ABSTRACT
In the present study, we examined the effects of fluvoxamine on nerve growth factor (NGF)-induced neurite outgrowth inhibition by dexamethasone (DEX) in PC12 cells. Fluvoxamine increased NGF-induced neurite outgrowth. Compared with co-treatment with NGF and fluvoxamine, p-Akt levels were higher than the values without fluvoxamine. The phosphorylated extracellular regulated kinase 1/2 levels were slightly increased by co-treatment with NGF and fluvoxamine. Fluvoxamine concentration-dependently improved NGF-induced neurite outgrowth inhibition by DEX. Fluvoxamine also improved the decrease in the NGF-induced p-Akt level caused by DEX. Interestingly, the sigma-1 receptor antagonist NE-100 blocked the improvement effects of fluvoxamine on NGF-induced neurite outgrowth inhibition by DEX. The selective sigma-1 receptor agonist PRE-084 also improved NGF-induced neurite outgrowth inhibition by DEX, which is blocked by NE-100. These results indicate that the improvement effects of fluvoxamine on NGF-induced neurite outgrowth inhibition by DEX may be attributable to the phosphorylation of Akt and the sigma-1 receptor.
Graphical Abstract
Fluvoxamine improved NGF-induced neurite outgrowth inhibition by DEX.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that is widely used for patients with major depression in many countries. However, mechanisms of its therapeutic effects are not well understood. Several studies have reported that there is an intimate relationship between depressive disorders and glucocorticoids other than serotonin and noradrenaline [Citation1–Citation4]. Previous studies revealed that glucocorticoids are significant mediators of the stress response in neuronal systems [Citation5,Citation6]. In addition, glucocorticoids are implicated in cell proliferation, production of neurotransmitters, neuronal differentiation and neuronal death; however, synthetic glucocorticoids including dexamethasone (DEX), which is used in clinical therapy, increased the risk of depressive moods in patients. Marques et al. reported that glucocorticoids and elevation of cortisol levels may cause psychiatric disorders including depression [Citation7]. It has been reported that glucocorticoid levels in depressive patients are acutely elevated and do not return to the basal level [Citation8]. In addition, Wrobel et al. reported that both single and repeated administrations of DEX resulted in the depressive-like behavior in mice [Citation9]. They also showed that antidepressant drugs such as imipramine, amitriptyline, and moclobemide are effective against depressive-like behavior in the forced swimming test [Citation9]. Recently, Terada et al. reported that pretreatment with DEX inhibited nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells through glucocorticoid receptors and the inhibition of phosphorylated Akt and extracellular regulated kinase 1/2 (ERK1/2) [Citation10]. They proposed that this model may be useful for clarifying the part of the central nervous system invo lved in depressive mechanisms of action of DEX and other glucocorticoids [Citation10]. Terada et al.’s findings should be highly evaluated from the perspective of discovering the signaling pathway through NGF-induced neurite outgrowth and the glucocorticoid receptor [Citation10]. However, Nishimura et al. reported that the stimulation of sigma-1 receptors is associated with effects of fluvoxamine [Citation11]. This suggests that sigma-1 receptors are also associated with the process of depression.
In the present study, therefore, the effects of fluvoxamine, a representative SSRI, on the inhibition of NGF-induced neurite outgrowth in PC12 cells by DEX were investigated to clarify the mechanism of depression induced by glucocorticoids. The relationship between the sigma-1 receptor and an inhibition of NGF-induced neurite outgrowth by DEX was also studied using a sigma-1 receptor antagonist.
Materials and methods
Materials
The reagents used in the experiments were obtained from the sources shown in parentheses: Dexamethasone sodium phosphate (DEX; Wako, Osaka, Japan), nerve growth factor (NGF; murine NGF (mNGF) 2.5s derived from mouse submaxillary glands, Alomone Labs, Jerusalem, Israel). Fluvoxamine hydrochloride (Fluvoxamine; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), NE-100 hydrochloride (NE-100; Santa Cruz Biotechnology, Inc., CA, USA), PRE-084 hydrochloride (PRE-084; Santa Cruz Biotechnology), anti-phospho-Akt (Ser473) (p-Akt) antibody (Cell Signaling Technology, Beverly, MA, USA), anti-Akt antibody (Cell Signaling Technology), anti-phospho-ERK1 (T202/Y204)/ERK2 (T185/Y187) (p-ERK1/2) antibody (R&D Systems, Minneapolis, MN, USA), and anti-ERK1/2 antibody (R&D Systems).
