ABSTRACT
Theanine (γ-glutamylethylamide) is an amino acid analog that reduces blood pressure and improves immune responses. The ϒ-glutamyltranspeptidase (GGT) from Pseudomonas nitroreducens IFO12694 (PnGGT) has a unique preference for primary amines as ϒ-glutamyl acceptors over standard L-amino acids and peptides. This characteristic is useful for the synthesis of theanine. We used X-ray crystallographic analysis to understand the structural basis of PnGGT’s hydrolysis and transpeptidation reactions and to characterize its previously unidentified acceptor site. Structural studies of PnGGT have shown that key interactions between three residues (Trp385, Phe417, and Trp525) distinguish PnGGT from other GGTs. We studied the roles of these residues in the distinct biochemical properties of PnGGT using site-directed mutagenesis. All mutants showed a significant decrease in hydrolysis activity and an increase in transpeptidase activity, suggesting that the aromatic side chains of Trp385, Phe417, and Trp525 were involved in the recognition of acceptor substrates.
Abbreviations: ϒ-glutamyl peptide, theanine, X-ray crystallography.
Graphical Abstract
Crystal structure analysis and enzymatic characterization of ϒ-glutamyltranspeptidase from Pseudomonas nitroreducens
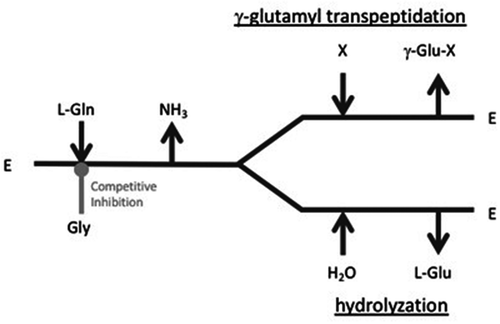
ϒ-Glutamyltranspeptidase (GGT) is a ubiquitous enzyme that catalyzes the hydrolysis of the γ-glutamyl linkages of γ-glutamyl compounds and the transfer of their γ-glutamyl moieties to acceptor substrates [Citation1–Citation3]. In the first step of the catalysis, the cleaved γ-glutamyl group from the donor substrate reacts with the hydroxy group of a nucleophilic Thr residue to form a γ-glutamyl-enzyme intermediate. The catalytic cycle is completed by the transfer of the γ-glutamyl moiety to water in hydrolysis reactions or to an acceptor substrate in transpeptidation reactions (). GGT belongs to the N-terminal nucleophile hydrolase superfamily. The enzyme is produced as an inactive precursor that undergoes autocatalytic cleavage to generate the mature enzyme, which usually comprises a large and a small subunit. The catalytic residue Thr at the N-terminal end of the small subunit is also essential for precursor autoprocessing. Theanine (ϒ-glutamylethylamide), a Food and Drug Administration-approved food supplement, is amino acid analog that reduces blood pressure and improves immune responses [Citation4,Citation5]. Unlike other GGTs, the GGT of Pseudomonas nitroreducens IFO12694 (PnGGT) prefers primary amines as γ-glutamyl acceptors over standard L-amino acids and peptides [Citation6].
Although PnGGT has been used to synthesize theanine at industrial scale, it exhibits higher hydrolysis activity than transpeptidase activity at neutral pH. Human, Helicobacter pylori and Bacillus anthracis GGTs (HsGGT, HpGGT, CapD) catalyze transpeptidation 180-fold, 2-fold, and 12-fold faster than hydrolysis [Citation7], respectively. The low activity of PnGGT in γ-glutamyl transfer is a fundamental problem in the industrial production of theanine.
To understand the structural basis for hydrolysis and transpeptidation reactions, we have determined the X-ray crystal structure of PnGGT and compared it with the structures of known GGTs. The results suggested that three key amino acid residues, Trp385, Phe417, and Trp525 distinguish PnGGT from other GGTs and define its recognition of acceptor substrates.
Materials and methods
Chemicals and reagents
Crystal Screen was obtained from Hampton Research (Aliso Viejo, CA, USA). Other chemicals were of analytical-grade, and were used without modification unless otherwise stated.
Preparation and purification of PnGGT
The PnGGT protein was overexpressed and purified as described previously [Citation6]. Purity of the enzyme was confirmed by SDS-PAGE, and the protein concentration was determined using the Lowry method.
