ABSTRACT
This study investigated the effects of proanthocyanidins derived from Acacia (Acacia mearnsii) bark extract in healthy Japanese adult subjects experiencing uncomfortable skin symptoms. All subjects were randomly allocated into two groups (n = 33 each) using a computerized random-number generator. The subjects received either Acacia bark extract tablets or placebo for 8 weeks. Evaluations included water content in the stratum corneum, transepidermal water loss (TEWL), Skindex-16, dermatology life quality index (DLQI), visual analog scale for desire to scratch, and blood tests. At 4 weeks, the symptom/feeling score of DLQI, subjective symptoms related to uncomfortable skin, and the desire to scratch were significantly reduced in the intervention group than in the placebo group. At 8 weeks, the intervention group exhibited significantly lower TEWL on facial skin than that in the placebo group. In conclusion, the intake of Acacia bark extract tablets reduced TEWL and improved dry and uncomfortable skin.
Graphical Abstract
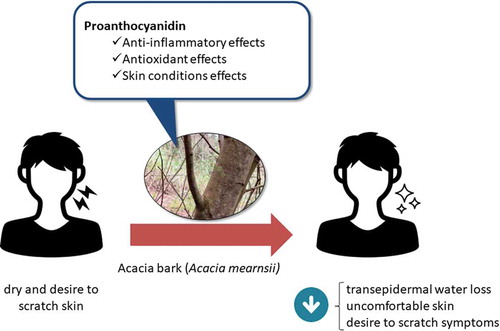
Proanthocyanidins-derived from Acacia bark reduced TEWL and alleviated the symptoms of uncomfortable, dry skin (in Japanese: “muzumuzu” feeling) in healthy Japanese adult subjects.
The presence and progression of dry skin reduces protection against external pathogens, stimuli, endotoxins, and allergens [Citation1–Citation3]. As dry skin worsens, redness, itching, and edema of the skin surface can occur, leading to elevated levels of immunoglobulin E (IgE) in the blood [Citation2,Citation3]. Reportedly, itching caused by external agents leads to secretions of histamine, acetylcholine, and/or proteinases, even in healthy individuals [Citation4]. Apart from external stimuli, the causes of dry skin include artificial sources. For example, use of domestic air conditioners lead to a dry environment, which affects the skin, mucosa of the eyes and nose, and potentially causes respiratory distress [Citation5]. Moreover, evidence has demonstrated that itching is exacerbated by stress and scratching behavior [Citation6], which may interfere with an individual’s sleep, work, or home routine and consequently reduce quality of life (QOL). It has been demonstrated that a decrease in QOL caused by chronic itching is equivalent to that caused by chronic pain [Citation7]. Therefore, therapeutic solutions are required to relieve skin discomfort.
Polyphenols present in food, such as catechins, have been shown to effectively improve skin conditions [Citation8–Citation10]. These polyphenols inhibit allergic reactions, such as the secretion of histamine [Citation11,Citation12] and expression of high–affinity IgE receptor [Citation13], thereby improving skin conditions. In addition to their anti-inflammatory properties, polyphenols reduce skin damage from ultraviolet irradiation through their antioxidant activity [Citation14,Citation15].
This study focused on the effectiveness of Acacia (Acacia mearnsii) bark extract, which contains an abundance of proanthocyanidins (a type of polyphenols), in the prevention of inflammation and allergic reactions.
The extract used in this study was obtained through hot water extraction from the bark of the Acacia tree. It comprises a mixture of monomer, dimer, trimer, and polymer compounds with structures based on flavan-3-ol, such as gallocatechin and robinetinidol [Citation16,Citation17]. Approximately 80% of these compounds are polyphenols [Citation18,Citation19]. A previous study demonstrated that Acacia bark extract possesses more powerful antioxidant properties than both vitamin C and catechin [Citation20]. In addition, intake of Acacia bark extract prevents a decrease in ceramide levels, which are important for maintaining moisture in the skin. In mice with atopic dermatitis, Acacia bark extract suppressed the mRNA expression of ceramidase, a ceramide-degrading enzyme [Citation21]. Thus, polyphenols in Acacia bark extract may prevent the desire to scratch, which is associated with atopic dermatitis, by preventing the development of dry skin. Although the ingestion of Acacia bark extract has been associated with a relief from the desire to scratch, previous studies have not provided sufficient evidence regarding the effectiveness of Acacia bark extract on humans with healthy skin. Therefore, the present study investigated the effect of proanthocyanidins derived from Acacia bark extract on the reduction of dryness in healthy Japanese adult subjects who were experiencing uncomfortable skin symptoms (e.g. a desire to scratch, which was not caused by an allergic reaction).