Cell culture and assessment of neurite outgrowth
Cell culture and assessment of neurite outgrowth were performed according to the method shown by Terada et al. [Citation10]. PC12 cells (rat pheochromocytoma) were obtained from Riken Cell Bank (Ibaraki, Japan). PC12 cells were cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F-12; Gibco-Life Technologies, Gaithersburg, MD, USA) including 10% (v/v) fetal bovine serum (FBS; Gibco-Life Technologies) and 1% (v/v) penicillin/streptomycin (Nakalai Tesque, Kyoto, Japan). Cells were kept in an incubator at 37°C with 5% CO2 humidified atmosphere.
The neurite outgrowth of PC12 cells was measured following the method described by Terada et al. [Citation10]. Cells were seeded in type I collagen-coated 60-mm tissue the culture dishes (Iwaki, Tokyo, Japan) in DMEM/F-12 containing 10% FBS for 24 h. Then, cells were pretreated with DEX (1 μM) for 24 h, washed with Dulbecco’s phosphate buffered saline (D-PBS; Nakalai Tesque), and treated with DMEM/F-12 containing 5% FBS and NGF (50 ng/mL) and fluvoxamine for another 24 h. Neurite outgrowth was measured using a previously described method [Citation10] with a slight modification. After 24 h of treatment, images of five randomly selected fields, each containing 10–15 cells, were captured using an inverted phase-contrast microscope (Nikon, Tokyo, Japan) equipped with a digital camera (Digital Sight DS-L2 system; Nikon), and 10 cells in each image were measured using ImageJ version 1.48v (National Institutes of Health, Bethesda, MD, USA). The neurite length is defined as the distance along a neurite, i.e., the distance from the soma to the end of the neurite. The total neurite length of 10 cells under each treatment condition was calculated to evaluate neurite outgrowth.
Western blot analysis
To study the time course changes of the phosphorylation of Akt and ERK1/2, cells were treated with 50 ng/mL NGF and fluvoxamine 10 μM. To study the concentration-dependent effects of fluvoxamine on the DEX-mediated inhibition of phosphorylation of Akt, cells were pretreated with DEX (1 μM) for 24 h, washed with D-PBS, and treated with 50 ng/mL NGF and 0.01–10 μM fluvoxamine for 10 min. Western blot analysis with the antibodies against Akt and ERK1/2 was conducted as described previously [Citation10].
Statistical analysis
Quantitative data are given as the means ± S.E.M. Statistical significance was analyzed using analysis of variance (ANOVA) followed by Tukey’s post-hoc test.
Results
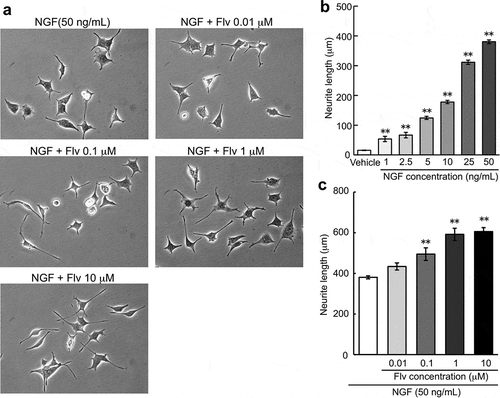
Effect of fluvoxamine on NGF-induced neurite outgrowth
Effects of NGF on neurite outgrowth in PC12 cells are shown in ). NGF caused concentration-dependent neurite outgrowth in PC12 cells, and significant effects were observed at the concentrations above 1 ng/mL. Fluvoxamine increased NGF (50 ng/mL)-induced neurite outgrowth concentration dependently. Significant effects were observed at concentrations of 0.1, 1, and 10 μM ().
Figure 1. Effect of fluvoxamine on NGF-induced neurite outgrowth in PC12 cells.
