ABSTRACT
We previously demonstrated that alterations in sugar partitioning affect the expression of genes involved in hormone biosynthesis and responses, including BRANCHED1 (BRC1), resulting in enhanced shoot branching in transgenic Arabidopsis plants overexpressing cyanobacterial fructose-1,6-bisphosphatase-II in the cytosol (AcF). The exogenous treatment of wild-type Arabidopsis plants with sugars showed the same transcript characteristics, indicating that sugars act as a signal for branching. We also found that the reductions induced in BRC1 expression levels in wild-type plants by the sugar treatments were suppressed in the knockout mutant of sugar transporter 1 (stp1-1). Intracellular sugar contents were similar in stp1-1 and wild-type plants following the sugar treatments, suggesting that STP1 acts as a factor for the regulation of shoot branching depending on extracellular sugar contents.
Abbreviations: BRC1: BRABCHED1; FBP/SBPase: fructose-1,6-/sedoheptulose-1,7-bisphosphatase; Glc: glucose; HXK: hexokinase; SnRK1.1/AKIN10: SNF1-RELATED PROTEIN KINASE 1.1; Suc: sucrose; SnRK1: sucrose non-fermenting 1-related protein kinase; STP: sugar transporter protein
Graphical Abstract
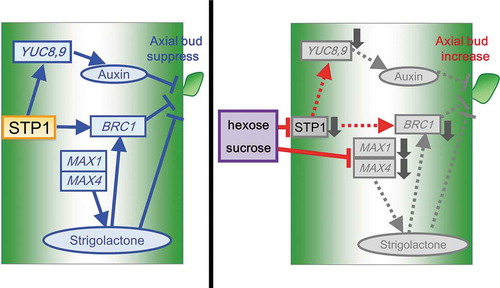
Sugar Transporter Protein 1 (STP1) contributes to regulation of shoot branching.
Shoot branching is a key factor influencing plant morphogenesis and affects plant productivity. The regulation of shoot branching is coordinately controlled by endogenous and environmental cues. The plant hormones, cytokinins and auxin, have been implicated in the modulation of plant morphogenesis. Auxin suppresses, whereas cytokinins promote the formation of lateral shoots [Citation1,Citation2]. Strigolactones have been identified as branching inhibitors [Citation3], and the activity of branching inhibition was also shown to be antagonistically regulated by auxin and cytokinins [Citation4]. Arabidopsis BRANCHED 1 (BRC1), which belongs to the TCP transcription family, functions as a negative regulator of shoot branching [Citation5,Citation6]. The number of axial buds increased when the expression level of BRC1 decreased [Citation5–Citation7]. In addition, the knockout mutant of BRC1 (brc1–1) exhibited excessive branch outgrowth [Citation5,Citation6]. Braun et al. (2012) proposed that BRC1 integrates the transcript levels of the strigolactones and cytokinins pathways to regulate shoot branching [Citation8].
Metabolism needs to be tightly coupled to regulatory mechanisms that control growth and development [Citation9]. Carbon metabolites are essential to the fundamental processes required for plant growth. Plants utilize the energy from light to convert carbon dioxide into carbohydrates. Among carbohydrates, glucose (Glc) and sucrose (Suc) act as signaling molecules to regulate cellular activity at multiple levels from transcription to post-translation [Citation9–Citation12]. Glc and Suc signaling pathways are related to plant morphogenesis. Mutants lacking and overexpressing genes involved in sugar sensing and signaling showed morphological abnormalities during seed germination and seedling growth in Arabidopsis [Citation9,Citation10,Citation13–Citation15]. Regarding sugar signaling, hexokinase (HXK) is recognized as an important Glc sensor that mediates sugar responses [Citation13,Citation14,Citation16,Citation17]. The gene knockout mutant of HXK1 (gin2) survived under a high concentration of Glc [Citation13]. Sucrose non-fermenting 1-related protein kinase (SnRK1) functions in sugar signaling when sugar levels are limited in plant cells [Citation18]. The overexpression of SNF1-RELATED PROTEIN KINASE 1.1 (SnRK1.1/AKIN10) prevented nutrient-deprivation-induced senescence in Arabidopsis [Citation19]. These sugar sensors have been suggested to integrate sugar signaling and plant hormones for the modulation of plant growth [Citation9]).