Methods
Study design
This was a randomized, double-blind, placebo-controlled, parallel-group study. The investigation was conducted at the Hiroo Dermatology Clinic & Mentors Inc. (Tokyo, Japan). The study protocol was approved by the ethical committee of the Takara Clinic, Medical Corporation Seishinkai (Tokyo, Japan), on July 10 2017 (approval number: 1707–1706-MZ01-03-TC). It was conducted in accordance with the principles of the Declaration of Helsinki (2013), the ethical guidelines for medical and health research involving human subjects in Japan, and broader medical ethics. Subjects were recruited from July 12 to September 9 2017, and the study was conducted from October 2 to December 16 2017. The protocol has been registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000028199). The trial data were reported according to the CONSORT 2010 statement.
Subjects
In this study, the inclusion criterion was healthy Japanese adult subjects experiencing desire to scratch, uncomfortable skin, and dry skin (in Japanese: “muzumuzu” feeling) on the face and hands. The exclusion criteria were as follows: (a) any medical history of a malignant tumor, heart failure, or myocardial infarction; (b) undergoing treatment for any of the following chronic diseases: arrhythmia, liver failure, kidney failure, cerebrovascular disease, rheumatism, diabetes mellitus, hyperlipidemia, hypertension, or any other chronic diseases; (c) daily intake of “Food for Specified Health Uses” and/or “Foods with Function Claims”; (d) diagnosis of restless legs syndrome; (e) diagnosis of atopic dermatitis; (f) daily use of skin care products with the exception of a cream/essence, skin pack, skin lotion, milky lotion, sunscreen, and all-in-one products; (g) regular skin care treatments (massages) or use of beauty devices (facial equipment); (h) severe sunburn within one month prior to signing the study’s informed consent form, or plans to expose the skin to sun during the intervention period; (i) regular use of medications, including herbal medicines and/or supplements; (j) allergy to medications and/or products associated with the study substances, or having allergic skin; (k) pregnancy, lactation, or expected/planned pregnancy during the study period; (l) participation in another clinical study within the last 3 months prior to signing the study’s informed consent form; and (m) anyone judged as ineligible to participate in this study by the principal physician.
All subjects were enrolled through the website https://www.go106.jp/ operated by ORTHOMEDICO Inc. (Tokyo, Japan). The study protocol was comprehensively explained to all subjects. Written informed consent was obtained from all subjects prior to their participation in the study at ORTHOMEDICO Inc. office. No subject was part of the sponsors or funding companies.
Determination of sample size
Evidence regarding the effect of the intake of tablets containing Acacia bark extract on the water content of the stratum corneum water in humans is currently limited. Thus, the difference between the intervention and placebo group was hypothetically large in this study. According to a study conducted by Cohen [Citation22], the effect-size (d) was 0.80, α was set to 0.05 and β to 0.20. Consequently, the calculated sample size per group was determined to be 33 subjects (factoring in a dropout rate over the study period). Therefore, it was estimated that a total of 66 subjects would be required for this randomized, double-blind, placebo-controlled, parallel-group study.
Enrollment, randomization, and blinding
Sixty-six healthy subjects experiencing uncomfortable skin and dry skin symptoms (in Japanese: “muzumuzu” feeling) were enrolled in this study. The principal physician declared the subjects as eligible to participate. Among subjects with thymus and activation-regulated chemokine (TARC) levels <450 pg/mL and non-specific IgE levels <170 IU/mL in prior to baseline intake, those with relatively high levels of transepidermal water loss (TEWL) were selected. An allocation controller not directly involved in this study, randomly assigned subjects to either the intervention group (n = 33) or placebo group (n = 33) using a computerized random-number generator. The allocation was based on the mean value and standard deviation of TEWL, gender, and age. Furthermore, none of the persons related to this study (i.e. subjects, physicians, or assessor of outcomes) were aware of the group assignments or were involved in the allocation. The allocation controller stored the allocation data (assignment sheet) in a safety cabinet until key-opening day.
Intervention
The test tablets were obtained from mimozax Co., Ltd. (Hiroshima, Japan), and the Acacia bark extract present in the test tablet was prepared as follows: Acacia bark was chopped and extracted using boiling water, and the extract was dried using a spray dryer. The extract is rich in proanthocyanidins, which are known as wattle tannins [Citation23]. The Folin-Ciocalteu method was used to determine the total proanthocyanidins content of the tablet [Citation24]. In addition, the tablet was analyzed using high-performance liquid chromatography, and robinetinidol-(4α,8)-catechin concentration was analyzed according to a previously described qualitative analysis method [Citation24]. All subjects were administered Acacia bark extract tablets or placebo (six tablets per day) to be taken with water in the morning or after breakfast for a period of 8 weeks. The test tablets contained 180 mg (catechin-equivalent) or 245 mg (A. mearnsii proanthocyanidin-equivalent) of proanthocyanidins derived from Acacia bark as functional substance per day. The placebo tablets did not contain proanthocyanidins derived from Acacia bark but were indistinguishable in appearance.