PC12 cells were treated with NGF (50 ng/mL) or NGF plus fluvoxamine (0.01, 0.1, 1, and 10 μM). (a) Representative image of phase-contrast photomicrographs. (b, c) Quantification data on neurite length. The values represent the mean ± S.E.M. from three independent experiments. **: Significantly different from the NGF-treated group at p < 0.01. NGF: nerve growth factor, Flv: fluvoxamine
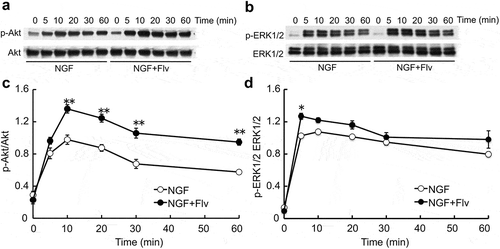
Effect of fluvoxamine on NGF-induced phosphorylation of Akt and ERK1/2
After adding NGF alone, the phosphorylated Akt (p-Akt) levels elevated and reached the maximum 10 min later. With fluvoxamine treatment, p-Akt levels were higher than with NGF alone. Significant effects were observed at 10, 20, 30, and 60 min after adding NGF in comparison with NGF alone (). The phosphorylated ERK1/2 (p-ERK1/2) levels were increased 5–60 min after adding NGF. With fluvoxamine treatment, p-ERK1/2 was somewhat higher than the value without fluvoxamine at 10–60 min after adding NGF. However, significant effects were observed only at 5 min after adding NGF with fluvoxamine in comparison with NGF alone ().
Figure 2. Time course changes in the effect of fluvoxamine on NGF-induced phosphorylation of Akt and ERK1/2.
PC12 cells were treated with NGF or NGF plus fluvoxamine. (a, b) Representative image of western blot. (c, d) Relative protein level quantification data from western blotting. The values represent the mean ± S.E.M. from three independent experiments. **: Significantly different from NGF-treated group at p < 0.01. *: Significantly different from NGF-treated group at p < 0.05. NGF: nerve growth factor, p-Akt: phosphorylated Akt, p-ERK1/2: phosphorylated ERK1/2, Flv: fluvoxamine
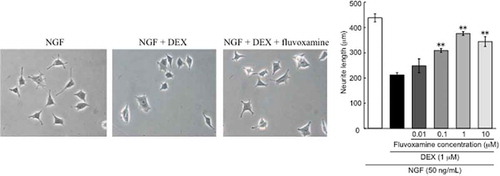
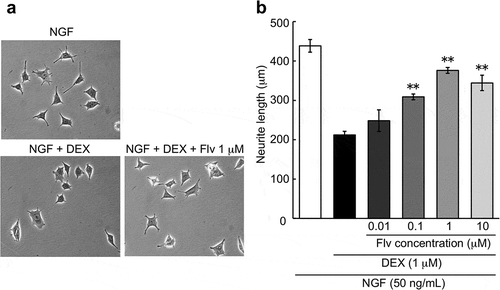
Effect of fluvoxamine on NGF-induced neurite outgrowth inhibition by DEX
Neurite outgrowth was inhibited by DEX (1 μM), which is consistent with results previously described by Terada et al. [Citation10] Fluvoxamine concentration-dependently improved NGF-induced neurite outgrowth inhibition by DEX, and significant effects were observed at concentrations of 0.1, 1, and 10 μM ().
Figure 3. Effect of fluvoxamine on inhibition of NGF-induced neurite outgrowth by DEX.
PC12 cells were pretreated for 24 h with vehicle or DEX (1 μM), followed by washing in D-PBS and treatment with NGF or NGF plus fluvoxamine for 24 h. (a) Representative image of phase-contrast photomicrographs. (b) Quantification data on neurite length. The values represent the mean ± S.E.M. from three independent experiments. **: Significantly different from NGF plus DEX-treated group at p < 0.01. NGF: nerve growth factor, DEX: dexamethasone, Flv: fluvoxamine.
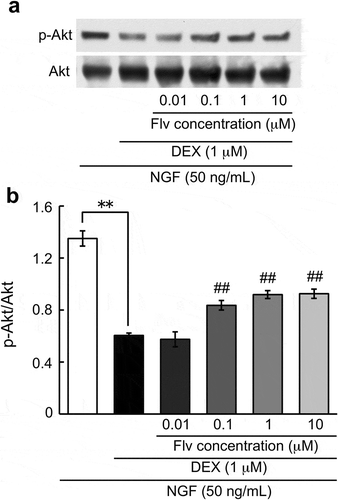
Effect of fluvoxamine on the decrease of NGF-induced phosphorylation of Akt by DEX
Next, we examined the change in phosphorylation of p-Akt in the effect of fluvoxamine on NGF-induced neurite outgrowth inhibition by DEX. DEX decreased the NGF-induced increase in p-Akt signaling. Fluvoxamine improved a decrease of NGF-induced p-Akt levels by DEX, and significant effects were observed at concentrations of 0.1, 1, and 10 μM of fluvoxamine ().