We previously reported that transgenic plants overexpressing the fructose-1,6-/sedoheptulose-1,7-bisphosphatase (FBP/SBPase) of Synechococcus elongatus PCC7942 in their chloroplasts had a higher CO2 assimilation rate and the greater accumulation of carbohydrates, resulting in an increased biomass production [Citation20–Citation23]. Furthermore, transgenic Arabidopsis plants overexpressing cyanobacterial fructose-1,6-bisphosphatase-II in the cytosol (AcF) had elevated Suc and hexose levels and an increased number of lateral shoots [Citation7]. In AcF plants, the transcript levels of MAX1 and MAX4, involved in SL metabolism, and YUC8 and YUC9, involved in auxin metabolism, were suppressed. In addition, the transcript levels of BRC1 were suppressed in AcF plants. These findings suggested that alterations in sugar levels affect the hormone balance in the regulation of shoot branching. Shoot branching via auxin, strigolactones, and BRC1 is potentially impacted by sugars [Citation7,Citation24–Citation26]. However, the mechanisms by which plants recognize various sugar levels and regulate growth and development by sugars via hormone regulation have not yet been elucidated. Therefore, the sugar signaling pathway that integrates the regulation of gene expression for shoot branching needs to be identified.
In the present study, we found that exogenous treatments with sugars affect the transcript levels of genes involved in the regulation of shoot branching in the basal tissues of Arabidopsis plants grown under hydroponic cultures. Among known sugar signaling factors, the present results indicate that sugar transporter 1 (STP1) contributes to regulation of shoot branching and carbohydrate metabolism.
Materials and methods
Plant materials and growth conditions
The Arabidopsis T-DNA insertion lines of STP1 (stp1-1; SALK_048848) [Citation27], HXK1 (hxk1-1; SALK_015782) [Citation28], cell wall invertase 1 (CWINV1) (cwinv-1-1; SALK_119499) [Citation29]), and BRC1 (brc1-1; SALK_091920C) were obtained from the Arabidopsis Biological Resource Center.
Arabidopsis (Arabidopsis thaliana) ecotype Colombia-0 seeds were sown on an Araponics® seed folder (Araponics SA, Liège, Belgium) filled with Grodan stone wool (Grodan® Inc., Roermond, Netherlands) under hydroponic conditions or sown on a 1:1 perlite/soil mix and stratified at 4°C for 2 days.
Plants were grown in non-sterile hydroponic medium in three independent growth chambers under a 12-h light/dark (23°C) cycle with a light intensity of 100–150 µmol photons m−2 s−1, 400 ppm CO2, and 60% relative humidity. The hydroponic nutrient solution, which was modified from the method described by Reda [Citation30], contained 0.5 mM Ca(NO3)2, 1.5 mM MgSO4, 2.5 mM KNO3, 0.13 mM NH4H2PO4, 0.023 mM 2NA(EDTA · 2Na), 1.25 mM K2SO4, 1.8 mM Na H2PO4, 0.03 mM H3BO4, 0.21 µM CuSO4, 2.03 µM MnCl2, 0.139 µM MoO3, 0.314 µM ZnSO4, 0.086 µM CoCl2, and 0.0224 mM FeSO4, was oxygenated with an air pump (non-noise S100, JAPAN PET DESIGN CO., LTD., Tokyo, Japan), and was renewed every week to avoid nutrient deficiencies.
Sugar treatments
Regarding sugar treatments, 5-week-old plants were transferred to hydroponic medium containing various sugars after 6 h in the light phase. Plants were treated with 50 mM Glc, 50 mM Suc, a mixture of 50 mM each of Glc and Suc, 50 mM sorbitol, and without sugar according to Sarianen et al. [Citation31] with some modifications. All plants were treated for 24 h to avoid secondary effects by a futile cycle or temporary Glc or Suc signaling. After these treatments, rosette leaves and basal tissues were cut, frozen in liquid nitrogen, and stored at −80°C for later analyses.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from the basal tissues of 5-week-old plants immediately after bolting treated with or without sugars [Citation7]. Frozen basal tissues were ground in liquid nitrogen and total RNA was extracted with ISOSPIN plant RNA (NIPPON GENE CO., LTD., Tokyo, Japan) according to the manufacturer’s instructions.
The primer sequences as well as accession numbers of genes are shown in Supplemental Table S1. Gene-specific primers were selected such that the resulting PCR products had an approximately equal size of 100 bp. qRT-PCR experiments were performed with a LightCycler 96 System (Roche, Basel, Switzerland) using FastStart Universal SYBR Green Master (ROX) (Roche, Basel, Switzerland) according to Otori et al. [Citation7]. Quantities were assessed from a standard curve and normalized to the amount of actin DNA. qRT-PCR experiments were repeated three times for cDNA prepared from three batches of plants.
Measurement of carbohydrate contents
Frozen rosette leaves (80–100 mg FW) were immediately placed into liquid N2, ground using a pestle and mortar with 1 mL of 6% perchloric acid, and centrifuged at 15,000 × g for 5 min. Pellets were used for starch measurements and supernatants were enzymatically examined for sugars (hexose and Suc), as described by Galtier et al. [Citation32].