Examination items
The schedule of this study is shown in . Examinations were conducted at baseline and 4 and 8 weeks after initiating the intervention. Subjects were requested to avoid alcohol consumption and excessive exercise for 24 h prior to the examination. Furthermore, subjects were requested not to consume any food or drinks (including test food), except water, for 6 h prior to the examination.
Table 1. The schedule of enrollment, intervention, and assessments in this study.
Primary outcome: objective evaluations of skin items
Objective evaluation of skin was performed through assessment of the water content in the stratum corneum (arbitrary unit; a.u.) and TEWL (g/h/m2) on the cheek (the intersection of the vertical line from the outer canthus of the right eye and the horizontal line in the middle of the nose) and back of the hand (between the thumb and index finger). The measurement was performed in triplicate at each examination and the mean was calculated. Subjects were requested to wash their face and hands and remain in a room at a temperature of 23 ± 5ºC with a humidity of 50 ± 15% for at least 10 minutes prior to the evaluation. Following at least 10 minutes of acclimation, the water content in the stratum corneum and TEWL were evaluated using the Corneometer® CM825 (Courage +Khazaka Electronic GmbH, Cologne, Germany) and Tewameter® TM300 (Courage +Khazaka Electronic GmbH, Cologne, Germany), respectively.
Secondary outcomes
Secondary outcomes included gloss (arbitrary unit; a.u.) and viscoelasticity (arbitrary unit; a.u.) on the cheek and back of the hand. Gloss and viscoelasticity of the skin were evaluated using a Skin-Glossymeter GL200 (Courage +Khazaka Electronic GmbH, Cologne, Germany) and Cutometer® dual MPA580 (Courage +Khazaka Electronic GmbH, Cologne, Germany), respectively.
The skin conditions 1 week prior to the examination were assessed using the Japanese version of Skindex-16 [Citation25,Citation26] and Dermatology Life Quality Index (DLQI) [Citation27,Citation28]. In addition, subjective symptoms of the desire to scratch were evaluated using the visual analog scale (VAS), in which a 100-mm horizontal line was printed. The left end (0 mm) was defined as the best condition (no desire to scratch), whereas the right end (100 mm) was defined as the worst condition (very frequent desire to scratch). The subjects were requested to draw a vertical line across the horizontal line at the point that most accurately described their desire to scratch.
Furthermore, the levels of TARC and IgE in the blood – biomarkers of atopic dermatitis and allergic reactions – were determined. This measurement was performed by LSI Medience Corporation (Tokyo, Japan).
Safety evaluation
The physical examination conducted included height, weight, body mass index, body fat percentage, systolic and diastolic blood pressures, and pulse rate. Height was measured once at baseline to calculate the body mass index. Urine samples were collected to evaluate levels of protein, glucose, urobilinogen, bilirubin, ketone bodies, pH, and occult blood. These measurements were performed by LSI Medience Corporation. Hematological testing was conducted to assess the following: leukocyte count, erythrocyte count, hemoglobin, hematocrit value, platelet count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and differentiation of white blood cells (percentage of neutrophils, lymphocytes, monocytes, eosinophils, and basophils). Furthermore, biochemical testing evaluated the following: AST, ALT, γ-GT, ALP, LD, LAP, total bilirubin, direct bilirubin, indirect bilirubin, cholinesterase, ZTT, total protein, urea nitrogen, creatinine, uric acid, calcium, serum amylase, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, glycoalbumin, serum iron, sodium, potassium, chloride, inorganic phosphorus, glucose, and hemoglobin A1c. Hematological and biochemical testing was perfomed by LSI Medience Corporation. Subjects were requested to complete a medical questionnaire to determine their health status at each assessment point. Additionally, subjects reported medication dosage and any changes in their physical condition on a daily basis.
Statistical analysis
All statistical analyses were two-sided without adjustment for multiple comparisons, and the significance level was set at 1% and 5%. Data analysis was performed using the SPSS Version 23.0 (IBM Japan, Ltd., Tokyo, Japan) and Microsoft Excel 2013 (Microsoft Japan Co., Ltd., Tokyo, Japan) software. Moreover, subjects with an ingestion rate <90% and/or those who violated the compliance of the study were excluded from the analysis.
Demographic data of the subjects included gender, age, and physical characteristics. The data at baseline and degree of change were analyzed using Student’s t-test, and the data after intake was analyzed using analysis of covariance (ANCOVA). ANCOVA was applied for comparisons between groups, using the baseline values as covariates. Furthermore, urinalysis data was analyzed using the chi-square test at baseline and at 4 and 8 weeks after intake.
