ABSTRACT
Ginkgo biloba, a natural biflavonoid isolated from Ginkgo biloba leaves, is reported to have strong anti-inflammatory and immunosuppressive properties. The aim of this study is to investigate the potential anti-inflammatory mechanisms of ginkgo flavonoids on cerebral ischemia/reperfusion (I/R) injury. Inflammatory-associated cytokines in cerebral ischemic hemispheres were determined by immunohistochemical staining, Western blot and enzyme-like immunosorbent assay (ELISA). Our results indicated that treatment with Ginkgetin significantly restored rat brain I/R-induced neurological deficit scores. Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in Ginkgetin treatment group (100 mg/kg) also significantly reduced. The expression inflammation-related protein prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-8 (IL-8) was also decreased in Ginkgetin treatment group. However, the expression of interleukin-10 (IL-10) was remarkably increased. Thus, this study demonstrates that Ginkgetin protects neurons from I/R-induced rat injury by down-regulating pro-inflammatory cytokines and blocking the TLR4/NF-κB pathway.
Graphical Abstract

Ginkgetin protects neurons from I/R-induced rat injury by down-regulating pro-inflammatory cytokines and blocking the TLR4/NF-κB pathway.
Stroke is one of the leading causes for adult disability and mortality worldwide, which makes patients loss of life quality and has poor prognosis for survival [Citation1–Citation3]. The main risk for stroke is high blood pressure and other risk factors include smoking, obesity, high blood cholesterol, diabetes mellitus and atrial fibrillation [Citation4]. Because the high recurrence rate, prevention is the key method to reducing the public health impact of cerebrovascuar disease [Citation2,Citation5]. Currently, the therapeutic strategies for stroke include thrombolysis and intra-artery therapy, which is only available method for minority of patients. Despite this, 15% of patients survived from stroke will become disability [Citation6]. Ischemic stroke is also called brain ischemia and cerebra ischemia and account for 87% of all strokes [Citation7,Citation8]. This type of stroke was caused by blockage of the blood to the brain by reducing the blood flow and oxygen to the brain and leading to damage or the death of the brain cells [Citation7,Citation8]. Since the incidence of ischemic stroke heavily affect the public health and life quality of the patients and still increasing [Citation9], exploring new therapeutic strategies is critical for treatment of ischemic stroke.
Ginkgo biloba, a biflavone extracted from the leaves of Ginkgo biloba tree, has been used for memory, depression, tinnitus and confusion [Citation10–Citation12]. In the past few decades, it has been briefly studies for preclinical use to treat either a small group of patients with stroke or the animal model of stroke [Citation13–Citation17].Currently several minor clinical trials are ongoing for the clinical treatment of stroke by using the Ginkgo biloba extract Egb761 that is a standard extract for Ginkgo biloba and altogether, the current results from the clinical trial suggested the significant improvement among treatment groups compared with controls [Citation18]. In the future, large scale of clinical trials is needed to confirm the results. Preclinical studies also support the use of Egb761 for ischemic stroke. The therapeutic mechanism of Egb761 includes increasing blood flow, neuroprotection and neuroregeneration [Citation15,Citation19,Citation20]. Different types of animal models were used by researchers to prove that Egb761 induced neuroprotective effect on ischemic stroke by reduction of infarct volume and apoptosis, as well as improved neurological function [Citation20,Citation21]. Ginkgetin, a biflavone, extracted from Ginkgo biloba leaves, was reported plays an anti-inflammatory role in arthritis and skin inflammation by suppressing proinflammatory gene expression such as COX-2 (cyclooxygenase-2) and inducible NO synthase (iNOS) [Citation22]. However, its anti-inflammatory role in cerebral ischemia is still not clear.
In this study, we focus on investigating the mechanism of the anti-inflammatory role of Ginkgetin in cerebral ischemia rat model. Our data showed that treatment with ginkgo biloba remarkably restored rat brain I/R-induced neurological deficit scores. Moreover, the Ginkgetin treatment significantly decreased the expression of toll-like receptor 4 (TLR4), inhibited the IκBα degradation and nuclear factor κB nuclear translocation (NF-κB) activation, as well as inhibited intercellular adhesion molecule-1 (ICAM-1) expression. The anti-inflammatory related genes COX-2, iNOS expression were also reduced upon the treatment of rat cerebral ischemia model with Ginkgetin. Furthermore, the expression inflammation-related protein prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-8 (IL-8) were also decreased in Ginkgetin treatment group, except the expression of interleukin-10 (IL-10). Thus, this study demonstrates that Ginkgetin protects neurons from I/R-induced rat injury by down-regulating pro-inflammatory cytokines and inhibition the TLR4/NF-κB pathway.