Figure 4. Effect of fluvoxamine on the decrease of NGF-induced phosphorylation of Akt by DEX.
PC12 cells were pretreated for 24 h with vehicle or DEX (1 μM), followed by washing in D-PBS and treatment with NGF or NGF plus fluvoxamine for 10 min. (a) Representative image of western blot. (b) Relative protein level quantification data from western blotting. The values represent the mean ± S.E.M. from three independent experiments. **: Significantly different from NGF-treated group at p < 0.01. ##: Significantly different from NGF plus DEX group at p < 0.01. NGF: nerve growth factor, DEX: dexamethasone, Flv: fluvoxamine.
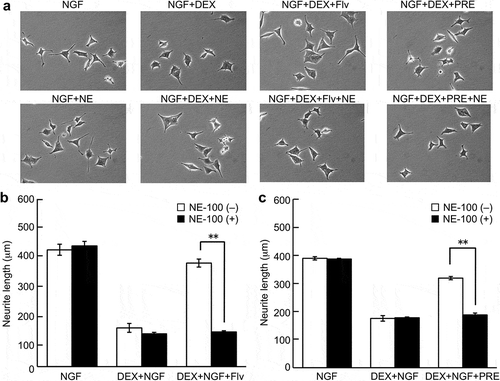
Effect of NE-100 on fluvoxamine’s improvement of DEX-mediated neurite outgrowth inhibition
The effect of fluvoxamine in improving the NGF-induced neurite outgrowth-inhibitory action of DEX was blocked by the adding the sigma-1 receptor antagonist NE-100 (). NE-100 itself had no effect on either NGF-induced neurite outgrowth or DEX-mediated inhibition on NGF-induced neurite outgrowth. In addition, the sigma-1 receptor agonist, PRE-084 improved NGF-induced neurite outgrowth inhibition by DEX (). The effects of PRE-084 were completely blocked by adding NE-100 ().
Figure 5. Effect of NE-100 on fluvoxamine improvement of NGF-induced neurite outgrowth inhibition by DEX.
PC12 cells were pretreated for 24 h with vehicle or DEX (1 μM), followed by washing in D-PBS and pre-incubated in the presence or absence of NE-100 (10 μM) for 4 h. NGF, fluvoxamine (1 μM) or PRE-084 (1 μM) for 24 h. (a) Representative image of phase-contrast photomicrographs. (b, c) Quantification data on neurite length. The values represent the mean ± S.E.M. from three independent experiments. **: Significantly different from DEX plus NGF plus Flv group at p < 0.01. NGF: nerve growth factor, DEX: dexamethasone, Flv: fluvoxamine, PRE: PRE-084
Discussion
In this study, we found that fluvoxamine improved the inhibition of NGF-induced neurite outgrowth by DEX in PC12 cells, and fluvoxamine improved the inhibition of Akt phosphorylation by DEX. The effects of fluvoxamine were abolished by the sigma-1 receptor antagonist. These findings are the first report that fluvoxamine improves NGF-induced neurite outgrowth inhibition by DEX in PC12 cells via the sigma-1 receptor. First, NGF caused concentration-dependent neurite outgrowth in PC12 cells. NGF at 50 ng/mL showed the most potent effect among the concentrations used. Therefore, we used NGF at 50 ng/mL in the following study. Terada et al. also reported that NGF at 50 ng/mL in PC12 cells increased neurite outgrowth [Citation10]. Next, we found that fluvoxamine significantly increased NGF (50 ng/mL)-induced neurite outgrowth in PC12 cells in a concentration-dependent manner. The concentrations of fluvoxamine with significant effects on NGF-induced neurite outgrowth were 0.1, 1, and 10 μM. Similar findings were reported by Nishimura et al., although the concentration and period in culture of NGF were different from in the present study (Nishimura et al: NGF, 2.5 ng/mL; culture period, 5 days, the present study: NGF 50 ng/mL; culture period, 24 h) [Citation11]. Therefore, it was confirmed that fluvoxamine potentiated NGF-induced neurite outgrowth even with different experimental conditions.