Results
Subjects
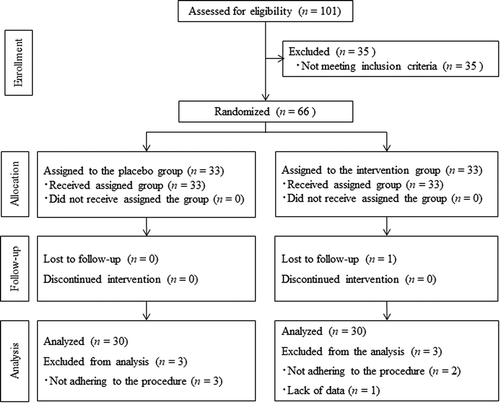
All subjects in this study had an ingestion rate ≥90%. However, six subjects total were excluded from the analysis; one and five subjects were excluded for missing data and violation of the compliance rules, respectively (). Therefore, this analysis was performed on the per-protocol dataset. The demographic characteristics of the subjects did not differ significantly between groups ().
Table 2. Subject background information.
Objective evaluations of skin items
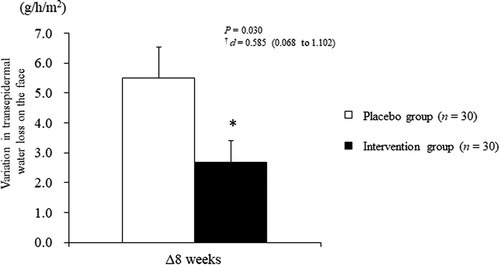
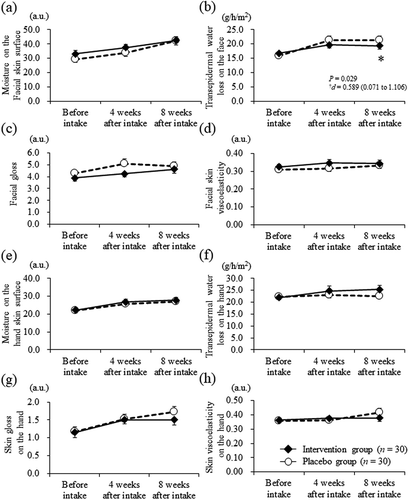
At baseline, there were no significant differences observed between the intervention and placebo groups. After 8 weeks of intake, the intervention group showed a significant reduction from 16.7 ± 4.9 to 19.4 ± 6.2 g/h/m2 in TEWL [the variation between baseline and 8 weeks of intake (Δ8 weeks); 2.7 ± 4.0 g/h/m2] compared with that reported in the placebo group (from 15.8 ± 4.6 to 21.3 ± 7.7 g/h/m2) (Δ8 weeks; 5.5 ± 5.7 g/h/m2; 8 weeks of intake, P = 0.029; Δ8 weeks, P = 0.030; ), ). For other assessments in the objective evaluation of skin items, there were no significant differences between groups throughout the intervention period (), –h)).
Figure 2. Objective evaluations on the skin items in the intervention (closed diamond, n = 30) and placebo (open circle, n = 30) groups at each assessment point. (a) moisture on the facial skin surface, (b) transepidermal water loss on the face, (c) facial gloss, (d) facial skin viscoelasticity, (e) moisture on the hand skin surface, (f) transepidermal water loss on the hand, (g) skin gloss on the hand, and (h) skin viscoelasticity on the hand. Bars display the standard error of the mean.
*: P < 0.05 vs. the Placebo group; †: Effect-size and 95% confidence interval
Skindex-16 score
There were no significant differences observed between the intervention and placebo groups at 4 and 8 weeks after intake ().