Methods and materials
Chemicals and reagents
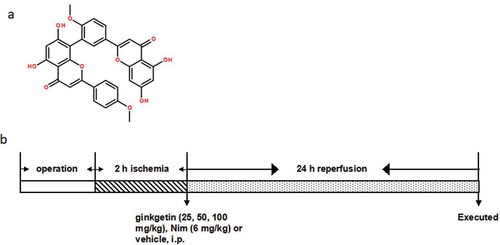
Ginkgetin (purity ≥98%) (The chemical structure of Ginkgetin was shown in )) was purchased form Nanjing Puyi Biological Technology Co., Ltd. (Nanjing, China). Nimodipine (Nim) was served as a positive drug obtained from Bayer Healthcare Company Ltd. Interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10) enzyme linked immunosorbent assay (ELISA) kits were purchased from ABGENT (San Diego, USA). Interleukin-8 (IL-8) ELISA kit was purchased from MR Biotech (China). Tumor necrosis factor alpha (TNF-α) ELISA kit was purchased from Ray Biotech (USA). Prostaglandin E2 (PGE2) ELISA kit was purchased from Beijing Xinfangcheng Biotechnology (China). Rabbit antibodies against toll-like receptor 4 (TLR4), IκBα, nuclear factor kappa B (NF-κB) (p65) and intercellular adhesion molecule-1 (ICAM-1) were obtained from Abcam (Cambridge, MA, USA). Rabbit antibodies against cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), β-actin and peroxidase-labeled goat anti-rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals and reagents were of analytical grade.
Figure 1. Chemical structure of Ginkgetin (a) and diagram of the experimental protocols (b). (a) Chemical structure of Ginkgetin (C32H22O10, molecular weight: 566.511). (b) Rats were subjected to 2 h of ischemia and 24 h of reperfusion. Ginkgetin (25, 50, 100 mg/kg), Nim (6 mg/kg) or vehicle (0.9% (w/v) NaCl solution) were administered i.p. 2 h after the onset of ischemia. The rats in sham and I/R groups received equal volumes of vehicle at the same time point. After 24 h of reperfusion, the rats were anesthetized and then decapitated.
Animals
Healthy, male Sprague-Dawley rats weighing 200–220 g were purchased from the Experimental Animal Center of Chongqing General Hospital, Chongqing, China. The rats were randomly housed in cages maintained at 23 ± 1 ℃ with a 12 h light-dark cycle and allowed free access to water and food. All experiments were approved by the Animal Care and Use Committee of Chongqing General Hospital and performed in accordance with the National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals.
Focal cerebral ischemia/reperfusion (I/R) model
Focal middle cerebral artery occlusion/reperfusion (MCAO/R, 2/24 h) procedure was performed according to the method described previously with minor modification [Citation23,Citation24]. Briefly, rats were anesthetized with 10% (w/v) chloral hydrate (350 mg/kg body weigh) intraperitoneally (i.p.), and the core body temperature was kept at 37 ℃ throughout the whole experiment. After the skin and muscle incision, the left common carotid artery (CCA) was exposed and clipped with artery clamp, and the external carotid artery (ECA) was isolated and ligated. A nylon monofilament (diameter criteria 0.25–0.28 mm) (Beijing Sunbio Biotech, Beijing, China) with a heparin-coated tip was introduced from the CCA into the internal carotid artery (ICA) until a resistance was encountered, thus blocking the origin of the middle cerebral artery. The cerebral cortex provided by the midbrain of the brain was measured by a laser Doppler flowmeter (LDF, PeriFlux 5000 Perimed Co., China), and local cerebral blood flow (rCBF) was reduced to baseline <20%, indicating successful occlusion of the artery. After 2 h of middle cerebral artery occlusion (MCAO), reperfusion was performed by withdrawing of the monofilament. The sham operation group underwent the MCAO surgical procedure, except that the thread was not inserted into the CCA. Efforts were made to minimize suffering and reduce the number of animals used.