Previous studies have shown that the Ras/ERK and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways play important roles in NGF-induced neurite outgrowth in PC12 cells based on the activation of TrkA receptors [Citation12–Citation15]. In addition, Moriguchi et al. reported that the reduction of Akt phosphorylation in CaMKIV-deficient mice is significantly improved by fluvoxamine via its sigma-1 receptor-activating effect [Citation16]. In the present study, NGF elevated the p-Akt levels. With fluvoxamine treatment, p-Akt levels were higher than the values without fluvoxamine, and significant effects were observed at 10, 20, 30, and 60 min after adding fluvoxamine. p-ERK1/2 levels were also increased 5–60 min after adding NGF. With fluvoxamine treatment, p-ERK1/2 levels were high compared with NGF alone, but significant effects were observed only at 5 min after adding NGF. From these findings, it was considered that the potentiating effect of fluvoxamine on NGF-induced neurite outgrowth in PC12 cells may involve the activation of p-Akt rather than p-ERK1/2.
Terada et al. reported that pretreatment with DEX inhibited the NGF-induced promotion of neurite outgrowth in PC12 cells [Citation10]. Moreover, they demonstrated that DEX decreased the p-Akt and P-ERK1/2 in the neurite outgrowth signaling cascade initiated by NGF [Citation10]. The DEX-mediated inhibition of these processes was counteracted by adding a glucocorticoid receptor (GR) antagonist, RU38486. From these findings, Terada et al. demonstrated that the binding of DEX to GR impaired NGF-promoted neurite outgrowth by interfering with the phosphorylation of Akt and ERK1/2 [Citation10]. In the present study, we demonstrated that fluvoxamine increased NGF-induced neurite outgrowth and p-Akt levels. Therefore, the possibility is raised that fluvoxamine modifies NGF-induced neurite outgrowth inhibition by DEX. As shown in the results, a representative SSRI, fluvoxamine, improved NGF-induced neurite outgrowth inhibition by DEX in PC12 cells. Fluvoxamine also improved the decrease in the NGF-induced p-Akt level caused by DEX. These findings suggest that fluvoxamine exerts an improving effect on DEX via the phosphorylation of Akt.
Fluvoxamine shows a high affinity for sigma-1 receptors [Citation17,Citation18]. Sigma-1 receptors are considered to play an important role in psychiatric disorders [Citation19–Citation21] and sigma-1 receptors agonists such as SA4503 shown an antidepressant-like activity [Citation22]. Nishimura et al. reported that SA4503, a sigma-1 receptor agonist, enhanced NGF-induced neurite outgrowth in PC12 cells and that this effect was antagonized when the PI3K inhibitor and ERK1/2 inhibitor were combined [Citation11]. These results indicate that the Ras/ERK1/2 pathway and the PI3K/Akt pathway may play an important role as the intracellular signaling pathway of the sigma-1 receptor. Hence, the Akt pathway is considered important for the improvement effect of fluvoxamine on DEX-mediated inhibition of NGF-induced neurite outgrowth. Clinically, several antidepressants inhibit psychiatric symptoms caused by glucocorticoids. As a conceivable mechanism, tricyclic antidepressants such as imipramine have been reported to inhibit GR mediated genes transcription [Citation23]. Moreover, cortisol synthesis inhibitors have been reported to exhibit an antidepressant effect [Citation24]. These results indicate that the antidepressant action may be involved in the GR function. However, the relationship between fluvoxamine and GR has not yet been reported. Further studies are needed on the action of fluvoxamine on GR.
Moreover, we investigated whether the sigma-1 receptor involves in the improvement effect of fluvoxamine. NE-100 is a representative sigma-1 receptor antagonist [Citation25]. It was found that the improvement effect of fluvoxamine on NGF-induced neurite outgrowth inhibition by DEX was blocked by adding the sigma-1 receptor antagonist NE-100. However, regarding the inhibitory action of DEX on NGF-induced neurite outgrowth, no suppression was observed by adding NE-100. Furthermore, the effect of the selective sigma-1 receptor agonist PRE-084 was investigated. PRE-084 improved NGF-induced neurite outgrowth inhibition by DEX in PC12 cells. It was also found that the improvement effect of PRE-084 on NGF-induced neurite outgrowth inhibition by DEX was suppressed by adding the sigma-1 receptor antagonist NE-100, which had a similar result to adding fluvoxamine. This is the first study showing the improvement effect of the selective sigma-1 receptor agonist on NGF-induced neurite outgrowth inhibition by DEX. Therefore, the improvement effect of fluvoxamine on the inhibitory action of DEX on NGF-induced neurite outgrowth was closely associated with sigma-1 receptors. The results of this study further support the possibility that the sigma-1 receptor plays at least a partial role in the antidepressant effect of fluvoxamine. Furthermore, this model may be useful for elucidating the neurogenesis mechanism of DEX and other glucocorticoids in the central nervous system.