DLQI
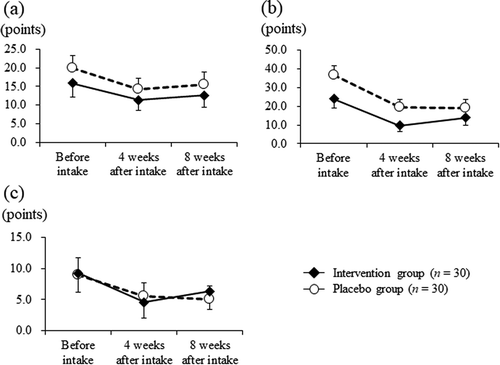
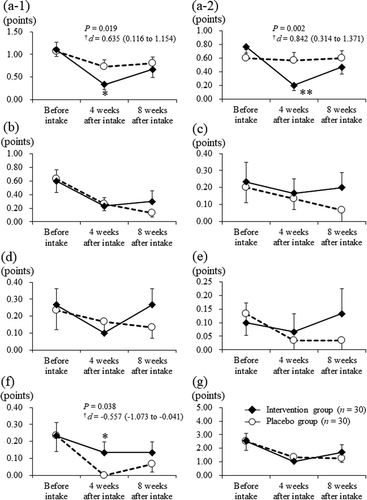
At baseline, there were no significant differences observed between the intervention and placebo groups. Moreover, at 4 weeks after intake, the intervention group showed significantly reduced subjective symptoms according to the symptom/feeling score (from 1.10 ± 0.80 to 0.33 ± 0.61 points) compared with the placebo group (from 1.07 ± 1.08 to 0.73 ± 0.83 points; P = 0.019, -)). Furthermore, the intervention group showed significantly reduced subjective symptoms based on the symptom/feeling score in response to the question: “Over the last week, how itchy, sore, painful, or stinging has your skin been?” (from 0.77 ± 0.50 to 0.20 ± 0.41 points) compared with the placebo group (from 0.60 ± 0.67 to 0.57 ± 0.63 points; P = 0.002, -)). At 4 weeks after intake, the intervention group presented a significantly higher treatment subscale score of the DLQI (from 0.23 ± 0.43 to 0.13 ± 0.35 points) than that of the placebo group (from 0.23 ± 0.50 to 0.00 ± 0.00 points; P = 0.038; )). There were no significant differences for other assessments in the DLQI between groups throughout the intervention period (), ), ))
Figure 5. Scores of DLQI in the intervention (closed diamond, n = 30) and placebo (open circle, n = 30) groups at each assessment point. (a-1) symptom/feeling, (a-2) question 1 “Over the last week, how itchy, sore, painful or stinging has your skin been?” (the subscale scores of symptom/feeling), (b) daily activities, (c) leisure, (d) work/school, (e) personal relationships, (f) treatment, and (g) total score. Bars display the standard error of the mean.
*: P < 0.05, **: P < 0.01 vs. the Placebo group; †: Effect-size and 95% confidence interval. DLQI, Dermatology Life Quality Index
Desire to scratch and blood tests
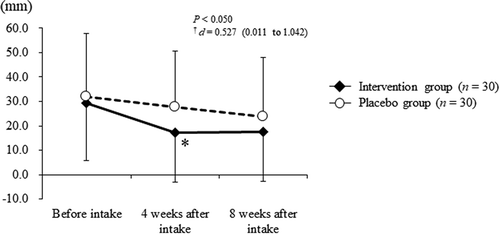
At 4 weeks after intake, the intervention group exhibited significantly reduced subjective symptoms with regard to the desire to scratch (from 29.5 ± 23.7 to 17.1 ± 20.2 mm) compared with those reported in the placebo group (from 31.8 ± 26.2 to 27.7 ± 22.8 mm; P < 0.050, ). No significant differences were observed between the intervention and placebo groups at baseline and at 8 weeks after intake. Furthermore, the levels of TARC and IgE remained within the normal range (TARC: <450 pg/mL; IgE level: ≤170 IU/mL, data not shown) and did not change significantly between the groups.
Figure 6. VAS results of desire to scratch in the intervention (closed diamond, n = 30) and placebo (open circle, n = 30) groups at each assessment point. Bars display the standard error of the mean.
*: P < 0.05 vs. the Placebo group; †: Effect-size and 95% confidence interval. VAS, Visual Analogue Scale
Safety evaluation
Safety evaluation demonstrated no significant differences in physical examination and urinalysis. Although the intervention group (89.1 ± 33.8 μg/dL) showed a significantly lower serum iron level at 8 weeks than that in the placebo group (109.5 ± 44.7 μg/dL, P = 0.009), it was within the reference range (–).
Table 3. The results of the safety evaluation (physical examination).
Table 4. The results of the safety evaluation (urinalysis).
Table 5. The results of the safety evaluation (blood tests).
Discussion
The present study showed that an eight-week intake of tablets containing proanthocyanidins derived from Acacia (A. mearnsii) bark extract (Acacia bark extract) significantly reduced TEWL on facial skin. Although there were no significant differences reported in the water content of the stratum corneum between the two groups, the mean score was increased in the intervention group during the study (data not shown). The reduction of TEWL is one of the most effective approaches in retaining the water content of the stratum corneum [Citation29] and indicates the prevention of dry skin. Furthermore, strictinin, a type of tea polyphenol, inhibits the production of specific IgE [Citation30]. The present study showed that the ingestion of the proanthocyanidins present in Acacia bark extract may lead to a decrease in the production of IgE. However, there were no significant changes observed in the production of non-specific IgE in healthy Japanese adult subjects in this study. Hence, the effect of Acacia bark extract on IgE may be minimal. Guo et al. [Citation31] reported that in hairless mice, an increase in the levels of tumor necrosis factor (TNF)-α and inflammation induced by human sebum resulted in a significant increase in TEWL. However, levels of IgE in the serum remained the same. These results suggested that inflammation induced by the presence of excess sebum is more likely an irritant than an indicator for allergic contact dermatitis. Consequently, the intake of Acacia bark extract tablets significantly decreased TEWL in humans without atopic dermatitis through a mechanism independent of IgE.