Crystallization and X-ray diffraction
PnGGT solution was dialyzed against 0.1 M HEPES (pH 7.0) before crystallization trials. The purified wild-type PnGGT enzyme was concentrated to 14 mg/mL using an Amicon Ultra centrifugal filter. Initial crystallization trials were performed with a Crystal Screen. All crystallization trials were conducted using the sitting-drop vapor-diffusion method in 24-well crystallization Cryschem M plates (Hampton Research). All drops were prepared by mixing 1 µL of protein solution with 1 µL of reservoir solution and were equilibrated against 500 µL of reservoir solution. The plates were sealed with Crystal Clear Sealing Tape (Hampton Research), and the crystals were allowed to grow at 20°C for approximately 1 week. Among the 48 potential reservoir solutions, crystals were observed in the drop of reservoir solution consisting of 0.2 M magnesium acetate tetrahydrate, 0.1 M sodium cacodylate trihydrate, pH 6.5, and 20% (w/v) polyethylene glycol 8000 (PEG-8000). After several crystallization trials, the crystals suitable for X-ray analysis were obtained under the following conditions: 16% PEG-8000, 15% PEG-400, 0.1 M HEPES, pH 7.0, 50 mM glycylglycine (Gly-Gly), and 3–5 mg/mL PnGGT protein. The single crystals were soaked for 30 s at 20°C in a cryoprotectant solution containig 5%(w/v) glycerol, 16% PEG-8000, 15% PEG-400, 0.1 M HEPES, pH 7.0, and 50 mM Gly-Gly. The crystals were placed under a cold nitrogen gas stream at 100 K. X-ray diffraction data of the PnGGT crystals were collected on beamline BL-17A at the Photon Factory (Japan), and processed to a resolution of 1.57 Å (). X-ray diffraction images of the crystals were mainly collected using ADSC Quantum 270 CCD X-ray detectors (Poway, CA, USA). Images were processed using the HKL-2000 program [Citation8] ().
Table 1. Data collection and refinement statistics for PnGGT structures.
Structure determination and refinement
The initial model of PnGGT was determined using the molecular replacement (MR) method within the PHASER program [Citation9] and an existing structure of Escherichia coli GGT (EcGGT) deposited in the RCSB Protein Data Bank (PDB) as the template (PDB entry: 2DG5). The initial model was refined using ARP/wARP automated protein model building software [Citation10]. Reflection data in the resolution range between 50 and 1.70 Å were used for the refinement. The model was refined using Phenix [Citation11] and manually rebuilt using Coot [Citation12]. Figures were prepared using PyMol (DeLano Scientific, Palo Alto, CA, USA) and schematic drawings were prepared by LigPlot+ [Citation13]. Superposition of the PnGGT structural model with other GGTs was conducted using GASH [Citation14].
Site-directed mutagenesis
A previously-constructed plasmid (pPnGGT2) [Citation6] was used as the template for site-directed mutagenesis. Overlapping complementary primers were designed bearing the desired nucleotide changes for each mutant (). The reaction mixtures (50 µL) contained 1 µL of template DNA, 1 µL each of the forward and reverse primers, 5 µL of PCR buffer, 3 µL of MgSO4 solution, 4 µL of each dNTP, and 0.5 µL of KOD Plus DNA polymerase. Following 20 cycles of the thermal cycling program (95°C for 30 s, 58°C for 1 min, and 68°C for 10 min), the PCR products were digested with DpnI endonuclease for 1 h at 37°C. E. coli XL10-Gold competent cells were transformed with the digested products, and single colonies were picked from LB plates to verify the mutations by DNA sequencing.
Table 2. Overlapping complementary primers for the site-directed mutagenesis.
Enzyme activity assay
Hydrolysis activity assays were performed at 30°C for 5–10 min in 100 mM imidazole buffer, pH 9.0, using diluted enzyme and 2.5 mM ϒ-glutamyl-p-nitroanilide (GlupNA) as a substrate. The release of p-nitroaniline was monitored by measuring the absorbance at 410 nm. One unit of GGT enzyme activity (Uhydro) was defined as the amount of enzyme needed to produce 1 µmol of p-nitroaniline per min under standard assay conditions.