From these findings, it can be concluded that the effect of fluvoxamine on NGF-induced neurite outgrowth inhibition in PC12 cells by DEX occurred via mechanisms involving p-Akt and sigma-1 receptors.
Author contributions
Y. M., K. T., C. K. and Y. S conceived and designed the experiments. Y. M. and K. T. performed the experiments. Y. M., K. T., C. K. and Y. S. wrote the paper. Y. M., K. T., J. T., Y. K., C. K. and Y. S. discussed the results, commented on the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We acknowledge with thanks the assistance of Takayuki Watanabe and Mizuho Hirose.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dinan TG. Glucocorticoids and the genesis of depressive illness. A psychobiological model. Br J Psychiatry. 1994;164:365–371.
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23.
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935.
- Holsboer F, Lauer CJ, Schreiber W, et al. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347.
- Liu HH, Payne HR, Wang B, et al. Gender differences in response of hippocampus to chronic glucocorticoid stress: role of glutamate receptors. J Neurosci Res. 2006;83:775–786.
- Sousa N, Lukoyanov NV, Madeira MD, et al. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266.
- Marques AH, Silverman MN, Sternberg EM. Glucocorticoid dysregulations and their clinical correlates: from receptors to therapeutics. Immunology. 2009;1179:1–18.
- Kunugi H, Ida I, Owashi T, et al. Assessment of the dexamethasone/CRH test as a state-ependent Mamrker for hypothalamic-pituitary-adrenal (HPA) xis Abanormalities in major depressive episode: A multicenter study. Neuropsychopharmacology. 2005;31:212–220.
- Wróbel A, Serefko A, Wlaź P, et al. The depressogenic-like effect of acute and chronic treatment with dexamethasone and its influence on the activity of antidepressant drugs in the forced swim test in adult mice. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:243–248.
- Terada K, Kojima Y, Watanabe T, et al. Inhibition of nerve growth factor-induced neurite outgrowth from PC12 cells by dexamethasone: signaling pathways through the glucocorticoid receptor and phosphorylated Akt and ERK1/2. PLoS One. 2014;9:e93223.
- Nishimura T, Ishima T, Iyo M, et al. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS One. 2008;3:e2558.
- Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol. 2007;621:1–16.
- Hafner A, Obermajer N, Kos J. γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem J. 2012;443:439–450.
- Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–2984.
- Zhao J, Cheng YY, Fan W, et al. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt ignaling athways in the neurite extension process. CNS Neurosci Ther. 2015;21:61–70.
- Moriguchi S, Sakagami H, Yabuki Y, et al. Stimulation of sigma-1 receptor ameliorates depressive-like behaviors in CaMKIV null mice. Mol Neurobiol. 2015;52:1210–1222.
- Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167–173.
- Narita N, Hashimoto K, Tomitaka S, et al. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307:117–119.
- Furuse T, Hashimoto K. Fluvoxamine monotherapy for psychotic depression: the potential role of sigma-1 receptors. Ann Gen Psychiatry. 2009;8:6–8.
- Hindmarch I, Hashimoto K. Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum Psychopharmacol. 2010;25:193–200.
- Ishikawa M, Hashimoto K. The role of sigma-1 receptors in the pathophysiology of neuropsychiatric diseases. J Receptor Ligand Channel Res. 2010;3:25–36.
- Maurice T, Su T-P. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206.
- Budziszewska B, Jaworska-Feil L, Kajta M, et al. Antidepressant drugs inhibit glucocorticoid receptor-mediated gene transcription - a possible mechanism. Br J Pharmacol. 2000;130:1385–1393.
- Murphy BEP. Antiglucocorticoid therapies in major depression: A review. Psychoneuroendocrinology. 1997;22:125–132.
- Okuyama S, Nakazato A. NE-100: A novel sigma receptor antagonist. CNS Drug Rev. 1996;2:226–237.