Generation of transgenic plants overexpressing STP1
In order to generate Arabidopsis plants overexpressing STP1, the cDNA encoding Arabidopsis STP1 was fused with CaMV35S promoter in pRI101-AN (Supplemental Fig. S1). The resultant plasmid was then introduced into wild-type Arabidopsis by Agrobacterium tumefaciens (C58)-mediated transformation utilizing the floral dip method [Citation33]. Transgenic plants were selected on MS medium plate containing 0.8% (w/v) agar and 50 μg mL−1 kanamycin. Homozygous T3 generation plants harboring the transgene were used in further analyses.
Statistical analysis
Statistical analyses were performed using Excel (Microsoft) or EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [Citation34]. Two-tailed unpaired t-tests were performed to measure differences from at least three independent biological replicates. More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Results
Effects of exogenous treatments with sugars on transcript levels of genes involved in shoot blanching in wild-type plants
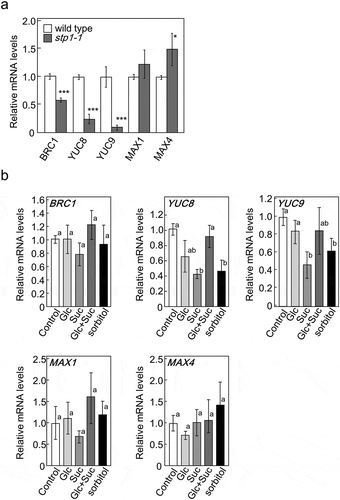
We previously reported that AcF plants expressing FBPase-II in the cytosol had higher sugar contents and, thus, more shoots branching than wild-type plants. The transcript levels of genes involved in shoot branching and plant hormone biosynthesis were reduced in AcF plants [Citation7]. To clarify the relationship between shoot branching and sugar signaling, 35-day-old Arabidopsis plants grown in hydroponic medium were treated with Glc and/or Suc. We examined the expression of genes involved in hormone biosynthesis and its signaling using qRT-PCR. After being treated with sugars, the transcript levels of BRC1, YUC8, YUC9, MAX1, and MAX4 were significantly lower than those in control plants (Ctrl) (). The transcript levels of YUC8 and YUC9, related to auxin biosynthesis, were reduced more by the Glu + Suc treatment than that with Glc or Suc alone. These results suggest that Glc and Suc act as a signal and regulate the expression of genes involved in plant hormone metabolism and responses.
Figure 1. Expression of BRC1 and genes involved in biosynthesis and its signaling of auxin or strigolactones in 5-week-old basal tissues of wild-type plants treated with various sugars. The transcript levels of these genes were measured by qRT-PCR. ActinII was used as the internal reference gene. Error bars indicate SE (n= 6–9). Bars with the same letter are not significantly different from each other (ANOVA, Tukey’s HSD test).
Identification of a sugar signaling factor involved in shoot branching
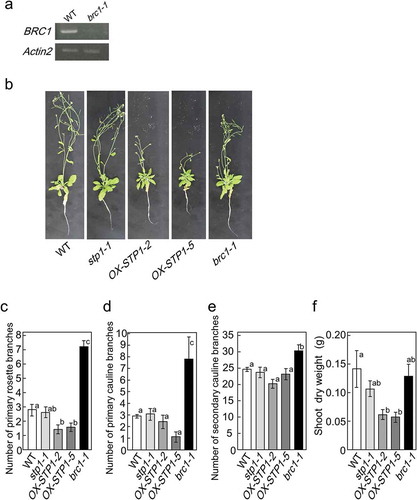
Several sugar signaling factors have been identified to date [Citation9,Citation16,Citation35–Citation37]. In order to clarify the contribution of these sugar signaling factors to the regulation of shoot branching, we initially isolated their gene knockout mutants. We obtained the T-DNA insertion lines of hexokinase 1 (hxk1-1: SALK_015782), cell wall invertase 1 (cwinv1-1: SALK_119499), and sugar transporter 1 (stp1-1: SALK_048848). The sites of T-DNA insertion for these mutants were confirmed by the sequencing of genomic DNA extracted from the mutants (data not shown). A semi-quantitative RT-PCR analysis revealed that these lines were null mutants ()).
Figure 2. Identification of a sugar signaling factor involved in BRC1 regulation. The steady-state amount of HXK1 mRNA in the basal tissues of hxk1-1 mutants, CWINV1 mRNA in cwinv1-1, and STP1 mRNA in stp1-1 mutants relative to those in wild-type plants by RT-PCR (a). Similar results were obtained in three independent experiments. Transcript levels of BRC1 in basal tissues of 5-week-old wild-type and mutant plants treated for 24 h with hydroponic medium containing a mixture of glucose and sucrose or no sugar (b). Error bars indicate SE (n = 3 or 4). Asterisks indicate genotypes that are significantly different from each control plant (ANOVA, Dunnett’s test, **P < 0.01, ***P< 0.001).
