and soy isoflavone (SI) attenuate ear swelling and edema in contact hypersensitivity (CHS) mice.(a) Ear swelling. NC, non-CHS control group; CC, CHS control group; SI, SI-treated group; PP, PP-treated group; PS, PP and SI-treated group. The data are presented as the means ± SEM of the data from two independent experiments [n = 6 mice per group, except for the non-CHS group (n = 3)]; **p < 0.01 vs. CC, #p < 0.05 PS vs. SI. (b) Hematoxylin and eosin-stained images of auricle tissues. Scale bars, 500 μm.
![Figure 1. Dietary pomegranate polyphenol (PP) and soy isoflavone (SI) attenuate ear swelling and edema in contact hypersensitivity (CHS) mice.(a) Ear swelling. NC, non-CHS control group; CC, CHS control group; SI, SI-treated group; PP, PP-treated group; PS, PP and SI-treated group. The data are presented as the means ± SEM of the data from two independent experiments [n = 6 mice per group, except for the non-CHS group (n = 3)]; **p < 0.01 vs. CC, #p < 0.05 PS vs. SI. (b) Hematoxylin and eosin-stained images of auricle tissues. Scale bars, 500 μm.](/cms/asset/c9bf700c-70d3-4756-9713-c71b68ccbf28/tbbb_a_1543013_f0001_oc.jpg)
Dietary PP and SI reduce the infiltration of Gr-1 positive cells
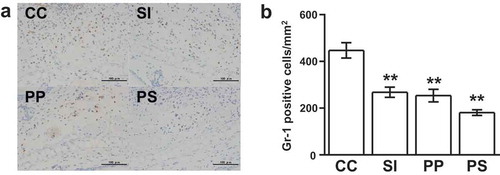
Immunohistochemical staining for Gr-1, a marker of granulocytes and monocytes, was conducted to evaluate the degree of infiltration of myeloid cells into the auricle tissues ()). Compared to the CHS controls (450 ± 30 cells/mm2), the SI- (270 ± 20 cells/mm2), PP- (250 ± 30 cells/mm2), and PP and SI- (180 ± 10 cells/mm2) treated mice exhibited significantly reduced numbers of Gr-1-positive cells infiltrating into auricle tissues (40%, 44%, and 60% decrease; p < 0.01) ()).
Figure 2. Dietary pomegranate polyphenol (PP) and soy isoflavone (SI) reduce the infiltration of myeloid cells in contact hypersensitivity (CHS) mice.(a) Immunohistochemistry images of auricle tissues stained with an anti-Gr-1 antibody. Dark brown, Gr-1-positive cells; blue, nuclei stained with hematoxylin. CC, CHS control group; SI, SI-treated group; PP, PP-treated group; PS, PP and SI-treated group. Scale bars, 100 mm. (b) The number of Gr-1-positive cells infiltrating the auricle tissues. The data are presented as the means ± SEM of the data obtained from two independent experiments (n = 6 mice per group); **p < 0.01 vs. CC.
Dietary PP and SI inhibit CXCL2 and MCP-5 production
To examine the secretion of cytokines in the auricle tissues of the PP and SI-treated and CHS control mice, we used two kinds of antibody arrays, cytokine and chemokine antibody arrays, which can detect 40 cytokines and 25 chemokines, respectively. The cytokine antibody array indicated that the cytokines with the greatest reduction in the mice fed a mixture of PPs and SIs were macrophage colony-stimulating factor (M-CSF), CXCL9, CXCL2, complement component 5a (C5/C5a), and CXCL10 (). In addition, per the chemokine antibody array, the most suppressed chemokines in the PP and SI-treated mice, listed in order from the greatest to the least reduction, were CXCL10, C-C motif chemokine ligand 5 (CCL5), Chemerin, CCL21, and MCP-5 ().
Table 1. Downregulated cytokines, as determined by cytokine antibody array, in mice fed a diet containing a mixture of PPs and SIs.
Table 2. Downregulated chemokines, as determined by chemokine antibody array, in mice fed a diet containing a mixture of PPs and SIs.
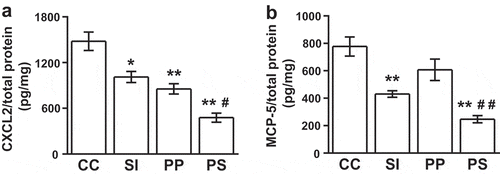
Furthermore, ELISA was conducted to determine the secretion levels of CXCL2 and MCP-5 in the auricle tissues. The production levels of CXCL2 were more reduced in the SI (p = 0.021), PP (p < 0.01), and PP and SI (p < 0.01) treated mice than in the CHS controls. There was also a significant difference between the SI- and PP and SI-treated mice (p = 0.023, )). In contrast, the levels of MCP-5 production decreased in the mice fed a diet containing both SI and PP, as well as those fed SI only, compared with the CHS controls; however, no significant difference was observed between the PP-treated and CHS control mice. There was a significant difference between the PP- and PP and SI-treated mice (p < 0.01, )).