Groups and drug administration
A total of 126 rats were randomly divided into 6 groups: (1) sham group (n = 21), rats were treated with MCAO surgery, except that CCA was not inserted; (2) I/R group (n = 21), connected Rats were subjected to cerebral ischemia by ligation for 2 hours and followed by 24 h of reperfusion; (3–5) low doses of Ginkgetin (25 mg/kg) (n = 21), medium dose of Ginkgetin (50 mg/kg) (n = 21) and High-dose Ginkgetin (100 mg/kg) (n) = 21) group, Ginkgetin (25, 50, 100 mg/kg) was injected intraperitoneally (ip) 2 hours after the onset of ischemia. (6) Nim (positive drug) group (n = 21), the rats were slowly injected i.p. with Nim (6 mg/kg) 2 h after the onset of ischemia. The rats in sham and I/R groups received equal volumes of vehicle (0.9% (w/v) NaCl solution) at the same time point. After 24 h of reperfusion, the rats were anesthetized and then decapitated. The specific experimental design for this study was showed in ).
Evaluation of neurological deficits
Neurological deficits were evaluated by an observer blinded to the animals treatment (n = 6) after 24 h reperfusion according to the methods previously described [Citation25]: 0 point, rats behave normally; 1 point, rats cannot fully stretch their left front legs; 2 points, rats turn around into a circle; 3 points, rats fall down to the left side; 4 points, rats cannot move by themselves, losing their consciousness.
Immunohistochemistry staining
After 24 h of reperfusion, rats (n = 3) were i.p. anesthetized with chloral hydrate (350 mg/kg) and transitorily perfused with 150 mL 4°C cold 0.9% NaCl followed by 300 mL 4% paraformaldehyde in phosphate buffered saline (PBS) (0.1 M, PH 7.4). The brain of each rat was rapidly removed and immersed in 10% formalin for fixation for 2 h, then washed three times with PBS. Ultimately, they were transferred to 30% sucrose in PBS solution at 4°C until precipitation. Each longitudinal section was cut at 5 μm from the beginning of 1.9 mm caudal to the bregma for immunohistochemistry staining. The antigen retrieval of paraffin sections was performed under high pressure after dewaxing and dehydration. Brain sections were firstly perforated in 3% triton solutions for 30 min at room temperature, and then washed three times with PBS for 10 min. The tissue was immersed in 1% H2O2 for 30 min to quench the endogenous peroxidase. After washing with PBS for three times, the sections were incubated with 5% goat serum for 30 min. Then they were incubated with rabbit polyclonal antibody against NF-κB (1: 50 dilution) for overnight at 4°C. After incubation, the tissue was rinsed in PBS for 3 times and 5 min for each time and then incubated in a biotinylated anti-rabbit secondary antibody at room temperature for 1 h. After another series of washing in PBS, the tissue was incubated with an AVIDIN-Bioyin for 20 min. The sections were washed and then placed in a solution of 0.5 mg/mL diaminobenzidine (DAB) for 5–10 min until the desired staining intensity was achieved. Finally, the tissue was washed and mounted onto super frost glass slides and left to dry. The three randomly positive area in each section was photographed under high-power magnification (bar = 20 μm) with microscope Olympus BX51 (Olympus, JP) by a blinded manner.
Enzyme linked immunosorbent assay
For enzyme-linked immunosorbent assay, rats (n = 6) were deeply anesthetized and decapitated at 24 h after reperfusion. The ischemic hemisphere tissue was quickly removed and completely grinded into brain tissue homogenate with saline (0.9% NaCl), then centrifugalized at 10,000 rpm for 15 min. The upper limpid liquid was collected and stored at −80°C to avoid repeated freeze-thaw cycles. The competitive ELISA was performed as previously described. We prepared all standard samples before starting the assay procedure. First, we ensured the desired amount of coated wells in the holder, and then added 50 μL standard samples to the appropriate wells of the antibody pre-coated microtiter plate. Secondly, we added 100 μL conjugate antibody to each well. Mix it well and incubate it for 1 h at 37°C. Microtiter plates were washed 5 times with PBS. Substrates A and B in 50 μL were added to each well. And then wells were covered and incubated with substrate for 15 min at room temperature. The wells were added with 50 μL Stop solution. Then the absorbance was measured with ELISA reader and analyzed and calculated the mean absorbance value of A450 for each set of reference standards and samples.