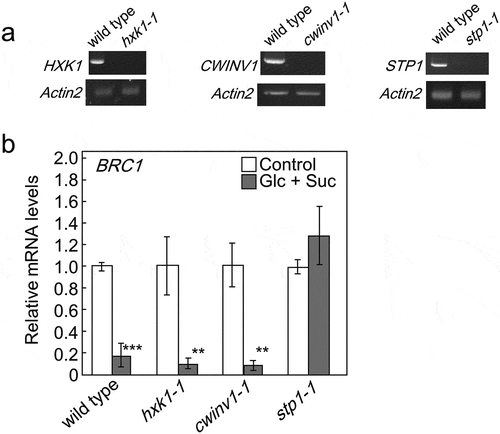
We then examined the transcript levels of BRC1 in mutants when plants were treated with Glc and Suc. The supplementation of sugars reduced the transcript levels of BRC1 in hxk1-1 and cwinv1-1 mutants, similar to those in wild-type plants. However, the transcript levels of BRC1 were not altered in stp1-1 mutants by the treatments with Glc and Suc ()), suggesting that STP1 acts as a factor that regulates the expression of BRC1.
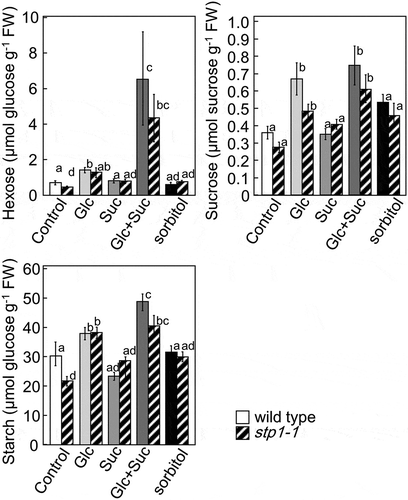
Effects of exogenous treatments with sugars on carbohydrate levels in wild-type and stp1-1 plants
STP1 has been identified as a hexose transporter that is located in the plasma membrane of Arabidopsis [Citation38]. In order to confirm whether the suppression of BRC1 in stp1-1 was due to the decreased incorporation of hexose or lack of other functions of STP1, we measured carbohydrate levels in the leaves of wild-type and stp1-1 plants (). The treatment with Glu increased the amounts of hexose, Suc, and starch in wild-type and stp1-1 plants. Furthermore, no significant differences were observed in the amounts of hexose, Suc, and starch between wild-type and stp1-1 plants. These results suggested that the lack of STP1 did not affect the hexose transport capacity in stp1-1 plants. On the other hand, the treatment with Suc did not affect the amount of carbohydrates, while the treatment with Suc and Glu markedly increased hexose levels in wild-type and stp1-1 plants. These results suggested that the treatment with Suc promoted the transportation of Glc into cells.
Effects of exogenous treatments with sugars on transcript levels of genes involved in the biosynthesis and signal transduction of plant hormones in stp1-1 plants
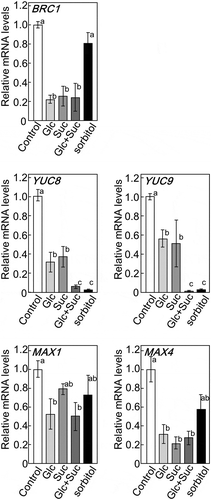
Furthermore, we examined the effects of the exogenous treatments with sugars on the transcript levels of genes involved in the biosynthesis and signal transduction of plant hormones in wild-type and stp1-1 plants. As shown in , the transcript levels of BRC1, YUC8, YUC9, MAX1, and MAX4 were reduced in wild-type plants treated with Glu, Suc, or Glu+Suc. However, these levels were mostly unaffected in stp1-1 plants following the sugar treatments (). The transcript levels of YUC8 and YUC9 in stp1-1 plants were decreased by the treatment with Suc (). These results suggested that Suc and Glc acted as a different signal to control the expression of these genes and STP1 did not function as a Suc signaling factor to regulate these genes.
Figure 4. Expression of BRC1 and genes involved in the biosynthesis and responses of auxin or strigolactones in 5-week-old basal tissues of wild-type plants and stp1-1. The expression of target genes in stp1-1 plants is represented relative to wild-type control plants (a) or those in stp1-1 plants treated with various sugars are represented relative to stp1-1 control plants (b). ActinII was used as the internal reference gene. The transcript levels of these genes were measured by qRT-PCR. Error bars indicate SE (n= 6–10). Bars with the same letter are not significantly different from each other (ANOVA, Tukey’s HSD test).