Figure 3. Dietary pomegranate polyphenol (PP) and soy isoflavone (SI) suppress CXCL2 and MCP-5 production in contact hypersensitivity (CHS) mice. (a) CXCL2, (b) MCP-5. CC, CHS control group; SI, SI-treated group; PP, PP-treated group; PS, PP and SI-treated group. The data represent the results of two independent experiments (n = 6 mice per group) and are presented as means ± SEM; *p < 0.05, **p < 0.01 vs. CC, #p < 0.05 PS vs. SI, ##p < 0.01 PS vs. PP.
Discussion
The results of this study demonstrate that CHS symptoms in mice were attenuated following consumption of diets containing a mixture of PPs and SIs at doses physiologically relevant to humans. We observed reduced edema, as evidenced by decreased ear swelling, reduced infiltration of Gr-1-positive cells, and downregulation of the expression of pro-inflammatory cytokines, including CXCL2 and MCP-5. Our previous studies showed that dietary supplementation with PPs or SIs alone at physiologically relevant doses reduced CHS symptoms [Citation16,Citation17]. Thus, in the present study, we investigated the CHS-inhibitory effects of diets containing a mixture of PPs and SIs compared to supplementation with PPs or SIs alone. Compared with the SI-treated mice, ear swelling decreased and the production of CXCL2 was suppressed in the PP and SI-treated mice. In addition, compared to the PP-treated mice, the levels of MCP-5 were reduced in the PP and SI-treated mice. These results suggest that dietary supplementation with a mixture of PPs and SIs may have ACD-preventive effects and may prove more beneficial than supplementation with PPs or SIs alone.
Chemokine and cytokine antibody arrays were used to elucidate the underlying mechanisms of the CHS-suppressive effects of supplementation with PPs and SIs. In a prior investigation, we found that the group fed a high-dose PP diet (0.2% PPs) exhibited decreased release (i.e. less than 70%) of three chemokines (CXCL5, MCP-5, and MCP-5) compared to the CHS control [Citation16]. We also previously observed that four cytokines (CXCL1, IL-1β, MCP-1, and CCL3) were suppressed to less than 70% in the high-dose SI (0.1% HG)-treated group compared with the CHS control [Citation17]. In the present study, five chemokines and nine cytokines were suppressed to less than 70% in the mice treated with a mixture of low-dose PP (0.01% PP) and SI (0.004% HG) compared to the CHS control. These results indicate that diets containing a mixture of PP and SI suppress more proinflammatory cytokines at lower doses than either PP or SI alone.
We also observed decreased production of CXCL2 and MCP-5 in the auricle tissues of the PP and SI-treated mice compared with CHS controls. In addition, the auricle tissues of the PP and SI-fed mice exhibited reduced infiltration of Gr-1-positive cells. CXCL2 and CXCL1 are neutrophil-recruiting chemokines. The blockage of a prostaglandin E2 receptor by an antagonist resulted in the downregulation of Cxcl2 and Cxcl1 and inhibited CHS during the elicitation phase [Citation23]. In contrast, mouse MCP-5 was found as a novel monocyte-recruiting chemokine involved in allergic inflammation, and its amino acid sequence is closely related to that of human MCP-1, with 66% identity [Citation24]. In a previous study, the levels of chemokine gene expression were investigated at the elicitation site of the CHS mice using a cDNA microarray [Citation25]. The expression of eleven genes was detected, and these belonged to four groups on the basis of their kinetic patterns. First, Cxcl2 and Cxcl1 gene expression increased within 4 h of hapten exposure. Second, the mRNA expression for members of the MCP group, such as Mcp-1 and Mcp-5, increased and peaked at 6–9 h post challenge [Citation26]. Therefore, the observed inhibitory effects of diets containing a mixture of PPs and SIs on CXCL2 and MCP-5 production correlate with reduced infiltration of neutrophils and monocytes into the contact allergic auricle tissues in the mice with CHS.
Cutaneous dendritic cells (DCs) and T cells are involved in CHS [Citation12]. In a previous study, dietary SIs suppressed allergic reactions to peanuts in mice. This was attributed to suppression of DC maturation by SIs in the mesenteric lymph node. In addition, SIs suppressed cholera toxin-treated activation of human monocyte-derived DCs [Citation27]. In our previous study, more splenic IL-10-producing CD4-positive T cells were observed in PP-treated mice than in the CHS control [Citation19]. We also previously investigated the effects of urolithin A (UA), an important metabolite of ETs, on splenic cell activation. UA decreased the number of INF-γ-producing splenocytes in mice with CHS [Citation16]. The mechanisms of CHS suppression described in prior studies suggest how consuming a mixture of PPs and SIs effects CHS, but further work is required to definitely elucidate an underlying mechanism.