Western blot analysis
Western blot analysis was performed to measure the expression of apoptosis-related proteins as described previously. Briefly, after 24 h reperfusion, brain tissue (n = 6) were homogenized in an ice-cold lysis buffer containing 10 mM Tris (pH 8.0), 150 mM NaCl, 10% (w/v) glycerol, 1% (v/v) NP-40, 5 mM EDTA and protease inhibitor cocktail. The resulting homogenates were centrifuged at 13,200 × g for 20 min at 4 ℃. The supernatant was collected and total protein content was determined using a BCA protein assay kit with bovine serum albumin as the standard (KeyGEN, Nanjing, China). Individual samples (50 μg each) were separated by 10% (w/v) SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane (Hybond ECL, Amersham Pharmacia Biotech, USA). The membranes were then blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline with 0.1% (v/v) Tween-20 (TBS-T) for 2 h, followed by incubations with the affinity purified rabbit anti-TLR4 (1: 1000 dilution), NF-κB (1: 1000 dilution), IκBα (1: 1000 dilution), ICAM-1 (1: 1000 dilution), COX-2 (1: 1000 dilution), iNOS (1: 1000 dilution), overnight at 4 ℃, respectively. After three rinses with TBS-T at 10 min intervals, the membranes were incubated for 2 h with peroxidase-labeled goat anti-rabbit IgG (1: 5000 dilution) at room temperature. After washed four times TBS-T, the specific proteins were visualized with chemiluminescence substrate (ECL Plus) and band intensities were analyzed by Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). Phosphorylation levels of the targeted proteins were evaluated by comparing with corresponding total proteins. β-actin was consider as a loading control.
Statistical analysis
Data were presented as the means ± standard deviation (SD). Statistical analysis of the data was evaluated by one-way analysis of variance (ANOVA) followed by a post hoc LSD test. P < 0.05 was considered statistically significant.
Results
Effect of Ginkgetin on I/R induced neurological damage
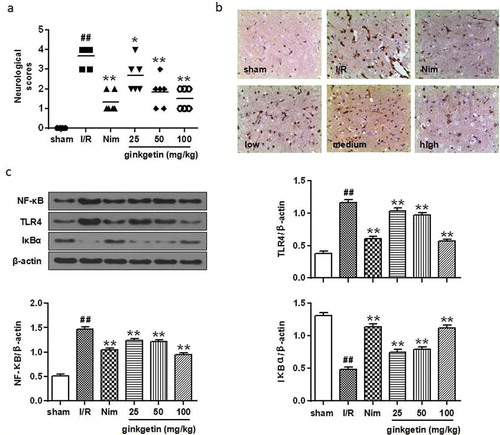
To detect the neuroprotective effect of Ginkgetin, rats were subjected to MCAO/R followed by Ginkgetin (25, 50, 100 mg/kg), Nim (6 mg/kg) or vehicle treatment. As shown in ), there were no neurological deficits detected in the sham group. After 24 h of reperfusion for, I/R treated rats showed severe neurological deficits (P < 0.01 vs sham group). However, treatment with Ginkgetin (25, 50, 100 mg/kg) and Nim (6 mg/kg) significantly reduced the neurological deficit score of cerebral I/R rats (P < 0.05, P < 0.01 vs I/R group) ()). Thus, these results proved that Ginkgetin plays an important neuroprotective role against ischemic attack.
Figure 2. Effect of Ginkgetin on the neurological deficit score and TLR4/NF-κB signaling pathway. Rats were subjected to 2 h of ischemia and 24 h of reperfusion. Ginkgetin (25, 50, 100 mg/kg), Nim (6 mg/kg) or vehicle (0.9% (w/v) NaCl solution) were administered i.p. 2 h after the onset of ischemia. (a) Protective effects of Ginkgetin on neurological deficit score. (b) Immunohistochemistry staining was performed to check the NF-κB expression in ischemic penumbra cortex from different groups 24 h after reperfusion (Scale bar = 20 μm). (c) Western blot analysis was performed and quantitative analysis was conducted for the densitometry of the expression of NF-κB, TLR4 and IκBα proteins. β-actin was consider as a loading control. Data were expressed as mean ± SD, n = 6. **P < 0.01 vs sham group; #P < 0.05 vs I/R group; ##P < 0.01 vs I/R group.
Effect of Ginkgetin on the TLR4/NF-κB signaling pathway
The expression of NF-κB in ischemic penumbra cortex was detected by immunohistochemistry staining and western blot. Immunohistochemistry staining indicated that the NF-κB expression was significantly increased in cortex of I/R group after 24 h of reperfusion ()). Western blot results also showed that NF-κB expression was upregulated in I/R group after 24 h of reperfusion (P < 0.01 vs sham group) ()). Treatment with Ginkgetin (25, 50, 100 mg/kg) or Nim (6 mg/kg) profoundly suppressed the expression of NF-κB (,c)) after 24 h of reperfusion (P < 0.01 vs I/R group). In addition, the expression levels of TLR4 and IκBα in ischemic penumbra cortex in the I/R group were abundantly increased compared with the sham group (P < 0.01), whereas TLR4 in the Ginkgetin (25, 50, 100 mg/kg) and Nim (6 mg/kg) treatment groups were dramatically decreased compared with the I/R group (P < 0.01) ()). Furthermore, Ginkgetin (25, 50, 100 mg/kg) treatment remarkably inhibited the degradation of IκBα (P < 0.01) ()). These results suggest that the TLR4/NF-κB signaling pathway is involved in the Ginkgetin triggered protective effect on cerebral I/R injury.