The phenotypes of STP1 mutant plants
In order to analyze the relationship between STP1 and shoot branching, the phenotypes of stp1-1 and wild-type plants were compared. No significant differences were observed in the dry weight of shoots, number of primary rosette branches, or number of lateral branches between wild-type and stp1-1 plants at 5 weeks (). For further analysis, we generated transgenic Arabidopsis plants overexpressing STP1 (OX-STP1) (supplemental Fig. S1). The number of primary rosette branches significantly decreased in OX-STP1 plants at 5 weeks ()). Accordingly, the total dry weights of OX-STP1 plants were approximately 60% smaller than that of wild-type plants ().
Figure 5. Phenotypes of wild-type, stp1-1, brc1-1 and transgenic plants (OX-STP1-2 and OX-STP1-5). Steady-state amount of BRC1 mRNA in basal tissues of brc1-1 mutants (SALK_091920C), BRC1 mRNA in brc1-1 mutants compared with those in wild-type plants by RT-PCR (a). Similar results were obtained in three independent experiments. Phenotypes of transgenic plants grown for 5 weeks on hydroponic culture (b). Number of primary rosette branches (c), number of lateral branches (d), number of rosette leaves (e) or shoot dry weights (f) of wild-type, stp1-1, brc1-1. Error bars indicate SE (n= 9–10). Bars with the same letter are not significantly different from each other (ANOVA, Tukey HSD test).
Loss of STP1 leads the up-regulation of STP13, but not STP4
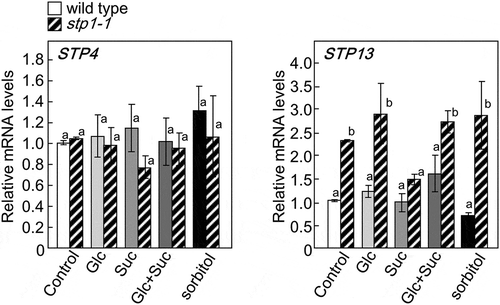
Among the 14 Arabidopsis STP transporters, STP4 and STP13 are dominantly expressed in the roots and leaves, respectively, and regulate the uptake of sugars [Citation38]. We speculated that STP4 and STP13 compensated for the loss of STP1 in stp1-1. To investigate this possibility, the transcript levels of STP4 and STP13 in stp1-1 plants were measured. The results obtained showed no significant differences in the expression level of STP4 between wild-type and stp1-1 plants, whereas the expression level of STP13 was higher in stp1-1 than in wild-type plants (). These results suggested that the loss of STP1 might be partially compensated for by the up-regulated expression of STP13, but not by that of STP4. We also examined whether the transcript levels of STP4 and STP13 were affected by the supply of sugars. The transcript levels of STP4 were not altered by the sugar treatments (). The transcript levels of STP13 were also not affected by sugars, except for sucrose that inhibited the up-regulation of this gene in stp1-1 plants ().
Figure 6. Expression of STP4 (left panel) and STP13 (right panel) in 5-week-old basal tissues of stp1-1 plants treated with various sugars. The transcript levels of these genes were measured by qRT-PCR. The expression of STP4 or STP13 is represented relative to wild-type control plants. ActinII was used as the internal reference gene. Error bars indicate SE (n= 9). Bars with the same letter are not significantly different from each other (ANOVA, Tukey’s HSD test).
Discussion
Sugars affect physiological and morphological aspects in plants [Citation9,Citation10,Citation12,Citation39]. We previously demonstrated that alterations in sugar metabolism in the cytosol affected the branching patterns of tobacco and Arabidopsis plants [Citation7,Citation40]. Furthermore, various genes involved in hormone biosynthesis and signal transduction were altered in AcF plants. These findings imply that sugars control shoot branching by regulating the biosynthesis and signal transduction of plant hormones. In order to reconfirm that sugars affect shoot branching, we analyzed the effects of exogenous treatments with sugars on the expression of genes involved in the biosynthesis and signal transduction of hormones. The results obtained showed that exogenous treatments with sugars resulted in the same transcript characteristics in wild-type Arabidopsis and AcF plants, suggesting that the changes observed in the expression of genes in AcF were due to altered sugar levels in organs.