Hydrolysis of L-glutamine (L-Gln) was determined using a high-performance liquid chromatography (HPLC). Reactions were conducted at 30°C in 0.4 mL reaction mixtures containing 40 mM L-Gln, 100 mM borate buffer, pH 10.5, and diluted enzyme. Reactions were stopped by the addition of an equal volume of 20% (w/v) trichloroacetic acid (TCA) and then boiled for 3 min. L-Gln and L-Glu in the reaction mixture were derivatized to a fluorescent compound using o-phthaldialdehyde (OPA) and N-tert-butyloxycarbonyl-L-cysteine (Boc) and analyzed by reversed-phase HPLC as follows: 5 μl of the filtered reaction mixture was mixed with 100 μl of OPA-Boc solution prepared by dissolving 10 mg OPA and 10 mg Boc in 1 ml methanol and 395 μl of 0.4 M borate buffer (pH 9.0). The mixture was incubated at room temperature for 10 min and 5 μl of the mixture was applied to HPLC. HPLC analysis was performed using Cosmosil 5C18-MS-II column (4.6 × 250 mm; Nacalai Tesque, Kyoto, Japan) and equilibrated with 0.1 M acetate buffer (pH 6.0) containing 7% acetonitrile and 3% tetrahydrofuran. The derivatized amino acids were eluted using a linear gradient of acetonitrile (7–47%). The elute was monitored by measuring its fluorescence at excitation and emission wavelength of 344 and 433 nm, respectively, and identified by their respective retention time using RF-10A Fluorescence Spectrophotometer (Shimadzu, Kyoto, Japan). In this case, one unit of GGT activity was defined as the amount of enzyme needed to produce 1 µmol of L-Glu per min.
For transfer activity, the reaction mixture contained 40 mM L-Gln, 20 mM hydroxylamine, 100 mM imidazole buffer (pH 7.5), and the enzyme in a final volume of 1 ml. After incubation at 30°C for 10 min, the reaction was terminated by adding 2 ml of 0.2 M FeCl3・6H2O, 0.12 M TCA, 0.25 M HCl, and water (8: 2: 1: 13). The release of γ-L-glutamyl-hydroxamate was monitored by measuring the absorbance at 540 nm. One unit of GGT enzyme activity (Utrans) was defined as the amount of enzyme needed to produce 1 µmol of γ-L-glutamyl-hydroxamate per min. Acceptor specificity was determined using a HPLC system as described above. Reactions were conducted at 30°C for 40 min in 0.4 mL reaction mixtures containing 40 mM L-Gln (donor), 100 mM of each acceptor substrate and 100 mM borate buffer, pH 10.5, and 0.08 Utrans PnGGT, and then stopped by the addition of an equal volume of 20%(w/v) TCA.
Results and discussion
Overall structure
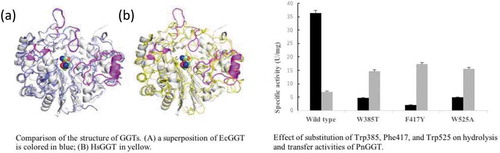
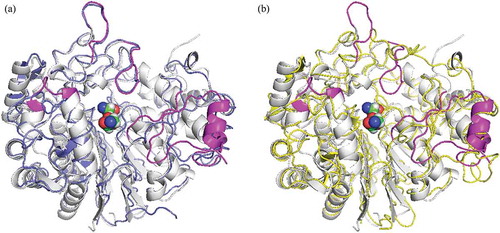
The obtained structure was refined at 1.57 Å resolution to R and Rfree factors of 0.168 and 0.201, respectively (). The mature GGT molecule is a heterodimer comprising a L (large) subunit (residues 26–363) and a S (small) subunit (residues 364–557) with no disordered loops. One side of the S subunit is surrounded by the large subunit, and a stacked αββα-core (two central β-sheets sandwiched by α-helices) is conserved with EcGGT [Citation15], HpGGT [Citation16], CapD [Citation17] and HsGGT [Citation18]. The main-chain atoms of PnGGT were superimposable with root-mean-square deviations of 1.38 (L subunit)/1.52 (S subunit) (EcGGT; PDB entry: 2DBU), 1.43/1.43 (HpGGT; 2QM6), 1.44/1.38 (CapD; 3WHQ) and 1.57/1.76 (HsGGT; 4Z9O) Å, respectively. The superposition of PnGGT with HsGGT, which has higher transpeptidation activity revealed that major structural differences were concentrated in four peripheral loops (residues 108–115, 177–184, 287–298, and 411–421) and a β-α-β motif (residues 496–525), which surrounded the mouth of the active-site pocket ().
