 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the current study, we isolated a proanthocyanidin oligomer from the hulls of red-kerneled rice. The structure of the oligomer was characterized based on spectral data and chemical reaction. Furthermore, two anthocyanins were isolated from the beards of the same source. The proanthocyanidins and beard extract showed more potent inhibitory and cleaving activities than those of positive controls, respectively.
Red-kerneled rice (Oryza sativa), which has been consumed in Japan for approximately 100 years, is cultivated at a limited number of areas such as Soja in Okayama, Tsushima in Nagasaki, and Tanegashima in Kagoshima and used in shrine rituals. Red-kerneled rice has recently been reported to show antioxidant [Citation1–Citation5] and anti-inflammatory [Citation6] activities as well as cancer inhibitory effects [Citation7]. The proanthocyanidin contained in the hulls of the rice is believed to contribute to its biological properties [Citation4,Citation7]. However, details of the chemical structure of the proanthocyanidin have not yet been reported. In addition, although red-kerneled rice possesses distinctive red-colored beards, little is known about the structures of the pigments responsible for this coloration.
In the present study, we characterized the proanthocyanidin from the hulls of red-kerneled rice and identified the pigments in the beards. Furthermore, the proanthocyanidin-rich fractions from several varieties of red-kerneled rice and the beard extract were evaluated for inhibition of advanced glycation end products (AGEs) formation and AGE-derived crosslink-cleaving effects.
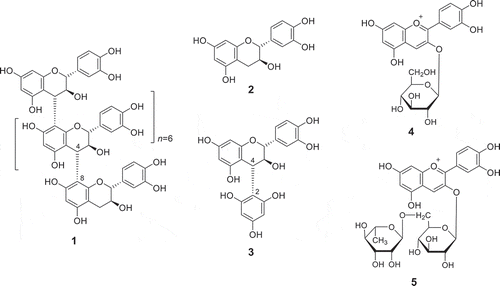
The hulls of red-kerneled rice (200 g) collected at Okayama Soja in 2012 and 2013 were extracted with 70% aqueous acetone (600 mL). The extract (3.0 g) was separated by column chromatography using a Diaion HP-20 column (50 × 6.0 cm i.d., Mitsubishi Chemical Co., Tokyo, Japan) with H2O and increasing amounts of MeOH (10→50→100% MeOH) and 70% aqueous acetone as the eluents. A fraction containing high levels of proanthocyanidin oligomer was obtained from the 50% MeOH eluate (0.5 g). The normal- and reversed-phase HPLC profiles of the proanthocyanidin-rich fraction displayed broad peaks characteristic of oligomeric proanthocyanidins. This fraction was heated with n-BuOH-HCl to produce cyanidins showing an absorbance maximum at 547 nm, indicating that the fraction contained procyanidin oligomers [Citation8]. The 13C-NMR spectrum of the proanthocyanidin oligomer (1) {[151 MHz, acetone-d6-D2O (1:1, v/v)] presented resonances at δC 152.4–156.9 (C-5, 7, 8a), 144.3 (C-3ʹ, 4ʹ), 131.0 (C-1ʹ), 120.3 (C-6ʹ), 115.7 (C-2ʹ, 5ʹ), 108.1 (C-8), 106.2 (C-4a), 96.5 (C-6), 81.9 (terminal C-2), 69.8–74.0 (C-2, 3, terminal C-3), 37.4 (C-4), and 29.9 (overlapped with the solvent signal, terminal C-4)} accompanied by low-resolution signals indicating a large-molecular-weight species with restricted rotation. The signal pattern of the oligomer was similar to those of catechin-(4α→8)-catechin dimer (procyanidin B-3) [Citation9] and its oligomer [Citation10]. The molecular weight of the proanthocyanidin oligomer (1) was estimated by gel permeation chromatography (GPC) analysis [two connected in linear columns, TSK-gel super AW3000 (150 × 6.0 mm i.d., Tosoh Co., Tokyo, Japan); solvent, 3 M ammonium formate-N,N-dimethylformamide (0.5:99.5, v/v); flow rate, 0.5 mL/min; detection, UV 280 nm at 40℃], using