Effects of Ginkgetin on ICAM-1, COX-2, and iNOS protein production
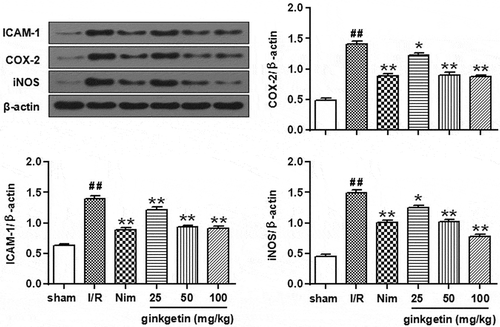
The expressions of ICAM-1, COX-2, and iNOS in the I/R group ischemic cortex were significantly increased compared with those of the sham-operated group after 24 h of reperfusion (P < 0.01) (). Administration with Ginkgetin (25, 50, 100 mg/kg) significantly decreased the expressions of ICAM-1, COX-2, and iNOS (P < 0.05, P < 0.01 vs I/R group) compared with I/R group. The effect of Ginkgetin-treated group (100 mg/kg) was similar to the Nim-treated group (6 mg/kg) (). These results showed that Ginkgetin treatment has the anti-inflammatory effects by inhibition of I/R induced various proinflammatory cytokines expression.
Figure 3. Effects of Ginkgetin on ICAM-1, COX-2, and iNOS protein expression. Rats were subjected to 2 h of ischemia and 24 h of reperfusion. Ginkgetin (25, 50, 100 mg/kg), Nim (6 mg/kg) or vehicle (0.9% (w/v) NaCl solution) were administered i.p. 2 h after the onset of ischemia. Westen blot was performed to check the protein level of ICAM-1, COX-2, and iNOS. β-actin was consider as a loading control. Data were expressed as mean ± SD, n = 6. **P < 0.01 vs sham group; #P < 0.05 vs I/R group; ##P < 0.01 vs I/R group.
Effects of Ginkgetin on PGE2 and TNF-α protein expression
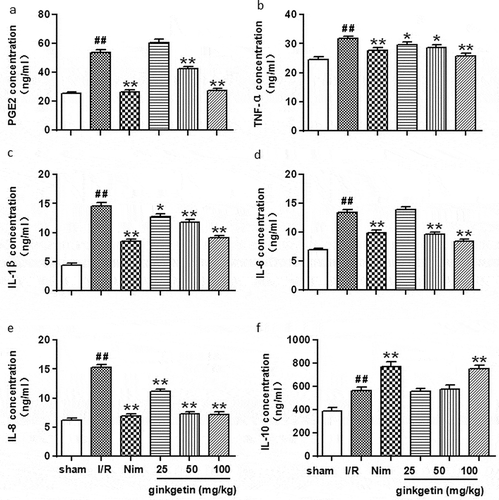
The expression level of PGE2 and TNF-α were remarkably increased in the I/R group compared with the sham-operated group after 24 h of reperfusion (P < 0.01) (,b)). ELISA assay results showed that the PGE2 protein level in the Ginkgetin-treated groups (50, 100 mg/kg) and the Nim-treated group (6 mg/kg) was significantly decreased by 20.97% (P < 0.05), 48.68% (P < 0.01), and 50.58% (P < 0.01) respectively, when compared with I/R group ()). Although PGE2 protein level was slightly increased in the Ginkgetin-treated group (25 mg/kg), but it was not statistically significant ()). The TNF-α protein level in the Ginkgetin-treated groups (25, 50, 100 mg/kg) and the Nim-treated group (6 mg/kg) was also decreased by 6.75% (P < 0.05), 9.97% (P < 0.05), 19.00% (P < 0.01), and 12.94% (P < 0.01) respectively compared with I/R group ()). These results suggested that Ginkgetin treatment may inhibit the activation of NF-κB to downregulate I/R-induced injury induced downstream inflammatory factor PGE2 and TNF-α expression.