According to a study conducted by Ikarashi et al. [Citation32], the intake of Acacia bark extract suppressed the expression of TNF-α in white adipose tissue of obese diabetic KKAy mice. Furthermore, some food ingredients other than Acacia bark extract have been shown to suppress the expression of TNF-α, leading to the alleviation of inflammation and reduction of TEWL. Reportedly, the intake of Korean red ginseng (KRG) significantly interrupted any increase in TEWL and significantly suppressed the expression of TNF-α in NC/Nga mice treated with 2,4,6-trinitrochlorobenzene [Citation33]. In addition, a study conducted by Lee and Cho [Citation34] demonstrated that KRG possesses anti-inflammatory properties, which suppress the mRNA levels of TNF-α. Furthermore, the intake of KRG protected against the onset and progression of dry skin through TEWL reduction. In another study, the intake of nutmeg extract suppressed the expression of TNF-α mRNA and reduced TEWL in mice with atopic dermatitis-like skin lesions induced through American house dust mite extract [Citation35]. Kim B and Kim HS [Citation36] reported that a novel peptide B (peptide sequences; RRFSLLRY), a protease-activated receptor 2 antagonist, reduced the expression of inflammatory cytokines such as TNF-α and interleukin-6 and decreased TEWL in hairless mice. In a clinical study conducted by Zhao et al. [Citation37], an ointment containing Portulaca oleracea extract combined with calcipotriol suppressed the expression of TNF-α and reduced TEWL in patients with psoriasis. The findings of these previous studies suggest that the mechanism for the reduction of TEWL and inflammation using proanthocyanidins derived from Acacia bark involves the suppression of TNF-α.
Furthermore, a previous study demonstrated that the intake of proanthocyanidins derived from Acacia (A. mearnsii) bark increased the moisture content of the skin and decreased TEWL in mice with atopic dermatitis. The intake of proanthocyanidins derived from Acacia bark suppressed the mRNA expression of ceramidase (the enzyme which degrades ceramide), preventing a decrease in the levels of ceramide [Citation21]. Ceramide is present in the stratum corneum and acts as a defense mechanism by retaining moisture between cells and preventing excess water permeation [Citation38,Citation39]. In this study, Acacia bark extract may suppress the expression of ceramidase even in skin without atopic dermatitis, resulting in decreased TEWL.
The intake of Acacia bark extract tablets improved QOL related to skin conditions, as assessed by the symptom/feeling score and question one of the DLQI: “over the last week, how itchy, sore, painful or stingy has your skin been?” Although the treatment score in the intervention group was higher than that recorded in the placebo group, this score was similar to that reported by healthy subjects in a previous study [Citation27]; therefore, there was no clinical meaning. VAS assessment showed that Acacia bark extract tablets improved the desire to scratch at 4 weeks after intake. The desire to scratch is associated with the production of nitric oxide (NO) [Citation40]. A previous study demonstrated that Acacia bark extract possesses a five- to seven-fold higher scavenging activity for superoxides than that possessed by vitamin C and catechins, indicating the powerful antioxidant properties of Acacia bark extract [Citation20]. Another previous study demonstrated that green tea possesses free radical-scavenging activity based on the following chemical compounds: (−)-epigallocatechin 3-O-gallate, (−)-gallocatechin 3-O-gallate, (−)-epicatechin 3-O-gallate, (−)-epigallocatechin, (+)-gallocatechin, (−)-epicatechin, and (+)-catechin [Citation41]. These compounds have demonstrated direct scavenging activity against NO and superoxide and have a similar structure to that of proanthocyanidins derived from A. mearnsii bark, which are known for their powerful antioxidant properties [Citation20]. Andoh and Kuraishi [Citation40] suggested that NO induces responses related to the desire to scratch and is a key antipruritic agent. Furthermore, polyphenols reduce skin damage from solar ultraviolet radiation through their antioxidant and anti-inflammatory activities [Citation14,Citation15]. These findings suggested that the intake of Acacia bark extract suppressed the desire to scratch by scavenging NO and alleviating skin damage caused by solar ultraviolet radiation, resulting in improved QOL related to skin conditions.
Based on the present results, the intake of proanthocyanidins derived from Acacia bark reduced TEWL significantly on the facial skin of healthy Japanese adult subjects experiencing uncomfortable skin symptoms through a mechanism independent of IgE. The following potential mechanism of TEWL regulation is proposed. Proanthocyanidins derived from Acacia bark may reduce TEWL and inflammation by suppressing TNF-α. Furthermore, proanthocyanidins derived from Acacia bark retain the levels of ceramide and possibly reduce TEWL on skin without atopic dermatitis by suppressing the expression of ceramidase. In addition, ingestion of proanthocyanidins derived from Acacia bark reduces skin damage caused by dry skin/desire to scratch and inflammation through the antioxidant and anti-inflammatory properties of polyphenols. Thus, the uncomfortable symptoms of dry skin were alleviated, resulting in improved QOL related to skin conditions.