We then attempted to identify the factors involved in the sugar signaling pathway that integrates the regulation of gene expression for shoot branching. Previous studies reported that many sugar signaling factors are involved in sugar sensitivity, sugar transport, the regulation of carbohydrate metabolism, hormone biosynthesis, senescence, or stress responses [Citation13,Citation14,Citation16,Citation17,Citation35,Citation41–Citation55]. HXK1 is recognized as an important Glc sensor that mediates sugar responses [Citation13,Citation14,Citation16,Citation17]. CWINV1 is expressed in stems, leaves and roots and contributes growth by regulating long-distance transport and partitioning of sugars [Citation35,Citation44,Citation46]. Hexose transporters such as STP1 act as a sugar sensor in various organisms [Citation56]. HXK1, CWINV1, and STP1 are strongly expressed, contribute to growth by regulating sugar partitioning, and function as sugar signaling factors in various aspects of the plant life cycle and the responses of plants to environmental stimuli [Citation13,Citation14,Citation16,Citation17,Citation35,Citation41–Citation46,Citation51–Citation53]. Accordingly, we focused on whether HXK1, CWINV1 and STP1 act as sugar signaling factors to regulate shoot branching via carbon partitioning. We analyzed the effects of exogenous sugar treatments on the transcript levels of BRC1 in the knockout mutants of these genes (hxk1-1, cwinv1-1, and stp1-1). Only the stp1-1 mutant did not show the repression of BRC1 when treated with Glc and/or Suc, suggesting that STP1 is a candidate signaling factor in sugar signaling to regulate the expression of BRC1 in Arabidopsis.
The stp1-1 mutant was well-analyzed and showed the same characteristics with other knockout lines of STP1 [Citation27,Citation38,Citation54,Citation57]. The phenotypes of stp1-1 were almost the same with those of wild-type plants (). In stp1-1 plants, transcript level of BRC1 was lower than that in the wild-type plants, suggesting that BRC1 negative regulation of bud outgrowth might be inhibited. On the other hand, OX-STP1 inhibited growth and branching (). In the present stage, we speculated that phenotypes of stp1-1 were maintained at normal phenotypes by other signaling pathways involved in auxin and cytokinins.
A previous study reported that a KO mutation in the STP1 gene resulted in a decreased uptake of exogenous monosaccharides in Arabidopsis seedlings, suggesting that STP1 functions in the plasma membrane to import monosaccharides from the apoplast [Citation57]. Accordingly, the repression of BRC1 in the stp1-1 mutant may not be caused by a break in sugar signaling, but by a decrease in hexose contents in cells with a reduced transport capacity. However, no significant differences were observed in the contents of Glc, hexose, and Suc between 5-week-old wild-type and stp1-1 plants. This contradiction might depend on plant stage because carbon metabolisms dramatically change at each developmental stage. In Arabidopsis plants with a family of at least 14 genes encoding putative STPs, the expression levels of STP1 were reported to be higher in leaves than other isoforms and were also found in other organs, including the stems, flowers, and roots [Citation38,Citation41]. As shown in , the transcript levels of STP13 were higher in stp1-1 plants than in wild-type plants, suggesting that increased STP13 protein levels compensate for the loss of the transport activity of hexose in stp1-1 plants. As described above, stp1-1 did not show the repression of BRC1 when treated with Glc and/or Suc. These results suggested that STP1 acts as not only a hexose transporter, but also a sensor of extracellular sugars or a signaling factor contributing to the regulation of BRC1 expression. In addition, the present results demonstrated that a break in sugar signaling by the loss of STP1 was not compensated for by the up-regulated expression of STP13. On the other hand, the repression of BRC1 by the treatment with Suc+Glc was stronger than that by the treatment with Glc (). As shown in , the treatment with Suc increased hexose contents in wild-type plants. These results imply that the increases induced in hexose by the Suc treatment resulted in a stronger signal for the regulation of BRC1 expression.
In stp1-1 mutants, the transcript levels of BRC1, YUC8, and YUC9 were lower than those in wild-type plants, while the transcript levels of MAX1 and MAX4 were higher than those in wild-type plants ()). These results support STP1 functioning upstream of these factors in the signaling pathway to regulate the expression of BRC1. On the other hand, the transcript levels of YUC8 and YUC9, involved in auxin synthesis, were decreased in wild-type plants by the treatment with sorbitol as well as that with Glc and/or Suc (). Naser and Shani (2016) proposed cross-talk between auxin and osmotic signaling because auxin levels were down-regulated under osmotic stress conditions [Citation58]. Therefore, the decreases observed in the transcript levels of YUC8 and YUC9 may be caused by osmotic conditions. However, the transcript levels of YUC8 and YUC9 in stp1-1 mutants were decreased by the treatment with sorbitol or Suc, indicating that the defect in STP1 was not related to the reregulation of YUC8 and YUC9 under osmotic conditions. Therefore, cross-talk does not appear to exist between the signaling pathway for osmotic stress and sugar signaling via STP1.