In conclusion, our data provide evidence that diets containing a mixture of PP and SI at doses physiologically relevant to humans attenuate CHS symptoms and reduce CXCL2 and MCP-5 production at CHS elicitation sites in mice. Diets containing a both PPs and SIs yielded greater beneficial effects in the CHS mice than supplementation with PPs or SIs alone. These results suggest that consuming pomegranate and soy based foods is of therapeutic value for ACD patients when included in their regular diets.
Author contributions
TN and HI conceived and designed the experiments; TN performed the experiments; TN wrote, and HI reviewed and edited the manuscript.
Acknowledgments
The authors thank N. Nishida (Morishita Jintan Co.) for kindly providing PPs. We would like to thank Editage (www.editage.jp) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aviram M, Rosenblat M. Pomegranate protection against cardiovascular diseases. Evid Based Comp Alter Med. 2012;2012:382763.
- Bakkalbaşi E, Menteş Ö, Artik N. Food ellagitannins–occurrence, Effects of processing and storage. Crit Rev Food Sci Nutr. 2008;49(3):283–298.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, et al. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48(10):4581–4589.
- Faria A, Calhau C. The bioactivity of pomegranate: impact on health and disease. Crit Rev Food Sci Nutr. 2011;51(7):626–634.
- Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269(2):262–268.
- Larrosa M, Garcia-Conesa MT, Espin JC, et al. Ellagitannins, ellagic acid and vascular health. Mol Asp Med. 2010;31(6):513–539.
- Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr. 2014;100:423S–430S.
- Nagata C, Mizoue T, Tanaka K, et al. Research group for the, J. Evaluation of cancer prevention strategies in, soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2014;44(3):282–295.
- Li SH, Liu XX, Bai YY, et al. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr. 2010;91(2):480–486.
- Alekel DL, Van Loan MD, Koehler KJ, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. 2010;91(1):218–230.
- Taku K, Melby MK, Kronenberg F, et al. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta-analysis of randomized controlled trials. Menopause. 2012;19(7):776–790.
- Honda T, Egawa G, Grabbe S, et al. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133(2):303–315.
- Kaplan DH, Igyártó BZ, Gaspari AA. Early events in the induction of allergic contact dermatitis. Nature Rev Immunol. 2012;12(2):114–124.
- Martin SF, Esser PR, Weber FC, et al. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy. 2011;66(9):1152–1163.
- Inagaki N, Nagai H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: mouse models for the development of remedies for human allergic dermatitis. J Pharmacol Sci. 2009;110(3):251–259.
- Nagano T, Ito H. Diet containing a polyphenol concentrate from pomegranate juice attenuates contact hypersensitivity in mice. J Funct Food. 2018;45:247–253.
- Nagano T, Katase M, Tsumura K. Inhibitory effects of dietary soy isoflavone and gut microbiota on contact hypersensitivity in mice. Food Chem. 2019;272:33–38.
- Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1–12.
- Nagano T, Nishida N, Ito H. The inhibitory effect of a polyphenol concentrate from pomegranate juice on 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Food Sci Technol Res. 2018;24(1):169–175.
- Ito H, Li P, Koreishi M, et al. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014;152:323–330.
- Kawakami K, Li P, Uraji M, et al. Inhibitory effects of pomegranate extracts on recombinant human maltase–glucoamylase. J Food Sci. 2014;79(9):H1848–H1853.
- Nagano T, Wu W, Tsumura K, et al. The inhibitory effect of soybean and soybean isoflavone diets on 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Biosci Biotechnol Biochem. 2016;80(5):991–997.
- Honda T, Matsuoka T, Ueta M, et al. Prostaglandin E2–EP3 signaling suppresses skin inflammation in murine contact hypersensitivity. J Allergy Clin Immunol. 2009;124(4):809–818.e2.
- Sarafi MN, Garcia-Zepeda EA, MacLean JA, et al. Murine monocyte chemoattractant protein (MCP)-5: A novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185(1):99–110.
- Mitsui G, Mitsui K, Hirano T, et al. Kinetic profiles of sequential gene expressions for chemokines in mice with contact hypersensitivity. Immunol Lett. 2003;86(2):191–197.
- Niwano Y, Mitsui G, Kohno M. Chemokines and their receptors as a target for the treatment of contact hypersensitivity. Anti-Inflamm Anti-Allergy Agents Med Chem. 2008;7(1):45–51.
- Masilamani M, Wei J, Bhatt S, et al. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J Allergy Clin Immunol. 2011;128(6):1242–1250e1.