Despite the significantly lower serum iron levels in the intervention group at 8 weeks, serum iron level was included as the reference value. A different study using the same tablet for 4 weeks but with a five-fold increase in dosage reported no effects in serum iron levels [Citation42]. Therefore, the significant difference in serum iron level observed in this study has no clinical relevance.
Our study design has several limitations. First, the sample size should be increased to obtain accurate results. Second, the face and hands were exposed to external factors; therefore, it is also necessary to verify the effect of Acacia bark extract on these exposed skin parts. Finally, further studies concerning these versatile aspects should investigate the fundamental underlying mechanism involved in reducing uncomfortable skin symptoms using Acacia bark extract to better understand our study. However, this study demonstrated that Acacia bark extract tablets containing proanthocyanidins derived from A. mearnsii significantly reduced TEWL from the facial skin of healthy Japanese adult subjects experiencing uncomfortable skin symptoms.
Conclusions
The eight-week intake of tablets containing proanthocyanidins derived from Acacia bark reduced TEWL and alleviated the symptoms of uncomfortable, dry skin (in Japanese: “muzumuzu” feeling) in healthy Japanese adult subjects. Furthermore, the intake of Acacia bark extract tablets was found to be completely safe under the conditions of the study.
Author Contribution
T. H., S. Y., and N. S. designed the study. S. O. was involved the study conceptions and managing the research expenses. Furthermore, A. B. drafted the manuscript. T. I. (MD) was the principal investigator and managed the physical conditions of all subject at his clinic. All authors reviewed and approved the final manuscript.
Acknowledgments
The authors would like to thank all the subjects and staff who participated in this study. Special appreciation is extended to Kenichi Kakino of the Evaluation Center of Health And Nutrition Inc. for his contribution.
Disclosure statement
This sponsor of this study, mimozax Co., Ltd. entrusted ORTHOMEDICO Inc., with conducting the study. S. O belongs to mimozax Co., Ltd. and T. H., S. Y., N. S., and A. B. all belong to ORTHOMEDICO Inc. T. I. (MD), belongs to Hiroo Dermatology Clinic & Mentors Inc., and is the principal investigator.
Additional information
Funding
References
- Fallon PG, Sasaki T, Sandilands A, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608.
- Moniaga CS, Egawa G, Kawasaki H, et al. Flaky tail mouse denotes human atopic dermatitis in the steady state and by topical application with Dermatophagoides pteronyssinus extract. Am J Pathol. 2010;176:2385–2393.
- Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485–493.e1.
- Hassan I, Haji ML. Understanding itch: an update on mediators and mechanisms of pruritus. Indian J Dermatol Venereol Leprol. 2014;80:106–114.
- Cao B, Shang Q, Dai Z, et al. The impact of air-conditioning usage on sick building syndrome during summer in China. Indoor Built Environ. 2013;22:490–497.
- Verhoeven EWM, de Klerk S, Kraaimaat FW, et al. Biopsychosocial mechanisms of chronic itch in patients with skin diseases: a review. Acta Derm Venereol. 2008;88:211–218.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life. Arch Dermatol. 2011;147:1153–1156.
- Hisano M, Yamaguchi K, Inoue Y, et al. Inhibitory effect of catechin against the superantigen Staphylococcal Enterotoxin B (SEB). Arch Dermatol Res. 2003;295:183–189.
- Noh SU, Cho EA, Kim HO, et al. Epigallocatechin-3-gallate improves Dermatophagoides pteronissinus extract-induced atopic dermatitis-like skin lesions in NC/Nga mice by suppressing macrophage migration inhibitory factor. Int Immunopharmacol. 2008;8:1172–1182.
- Kojima T, Akiyama H, Sasai M, et al. Anti-allergic effect of apple polyphenol on patients with atopic dermatitis: a pilot study. Allergol Int. 2000;49:69–73.
- Kanda T, Akiyama H, Yanagida A, et al. Inhibitory effects of apple polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci Biotechnol Biochem. 1998;62:1284–1289.
- Maeda-Yamamoto M, Inagaki N, Kitaura J, et al. O-Methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J Immunol. 2004;172:4486–4492.
- Fujimura Y, Tachibana H, Yamada K. Lipid raft-associated catechin suppresses the FcϵRI expression by inhibiting phosphorylation of the extracellular signal-regulated kinase1/2. FEBS Lett. 2004;556:204–210.
- Katiyar SK, Afaq F, Perez A, et al. Green tea polyphenol (–)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83.
- Roux D, Maihs E. Condensed tannins. 3. Isolation and estimation of (-)-7:3ʹ:4ʹ:5ʹ-tetrahydroxyflavan-3-ol, (+)-catechin and (+)-gallocatechin from black-wattle-bark extract. Biochem J. 1960;74:44–49.