Figure 1. Comparison of the structure of GGTs. PnGGT is colored in white and the ligands bound in the active site are represented with a space-filling model.
(a) a superposition of EcGGT is colored in blue; (b) HsGGT in yellow. The regions with major difference in PnGGT chain are represented in magenta.
Substrate binding sites
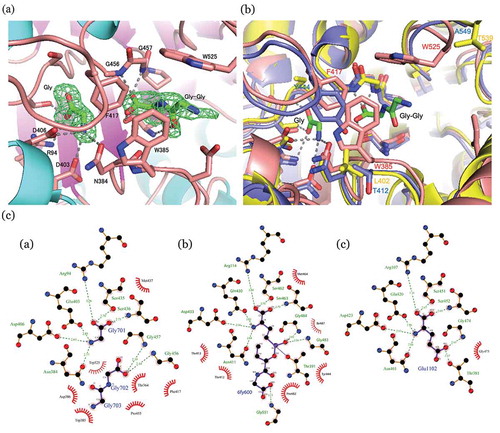
) shows the (Fo – Fc) map around Thr-364, the nucleophilic residue lying at the N-terminal of the S subunit. The two major peaks within the active-site pocket are clearly visible in the electron density map. The smaller peak located at the bottom of the pocket was identified as a free glycine molecule which was probably a hydrolyzed from Gly-Gly, a component in the crystallization solution. The carboxy group of the free glycine is bonded with Arg94 Nη, Ser435 Oϒ, and Ser436 N, and the α-amino group is bonded with Asn384 Oδ, Glu403 Oε1, and Asp406 Oδ2. These hydrogen bonds and salt bridges were well conserved in the other GGT structures in complex with donor substrates or their mimics [Citation15–Citation18]. Thus, the glycine-binding site likely corresponds to the donor substrate-binding site, consistent with the fact that glycine is a competitive inhibitor of PnGGT and displaces glutamine from the active site (data not shown). The larger peak of the (Fo – Fc) map was identified as a Gly-Gly molecule. The terminal carboxy group of Gly-Gly is bound to the hydroxy group of Thr364 and the α-amino groups of Thr364, Gly456, and Gly457 which are involved in forming an oxyanion hole. The N-terminal amino group is bound to the carboxy group of Asp386. There were no other polar interactions, but the aromatic side chains of Tyr170, Trp385, Phe417, and Trp525 were closely packed around Gly-Gly, which provides an advantage in accommodating this small molecule. It was noted that the terminal amino group of Gly-Gly was arranged in opposite orientation to the active center of nucleophilic attack. This binding mode probably relates to its reduced activity as a ϒ-glutamyl acceptor.
Figure 2. Local structures of substrate binding sites.
(a) Gly and Gly-Gly-bound PnGGT at 1.70 Å resolution. SIGMAA-weighted (Fo-Fc) omit maps for Gly and Gly-Gly (green). These maps are contoured at 3 σ. Ligands and major residues in the active site are drawn as stick models, and the protein is drawn as a line model. Hydrogen bonds are shown in a dashed line. (b) Comparison between the active site structures of PnGGT (pink), EcGGT (blue), and HsGGT (yellow). (c) Schematic diagrams of protein-ligand interactions for a) Gly and GlyGly in the PDB entry 5ZJB, b) peptidyl phosphonate inhibitor in the PDB entry 5B5T, and c) Glu in the PDB entry 5ZCG.
Comparison between the active site structures of PnGGT, EcGGT and HsGGT
) shows the superposition of PnGGT’s active site structures with those of EcGGT and HsGGT. All the residues around the donor substrate-binding site of PnGGT (Arg94, Asn384, Glu403, Asp406, Ser435, Ser436, Gly456, and Gly456) are conserved among the other GGTs ( () , , ). The EcGGT structure in complex with a peptidyl phosphonate inhibitor GGsTop [Citation19] indicated that the hydroxy-2-oxoethylamino-oxidanylidene-butane moiety of the inhibitor mimicked an acceptor substrate. The residues surrounding the postulated acceptor-binding pocket were much more structurally diverse than those around the donor substrate-binding site. As described in “Overall structure,” the structural difference between PnGGT and other GGTs are located on the four loops and the β-α-β motif around the mouth of the active-site pocket. Among these diverse regions, the loop (residues 411–421) and the β-α-β motif are involved in the contact with bound Gly-Gly, where a hydrophobic pocket is constructed by three aromatic residues: Trp385, Phe417 and Trp525.