In the present study, our data suggested that STP1 contributed to sugar signaling for the regulation of shoot branching by controlling the expression of genes involved in hormone biosynthesis and signal transduction in Arabidopsis. Transcript level of STP1 was regulated by sugars (supplemental Fig. S2) [Citation52]. Hexose transporters derived from human and yeast showed highly homology with Arabidopsis STP1 [Citation41,Citation56,Citation59,Citation60]. These hexose transporters functioned to maintain sugar contents regulated by its phosphorylation and ubiquitination addition to its transcription [Citation56,Citation60,Citation61]. In higher plants, the increase in photosynthate requires additional sink capacity. Accordingly, alteration of carbon partitioning might regulate plant morphogenesis via the regulation of gene expressions including STP1 and BRC1 involved in the shoot branching. On the other hand, STP1, which is located in the plasma membrane, cannot directly regulate the expression of BRC1. Therefore, we hypothesize that there are additional signaling factors that interact with STP1 and transfer the signal downstream of the signal transduction pathway to regulate the expression of BRC1. Further studies on these factors may reveal the underlying mechanisms of sugar signaling for the regulation of shoot branching via carbon partitioning.
Author contributions
M.T. and S.S. conceived and designed the research. K.O. and N.T. performed the experiments and analyzed the data. M.T. and K.O. wrote the manuscript with assistance of N.T. and S.S. All authors read and discussed the manuscript.
Otori_et_al.STP1_suppl.Fig.2.TIF
Download TIFF Image (238.5 KB)Otori_et_al.STP1_suppl.Fig.1.TIF
Download TIFF Image (359.5 KB)Otori_et_al.STP1.suppl_Table_S1.docx
Download MS Word (31.7 KB)Disclosure statement
Authors declare that they have no conflict of interest.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Sachs T, Thimann KV. The role of auxins and cytokinin in the release of buds from dominance. Am J Bot. 1967;54:136–144.
- Cline MG. Apical Dominance. Bot Rev. 1991;57:318–358.
- Umehara M, Hanada A, Yoshida S, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200.
- Ferguson BJ, Beveridge CA. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009;149:1929–1944.
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19:458–472.
- Finlayson SA. Arabidopsis TEOSINTE BRANCHED1-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol. 2007;48:667–677.
- Otori K, Tamoi M, Tanabe N, et al. Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci Biotechnol Biochem. 2017;81:1470–1477.
- Braun N, de Saint Germain A, Pillot JP, et al. The pea TCP transcription factor PsBRC1 acts downstream of Strigolactones to control shoot branching. Plant Physiol. 2012;158:225–238.
- Eveland AL, Jackson DP. Sugars, signalling, and plant development. J Exp Bot. 2012;63:3367–3377.
- Rolland F, Moore B, Sheen J. Sugar sensing and signalling in plants. Plant Cell. 2002;14:S185–S205.
- Rolland F, Baena-González E, Sheen J. Sugar sensing and signalling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709.
- Wind J, Smeekens S, Hanson J. Sucrose: metabolite and signaling molecule. Phytochemistry. 2010;71:1610–1614.
- Zhou L, Jang JC, Jones TL, et al. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Nat Acad Sci USA. 1998;95:10294–10299.
- Smeekens S, Ma J, Hanson J, et al. Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol. 2010;13:274–279.
- Gibson SI, Laby RJ, Kim DG. The sugar-insensitive1 (sis1) mutant of Arabidopsis is allelic to ctr1. Biochem Biophys Res Commun. 2001;280:196–203.
- Moore B, Zhou L, Rolland F, et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science. 2003;300:332–336.
- Ramon M, Rolland F, Sheen J. Sugar sensing and signaling. Arabidopsis Book. 2008;6:e0117.
- Simon NML, Sawkins E, Dodd AN. Involvement of the SnRK1 subunit KIN10 in sucrose-induced hypocotyl elongation. Plant Signal Behav. 2018;13:e1457913.
- Baena-González E, Rolland F, Thevelein JM, et al. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–942.
- Miyagawa Y, Tamoi M, Shigeoka S. Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nature Biotechnol. 2001;19:965–969.
- Yabuta Y, Tamoi M, Yamamoto K, et al. Molecular design of photosynthesis-elevated chloroplasts for mass accumulation of a foreign protein. Plant Cell Physiol. 2008;49:375–385.
- Ichikawa Y, Tamoi M, Sakuyama H, et al. Generation of transplastomic lettuce with enhanced growth and high yield. GM Crops. 2010;1:322–326.
- Otori K, Tanabe N, Maruyama T, et al. Enhanced photosynthetic capacity increases nitrogen metabolism through the coordinated regulation of carbon and nitrogen assimilation in Arabidopsis thaliana. J Plant Res. 2017;130:909–927.
- Mason MG, Ross JJ, Babst BA, et al. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Nat Acad Sci USA. 2014;111:6092–6097.
- Brewer PB, Dun EA, Gui RG, et al. Strigolactone inhibition of branching independent of polar Auxin transport. Plant Physiol. 2015;168:1820–1829.
- Barbier F, Péron T, Lecerf M, et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot. 2015;66:2569–2582.
- Yamada K, Saijo Y, Nakagami H, et al. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science. 2016;354:1427–1430.