Figure 4. Effects of Ginkgetin on PGE2, TNF-α, IL-1β, IL-6, IL-8, and IL-10 protein expression. Rats were subjected to 2 h of ischemia and 24 h of reperfusion. Ginkgetin (25, 50, 100 mg/kg), Nim (6 mg/kg) or vehicle (0.9% (w/v) NaCl solution) were administered i.p. 2 h after the onset of ischemia. (a) ELISA assay was performed to check the effects of Ginkgetin on the expression of PGE2, the expression of TNF-a (b), the expression of IL-1β (c), the expression of IL-6 (d), the expression of IL-8 (e) and the expression of IL-10 (f). Data were expressed as mean ± SD, n = 6. **P < 0.01 vs sham group; #P < 0.05 vs I/R group; ##P < 0.01 vs I/R group Data were expressed as mean ± SD, n = 6. **P < 0.01 vs sham group; #P < 0.05 vs I/R group; ##P < 0.01 vs I/R group.
Effects of Ginkgetin on IL-1β, IL-6, IL-8, and IL-10 protein expression
To evaluate the anti-inflammatory effect of Ginkgetin in rat brain after MCAO/R, we checked the inflammation-related cytokines (IL-1β, IL-6, IL-8, and IL-10) protein levels in the ischemic tissue after 24 h of reperfusion. As shown in , the protein level of IL-1β, IL-6, IL-8, and IL-10 were remarkably increased in I/R group after 24 h of reperfusion (P < 0.01 vs sham group). While, the IL-1β, IL-6, and IL-8 protein concentrations in the Ginkgetin-treated group (100 mg/kg) were significantly decreased by 37.51% P < 0.01), 37.64% (P < 0.01), and 52.95% (P < 0.01) respectively compared with I/R group (–e)). Interestingly, the IL-10 protein level in I/R group was upregulated, while the IL-10 protein level in Ginkgetin-treated group (100 mg/kg) or in Nim (6 mg/kg) treatment group was even higher than that of the I/R group (P < 0.01) ()). The elevation of IL-10 protein may be due to the compensatory mechanism for the ischemia stress in rats after 24 h of reperfusion. Therefore, these result indicated that Ginkgetin treatment inhibited the anti-inflammatory related protein expression except IL-10.
Discussion
Ginkgo biloba extracts have long been reported to protect against neuronal death caused by ischemia in animal models [Citation15–Citation17,Citation21]. The mechanism involved in this protection might rely on its role in anti-apoptosis and increasing cerebral blood flow [Citation15,Citation26]. Recently, some minor clinical trials have been ongoing and altogether results suggest the positive effect of Ginkgo biloba on ischemic stroke patients by a significant improvement in neurological function, which suggest that Ginkgo biloba extract can be used as a therapeutic strategy for the treatment of ischemia stroke [Citation17]. However, a large scale of trial with more patient participation is still needed to confirm the positive role of Ginkgo biloba extract effect on ischemia stroke. At the same time, preclinical studies have showed that Ginkgo biloba extract associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation [Citation27]. Specifically, the EGb 761 was used by different research groups to prove its effect on ischemia strokes. Altogether, these clinical trial and preclinical data all support that Ginkgo biloba extract has the great potential to be explored as a therapeutic method for treatment of ischemia stroke in the near future.
In this study, the Ginkgetin, a biflavone from Ginkgo biloba leaves, which was previously reported as a phospholipase A (2) inhibitor and potent anti-arthritic reagent [Citation28], was applied to cerebral ischemia/reperfusion-induced injury rat model. The results showed that Ginkgetin treatment markedly restored rat brain I/R-induced neurological deficit scores. Toll-like receptors are the member of the pattern recognition receptors (TLRs) which plays important role in transduce extracellular antigen information into cells and trigger the inflammatory response [Citation29,Citation30]. Among these TLRs, toll-like receptor 4 was reported to play a critical role in immune defense and immune regulations [Citation31]. Recently, studies proved that TLR4 can induce cell apoptosis in cerebral ischemia-reperfution inflammation injury model [Citation32,Citation33]. NF-κB signaling was involved in multiple cell functions which include inflammation, immune response, cell apoptosis and cell proliferation [Citation34]. NF-κB activation leads to the expression of different pro-inflammatory cytokines that include TNF-α, IL-6 and IL-8 [Citation35]. For ischemia stroke, one previous study proved that TLR4/NF-κB signaling was correlated with extent of the rat brain damage and inhibition of TLR4/NF-κB can alleviate the cerebral ischemia-reperfusion injury in rat [Citation35,Citation36]. In addition, upregulation of TLR4 was found in tubular epithelial cells and infiltration of leukocytes in the kidney following ischemia or ischemia-reperfusion injury [Citation37,Citation38]. Since the protection was related with a decrease in proinflammatory cytokine and chemokine secretion and decrease of leukocytes infiltration, downregulation or decreased expression of TLR4 signaling pathway could play a protection role in ischemia model. Our data showed that Ginkgetin treatment significantly decreased TLR4 expression, inhibited the IκBα degradation and nuclear factor κB nuclear translocation (NF-κB) activation, and inhibited intercellular adhesion molecule-1 (ICAM-1) expression. These results is in accordance with previous study and further confirmed the role of TLR4/NF-κB signaling in ischemia-reperfution injury and more importantly, these finding recovered the mechanism of how Ginkgetin works for treatment the ischemia stroke in animal model. In the future studies, we will detect the effect of TLR4 on leukocytes infiltration to uncover more details of the mechanism how Ginkgetin treatment reduced the ischemia-reperfusion injury.