In the active site of PnGGT, Phe417 in the 411–421 loop and Trp385 formed the side wall of the postulated acceptor-binding pocket ( (), () ). Although the main chain fold of the 411–421 loop is sandwiched in the clefts of the PnGGT and EcGGT active sites, the corresponding loop in HsGGT is left out of the cleft, such that the bacterial GGTs have a narrower active site pocket than human GGT. Trp525 at the N-terminal end of the β-α-β motif formed another side wall of the Gly-Gly-binding pocket and made hydrophobic contact.
shows the result of the multiple alignment of PnGGT, EcGGT and HsGGT amino acid sequences using ClustalW [Citation20]. Trp385 and Trp525 are unique to PnGGT and substituted with these residues with smaller side chains in other enzymes. Although Phe417 is relatively conserved among the GGTs, the corresponding residue in HsGGT probably can not interact with an acceptor substrate due to its position outside the active site. The hydrolysis activity of wild-type PnGGT is 30.2 U/mg [Citation6], approximately 22 times higher than that of EcGGT [Citation21]. These results suggested that Trp385, Phe417, and Trp525 were key residues that distinguish PnGGT from other GGTs with their higher hydrolysis activities.
Figure 3. Multiple sequence alignment of GGTs from Pseudomonas nitroreducens, Escherichia coli and human.
Arrows indicate three aromatic amino acids, Trp385, Phe417, and Trp525. Identical amino acid residues are highlighted by black background. Similar amino acid residues are highlighted by gray background.

Site-directed mutagenesis
Three amino acid residues, Trp385, Phe417 and Trp525, form a hydrophobic pocket where the substrate is expected to bind. To gain insight into the roles of Trp385, Phe417, and Trp525 in the enzyme activity of PnGGT, these residues were replaced with those at corresponding positions in the E. coli GGT sequence; Thr, Tyr, and Ala. Although wild-type PnGGT exhibited higher hydrolysis activity than transpeptidase activity, all mutants showed significantly decreased hydrolysis activity and increased transpeptidase activity (). Compared with a hydrolysis activity of 36.3 U/mg for the wild-type enzyme, the hydrolysis activities of all three mutants greatly decreased by 5%–14%. Since Trp385, Phe417, and Trp525 are located in the side walls of the Gly-Gly-binding pocket and help to shield the nucleophilic Thr364 from bulk solvent, the hydrophobic environment at this position was suggested to be important for activating hydrolysis.
Figure 4. Effect of substitution of Trp385, Phe417, and Trp525 on hydrolysis and transfer activity of PnGGT.
Black and gray bars represent the specific activity of ϒ-glutamyl-p-nitroanilide hydrolysis and ϒ-L-glutamylhydroxamate synthesis, respectively.
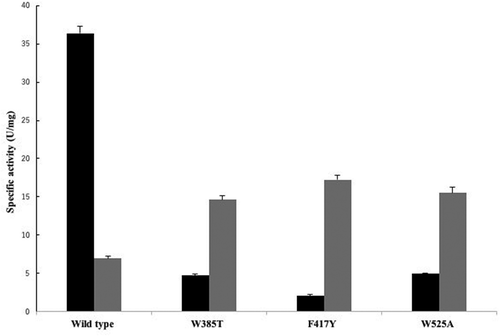
As for transpeptidation, the F417Y mutant exhibited the highest transpeptidase activity of 17 U/mg, 2.4 times higher than the wild-type PnGGT. The other mutants also had comparable transpeptidase activities. These results indicated that the aromatic side chains of Trp385, Phe417, and Trp525 were involved in the recognition of acceptor substrates. All the mutant enzymes prepared in this study showed a significant decrease in hydrolysis activity and an increase in transfer activity. In a preliminary experiment on theanine synthesis using one of Trp525 mutant enzymes, an excellent suppressive effect on hydrolysis of L-Gln to L-Glu has been observed. These suggest that sufficient improvement of PnGGT can be achieved by introducing mutation into these targeted aromatic amino acid residues. Therefore, further investigation is under progress to create mutant enzymes suitable for theanine synthesis based on the results. Furthermore, X-ray crystallographic analysis of PnGGT-L-Glu and/or inhibitor complex is now in progress. The detailed functions of these aromatic amino acid residues in catalysis will be investigated in the near future.