- Tamoi M, Tabuchi T, Demuratani M, et al. Point mutation of a plastidic invertase inhibits development of the photosynthetic apparatus and enhances nitrate assimilation in sugar-treated Arabidopsis seedlings. J Biol Chem. 2010;285:15399–15407.
- Bergareche D, Royo J, Muñiz LM, et al. Cell wall invertase activity regulates the expression of the transfer cell-specific transcription factor ZmMRP-1. Planta. 2018;247:429–442.
- Reda M. Regulation of nitrate reduction in Arabidopsis WT and hxk1 mutant under C and N metabolites. Physiol Plant. 2013;149:260–272.
- Sairanen I, Novák O, Pěnčík A, et al. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell. 2012;24:4907–4916.
- Galtier N, Foyer C, Murchie E, et al. Effects of light and atmospheric carbon dioxide enrichment onphotosynthesis and carbon partitioning in the leaves of tomato (Lycopersicon esculentum L.) plants over-expressing sucrosephosphate synthase. J Exp Bot. 1995;46:1335–1344.
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458.
- Sherson SM, Alford HL, Forbes SM, et al. Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot. 2003;54:525–531.
- Fragoso S, Espíndola L, Páez-Valencia J, et al. SnRK1 isoforms AKIN10 and AKIN11 are differentially regulated in Arabidopsis plants under phosphate starvation. Plant Physiol. 2009;149:1906–1916.
- Yadav UP, Ivakov A, Feil R, et al. The sucrose– trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot. 2014;65:1051–1068.
- Yamada K, Kanai M, Osakabe Y, et al. Monosaccharide absorption activity of Arabidopsis roots depends on expression profiles of transporter genes under high salinity conditions. J Biol Chem. 2011;286:43577–43586.
- Price J, Laxmi A, St Martin SK, et al. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell. 2004;16:2128–2150.
- Tamoi M, Hiramatsu Y, Nedachi S, et al. Increase in the activity of fructose-1,6-bisphosphatase in cytosol affects sugar partitioning and increases the lateral shoots in tobacco plants at elevated CO2 levels. Photosynth Res. 2011;108:15–23.
- Sauer N, Friedländer K, Gräml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. Embo J. 1990;9:3045–3050.
- Ehness R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–548.
- Stadler R, Büttner M, Ache P, et al. Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol. 2003;133:528–537.
- Roitsch T, González MC. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004;9:606–613.
- Brenner WG, Romanov GA, Köllmer I, et al. Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J. 2005;44:314–333.
- Quilliam RS, Swarbrick PJ, Scholes JD, et al. Imaging photosynthesis in wounded leaves of Arabidopsis thaliana. J Exp Bot. 2006;57:55–69.
- Geelen D, Royackers K, Vanstraelen M, et al. Trehalose-6-P synthase AtTPS1 high molecular weight complexes in yeast and Arabidopsis. Plant Sci. 2007;173:426–437.
- Chary SN, Hicks GR, Choi YG, et al. Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol. 2008;146:97–107.
- Lemoine R, La Camera S, Atanassova R, et al. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 2013;4:272.
- Lastdrager J, Hanson J, Smeekens S. Sugar signals and the control of plant growth and development. J Exp Bot. 2014;65:799–807.
- Sheen J. Master regulators in plant glucose signaling networks. J Plant Biol. 2014;57:67–79.
- Cordoba E, Aceves-Zamudio DL, Hernández-Bernal AF, et al. Sugar regulation of SUGAR TRANSPORTER PROTEIN 1 (STP1) expression in Arabidopsis thaliana. J Exp Bot. 2014;66:147–159.
- Veillet F, Gaillard C, Coutos-Thévenot P, et al. Targeting the AtCWIN1 gene to explore the role of invertases in sucrose transport in roots and during Botrytis cinerea infection. Front Plant Sci. 2016;7:1899.
- Yamada K, Osakabe Y, Yamaguchi-Shinozaki K. A C-terminal motif contributes to the plasma membrane localization of Arabidopsis STP transporters. PLoS One. 2017;12:e0186326.
- Durand M, Mainson D, Porcheron B, et al. Carbon source-sink relationship in Arabidopsis thaliana: the role of sucrose transporters. Planta. 2018;247:587–611.
- Moriya H, Johnston M. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc Nat Acad Sci USA. 2004;101:1572–1577.
- Sherson SM, Hemmann G, Wallace G, et al. Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. The Plant J. 2000;24:849–857.
- Crozet P, Margalha L, Confraria A, et al. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front Plant Sci. 2014;20:190.
- Naser V, Shani E. Auxin response under osmotic stress. Plant Mol Biol. 2016;91:661–672.
- Kayikci O, Nielsen J. Glucose repression in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;15:fov068.
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017.