Numerous studies proved that TLR4 signaling pathway attenuated inflammatory response and myocyte apoptosis following I/R injury [Citation39,Citation40]. Also, previous studies proved that TLR4 depletion or inhibition protected the kidney ischemia-reperfusion induced injury by reducing the leukocytes such as macrophages infiltration and pro-inflammatory cytokine production [Citation37,Citation38]. Pro-inflammatory cytokines such as TNF-α and IL-6 expressions were decreased by supressing TLR4/NF-Κb signaling pathways [Citation39,Citation40]. Therefore, we also checked the pro-inflammatory related genes and cytokines expression in this study. The results indicated that inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in Ginkgetin treatment group (100 mg/kg) also significantly reduced. The expression inflammation-related protein prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-8 (IL-8) was also decreased in Ginkgetin treatment group except the expression of interleukin-10 (IL-10) which was remarkably increased. Thus, the hypothesis for the Ginkgetin is Ginkgetin treatment inhibited TLR4/NF-κB signaling pathway activation which in turn decreased the expression of pro-inflammatory related genes and cytokine expression thus reduced the neuronal death and damage. Therefore, our data confirmed the neuronal protected role of Ginkgetin in cerebral ischemia/reperfusion-induced injury in rat model. These preclinical studies uncover the mechanism of how Ginkgo biloba extract works on ischemia stroke and confirmed that the great potential of Ginkgo biloba extract served as a therapeutic method for treatment of ischemia stroke in the future clinically.
Conclusions
Rats cerebral ischemia/reperfusion-induced injury model were subjected to ischemia for 2 hours and 24 hours of reperfusion. Ginkgetin treatment significantly restored rat brain I/R-induced neurological deficit scores. The Ginkgetin treatment also significantly decreased toll-like receptor 4 (TLR4) expression, IκBα degradation and nuclear factor κB nuclear translocation (NF-κB) activation, and inhibited intercellular adhesion molecule-1 (ICAM-1) expression. Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in Ginkgetin treatment group (100 mg/kg) also significantly reduced. The expression inflammation-related protein prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-8 (IL-8) was also decreased in Ginkgetin treatment group. However, the expression of interleukin-10 (IL-10) was remarkably increased. Thus, this study suggested that Ginkgetin treatment plays a protection role in I/R-induced rat injury through blocking the TLR4/NF-κB pathway and down-regulating pro-inflammatory cytokines.
Authors’ contributions
Q L and XM P designed and carried out the study. Q L, T Y and T L participated in experiments and statistical analysis. Q L wrote the manuscript. XM P revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics-2014 update a report from the American heart association. Circulation. 2014;129(3):399–410.
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2014 update a report from the American heart association. Circulation. 2014;129(3):E28–E292.
- Jauch EC, Saver HP, Adamsjr HP, et al. Guidelines for the early management of patients with acute ischemic stroke: executive summary a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44(3):870–948.
- Rundek T, Sacco RL. Risk factor management to prevent first stroke. Neurol Clin. 2008;26(4):1007–1045.
- Gallanagh S, Quinn TJ, Alexander J, et al. Physical activity in the prevention and treatment of stroke. ISRN Neurol. 2011;2011:953818.
- Davis S, Lees K, Donnan G. Treating the acute stroke patient as an emergency: current practices and future opportunities. Int J Clin Pract. 2006;60(4):399–407.
- Musuka TD, Wilton SB, Traboulsi M, et al. Diagnosis and management of acute ischemic stroke: speed is critical. Can Med Assoc J. 2015;187(12):887–893.
- Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;371(9624):1612–1623.