Author contribution
T.H., T.I., and M.W. designed research; T.H., M.I. and Y.S. performed research; T.H., T.I., M.I., Y.S., and M.W. analyzed data; T.H., T.I., and M.W. wrote the manuscript.
Acknowledgments
The synchrotron radiation experiments were performed at the BL-17A of the Photon Factory (Proposal No. 08G602). The authors would like to thank the staffs of the Photon Factory for the provision of synchrotron data collection facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Meister A, Tate SS, Griffith OW. γ-Glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253.
- Keillor JW, Castonguay R, Lherbet C. Gamma-glutamyl transpeptidase substrate specificity and catalytic mechanism. Methods Enzymol. 2005;401:449–467.
- Kadam PD. Rectocutaneous fistula with transmigration of the suture: a rare delayed complication of vault fixation with the sacrospinous ligament. Int Urogynecol J. 2016;27:155–157.
- Bindal S, Gupta R. L-theanine synthesis using gamma-glutamyl transpeptidase from Bacillus licheniformis ER-15. J Agric Food Chem. 2014;62:9151–9159.
- Chen X, Su L, Wu D, et al. Application of recombinant Bacillus subtilis γ-glutamyltranspeptidase to the production of l-theanine. Process Biochem. 2014;49:1429–1439.
- Imaoka M, Yano S, Okumura M, et al. Molecular cloning and characterization of gamma-glutamyltranspeptidase from Pseudomonas nitroreducens IFO12694. Biosci Biotechnol Biochem. 2010;74:1936–1939.
- Hu X, Legler PM, Khavrutskii I, et al. Probing the donor and acceptor substrate specificity of the γ-glutamyl transpeptidase. Biochemistry. 2012;51:1199–1212.
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326.
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, et al. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674.
- Langer G, Cohen SX, Lamzin VS, et al. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc. 2008;3:1171–1179.
- Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221.
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132.
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786.
- Toh H. Introduction of a distance cut-off into structural alignment by the double dynamic programming algorithm. Comput Appl Biosci. 1997;13:387–396.
- Okada T, Suzuki H, Wada K, et al. Crystal structures of gamma-glutamyltranspeptidase from Escherichia coli, a key enzyme in glutathione metabolism, and its reaction intermediate. Proc Natl Acad Sci U S A. 2006;103:6471–6476.
- Terzyan SS, Burgett AW, Heroux A, et al. Human gamma-glutamyl transpeptidase 1: structures of the free enzyme, inhibitor-bound tetrahedral transition states, and glutamate-bound enzyme reveal novel movement within the active site during catalysis. J Biol Chem. 2015;290:17576–17586.
- Ida T, Suzuki H, Fukuyama K, et al. Structure of Bacillus subtilis γ-glutamyltranspeptidase in complex with acivicin: diversity of the binding mode of a classical and electrophilic active-site-directed glutamate analogue. Acta Crystallogr D Biol Crystallogr. 2014;70:607–614.
- West MB, Chen Y, Wickham S, et al. Novel insights into eukaryotic gamma-glutamyltranspeptidase 1 from the crystal structure of the glutamate-bound human enzyme. J Biol Chem. 2013;288:31902–31913.
- Kamiyama A, Nakajima M, Han L, et al. Phosphonate-based irreverisble inhibitors of human γ-glutamyl transpeptidase (GGT). GGsTop is a non-toxic and highly selective inhibitor with critical electrostatic interaction with an active-site residue Lys562 for enhanced inhibitory activity. Bioorg Med Chem. 2016;24:5340–5352.
- Li KB. ClustalW-MPI: clustalW analysis using distributed and parallel computing. Bioinformatics. 2003;19:1585–1586.
- Suzuki H, Kumagai H, Tochikura T. ϒ-Glutamyltranspeptidase from Escherichia coli K-12: purification and properties. J Bacteriol. 1986;168:1325–1331.