- Botha JJ, Ferreira D, Roux DG. Condensed tannins: direct synthesis, structure, and absolute configuration of four biflavonoids from black-wattle bark (‘Mimosa’) extract. J Chem Soc Chem Commun. 1978;16:700–702.
- Zheng G, Lin Y, Yazaki Y. Comparing molecular size distribution of tannin extracts from Acacia mearnsii bark from different countries. Holzforschung. 1988;42:407–408.
- Yazaki Y, Zheng G, Searle S. Extractives yields and proflavonoid contents of Acacia mearnsii barks in Australia. Aust For. 1990;53:148–153.
- Yazaki Y. Utilization of flavonoid compounds from bark and wood: a review. Nat Prod Commun. 2015;10:513–520.
- Ikarashi N, Sato W, Toda T, et al. Inhibitory effect of polyphenol-rich fraction from the bark of Acacia mearnsii on itching associated with allergic dermatitis. Evidence-based complement. Altern Med. 2012;2012:120389.
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159.
- Kusano R, Ogawa S, Matsuo Y, et al. α-amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J Nat Prod. 2011;74:119–128.
- Ogawa S, Matsuo Y, Tanaka T, et al. Utilization of flavonoid compounds from bark and wood. III. Application in health foods. Molecules. 2018;23:1860.
- Chren -M-M, Lasek RJ, Sahay AP, et al. Measurement properties of skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105–110.
- Higaki Y, Kawamoto K, Kamo T, et al. The Japanese version of skindex-16: a brief quality-of-life measure for patients with skin diseases. J Dermatol. 2002;29:693–698.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216.
- Takahashi N, Suzukamo Y, Nakamura M, et al. Japanese version of the dermatology life quality index: validity and reliability in patients with acne. Health Qual Life Outcomes. 2006;4:46.
- Shishido Y, Torii K, Fujiwara T, et al. Studies on moisturizing effect of bath preparations. J Japan Soc Balneol Climatol Phys Med. 1989;52:97–103.
- Tachibana H, Kubo T, Miyase T, et al. Strictinin inhibits interleukin 4-induced STAT6 activation and antigen-specific IgE production.In: The Organizing Committee of 2001 International Conference on O-cha (tea) Culture and Science, editor. Proceedings of the 2001 International Conference on O-cha (tea) Culture and Science; 2001 Oct 5–8; Shizuoka, Japan. Shizuoka (Japan): O-cha Promotion Office, Shizuoka Prefectural Government; 2001. p. 234–237.
- Guo J-W, Lin T-K, Wu C-H, et al. Human sebum extract induces barrier disruption and cytokine expression in murine epidermis. J Dermatol Sci. 2015;78:34–43.
- Ikarashi N, Toda T, Okaniwa T, et al. Anti-obesity and anti-diabetic effects of acacia polyphenol in obese diabetic KKAy Mice fed high-fat diet. Evidence-based complement. Altern Med. 2011;2011:952031.
- Cho E, Cho SH. Effects of Korean red ginseng extract on the prevention of atopic dermatitis and its mechanism on early lesions in a murine model. J Ethnopharmacol. 2013;145:294–302.
- Lee HJ, Cho SH. Therapeutic effects of Korean Red Ginseng extract in a murine model of atopic dermatitis: anti-pruritic and anti-inflammatory mechanism. J Korean Med Sci. 2017;32:679.
- Chung H-C, Kim M-S, Mun S-Y, et al. Effect of oral administration of nutmeg extract on american house dust mite (Dermatophagoides farinae) extract-induced atopic dermatitis-like skin lesions in NC/Nga mice. Food Sci Biotechnol. 2012;21:559–564.
- Kim B, Kim H-S. Novel peptide inhibits inflammation by suppressing of protease activated receptor-2. Eur J Pharmacol. 2018;832:25–32.
- Zhao H, Li S, Luo F, et al. Portulaca oleracea L. aids calcipotriol in reversing keratinocyte differentiation and skin barrier dysfunction in psoriasis through inhibition of the nuclear factor κB signaling pathway. Exp Ther Med. 2015;9:303–310.
- Uchida Y, Hara M, Nishio H, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–2082.
- Imokawa G, Akasaki S, Kawamata A, et al. Water-retaining function in the stratum corneum and its recovery properties by synthetic pseudoceramides. J Soc Cosmet Chem. 1989;40:273–285.
- Andoh T, Kuraishi Y. Nitric oxide enhances substance P-induced itch-associated responses in mice. Br J Pharmacol. 2003;138:202–208.
- Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol. 2002;40:1745–1750.
- Ogawa S, Miura N. Safety evaluation study on overdose of acacia bark extract (Acacia Polyphenol) in humans ―A randomized, double‒blind, placebo‒controlled, parallel study―. Jpn Pharmacol Ther. 2017;45:1927–1934.