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–448.
- Smith JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64(4):465–472.
- Zimmermann M, Colciaghi F, Cattabeni F, et al. Ginkgo biloba extract: from molecular mechanisms to the treatment of Alzhelmer’s disease. Cell Mol Biol (Noisy-Le-Grand). 2002;48(6):613–623.
- Charemboon T, Jaisin K. Ginkgo biloba for prevention of dementia: a systematic review and meta-analysis. J Med Assoc Thai. 2015;98(5):508–513.
- Garg RK, Nag D, Agrawal A. A double blind placebo controlled trial of ginkgo biloba extract in acute cerebral ischaemia. J Assoc Physicians India. 1995;43(11):760–763.
- Anadere I, Chmiel H, Witte S. Hemorheological findings in patients with completed stroke and the influence of a Ginkgo biloba extract. Clin Hemorheol. 1985;5(5):411–420.
- Nash KM, Shah ZA. Current perspectives on the beneficial role of Ginkgo biloba in neurological and cerebrovascular disorders. Integr Med Insights. 2015;10:1–9.
- Liu JP. The use of Ginkgo biloba extract in acute ischemic stroke. Explor J Sci Heal. 2006;2(3):262–263.
- Li S, Zhang X, Fang Q, et al. Ginkgo biloba extract improved cognitive and neurological functions of acute ischaemic stroke: a randomised controlled trial. Stroke Vasc Neurol. 2017;2(4):189–197.
- von Gunten A, Schlaefke S, Uberla K. Efficacy of Ginkgo biloba extract EGb 761((R)) in dementia with behavioural and psychological symptoms: a systematic review. World J Biol Psychiatry. 2016;17(8):622–633.
- Krieglstein J, Beck T, Seibert A. Influence of an extract of Ginkgo biloba on cerebral blood flow and metabolism. Life Sci. 1986;39(24):2327–2334.
- Zhang WR, Hayashi T, Kitagawa H, et al. Protective effect of ginkgo extract on rat brain with transient middle cerebral artery occlusion. Neurol Res. 2000;22(5):517–521.
- Lee EJ, Chen HY, Wu TS, et al. Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague-Dawley rats. J Neurosci Res. 2002;68(5):636–645.
- Son JK, Son MJ, Lee E, et al. Ginkgetin, a Biflavone from Ginko biloba leaves, inhibits cyclooxygenases-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Biol Pharm Bull. 2005;28(12):2181–2184.
- Larpthaveesarp A, Gonzalez FF. Transient middle cerebral artery occlusion model of neonatal stroke in P10 rats. J Vis Exp. 2017;122:54830.
- Hill JW, Nemoto EM. Transient middle cerebral artery occlusion with complete reperfusion in spontaneously hypertensive rats. MethodsX. 2014;1:283–291.
- Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476.
- Guo M, Suo Y, Gao Q, et al. The protective mechanism of Ginkgolides and Ginkgo flavonoids on the TNF-alpha induced apoptosis of rat hippocampal neurons and its mechanisms in vitro. Heliyon. 2015;1(1):e00020.
- Jiang M, Li J, Peng Q, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflamm. 2014;11:167.
- Kwak WJ, Han CK, Son KH, et al. Effects of Ginkgetin from Ginkgo biloba Leaves on cyclooxygenases and in vivo skin inflammation. Planta Med. 2002;68(4):316–321.
- Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426(6):1246–1264.
- Galiana-Arnoux D, Imler JL. Toll-like receptors and innate antiviral immunity. Tissue Antigens. 2006;67(4):267–276.
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151.
- Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175(11):7661–7668.
- Hua F, Ma J, Ha T, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190(1–2):101–111.
- van Delft MA, Huitema LF, Tas SW. The contribution of NF-kappaB signalling to immune regulation and tolerance. Eur J Clin Invest. 2015;45(5):529–539.
- Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40.
- Zhao H, Chen Z, Xie LJ, et al. Suppression of TLR4/NF-kappaB signaling pathway improves cerebral ischemia-reperfusion injury in rats. Mol Neurobiol. 2018;55(5):4311–4319.
- Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117(10):2847–2859.
- Zhao H, Perez JS, Lu K, et al. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306(8):F801–11.
- Yang Y, Lv J, Jiang S, et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016;7:e2234.
- Wang C, Sun H, Song Y, et al. Pterostilbene attenuates inflammation in rat heart subjected to ischemia-reperfusion: role of TLR4/NF-kappaB signaling pathway. Int J Clin Exp Med. 2015;8(2):1737–1746.