catechin (tR: 5.60 min), procyanidin B-3 (tR: 4.88 min), and procyanidin undecamer from loquats [Citation11] (tR: 3.55 min) as molecular-weight markers and the relationship log MW = – 0.28 × tR + 5.71. The number (Mn) and weight (Mw) average molecular weights of the oligomer (tR: 3.90 min) were 2,194 and 3,924, respectively, indicating that the average degree of polymerization of the oligomer is approximately eight. The extension and terminal units of the oligomer (1) were identified by acid-degradation in the presence of phloroglucinol [Citation12]. A mixture of the oligomer (1 mg) and phloroglucinol (1 mg) was dissolved in 1% HCl-EtOH (1 mL) and left to stand overnight at ambient temperature. The resultant mixture was analyzed by HPLC [column, Inertsustain C18 (150 × 4.6 mm i.d., GL Sciences, Tokyo, Japan); solvent, H2O-acetonitrile-formic acid (93:2:5, v/v); flow rate, 1.0 mL/min; detection, UV 280 nm at 40℃], revealing a peak due to catechin (2). Preparative HPLC of the mixture (10 mg) using the above conditions furnished the catechin (4α→2) phloroglucinol adduct (3) (1.1 mg) (). The structure of compound 3 was identified based on NMR and MS analysesFootnote1 [Citation13]. The circular dichroism (CD) data for the oligomer (1) revealed a positive Cotton effect centered at 207 nm (∆ε +16.0 at 200 nm and ∆ε −20.0 at 221 nm), which is similar to those exhibited by the catechin oligomers procyanidins B-3 and C-2 [Citation14]. Based on these findings, the proanthocyanidin from the hulls of red-kerneled rice was concluded to be a procyanidin octamer (on average) composed of catechin-(4α→8)-catechin units, as represented by formula 1 in .
Figure 1. The structures of compounds isolated from the hulls and beards of red-kerneled rice and the proanthocyanidin degradation products.
The oligomeric features of the proanthocyanidins from several varieties of red-kerneled rice (akaonimochi, benizomemochi, tanegashima, akamai, and benimusume), obtained from 50% MeOH eluate on Diaion HP-20 column chromatography of 70% aqueous acetone extract of the hulls, were evaluated for Mn, Mw, and polydispersity index (Mw/Mn) by GPC analysis (). The Mn, Mw, and Mw/Mn of these proanthocyanidins fell in the ranges 2194–2888, 3924–5172, and 1.79–1.95, respectively, indicating that the differences in the oligomeric features among these proanthocyanidins is non-significant.
Table 1. Number (Mn) and weight (Mw) average molecular weights and polydispersity index (Mw/Mn) values of proanthocyanidin oligomers from several varieties of red-kerneled rice.
The 70% aqueous acetone homogenate of the beards (18.5 g) of red-kerneled rice collected at Okayama Soja in 2012 and 2013 was filtered and evaporated. The extract (1.0 g) was purified by column chromatography using a Toyopearl HW-40 column (50 × 2.2 cm i.d., Tosoh Co., Tokyo, Japan) with 30% aqueous MeOH containing 5% formic acid to yield cyanidins 3-O-glucoside (4) (6.0 mg) and 3-O-rutinoside (5) (2.2 mg) (). These pigments were identified by NMR, MS, and HPLC comparison with authentic specimens purchased from Tokiwa Phytochemical Co. Ltd. Chiba, Japan. To the best of our knowledge, this is the first report of the isolation and identification of anthocyanins from the beards of red-kerneled rice.
We evaluated the inhibitory effects of the proanthocyanidin oligomers from several varieties of red-kerneled rice and the beard extracts on the formation of AGEs according to the method described by Ito et al. [Citation15] with a slight modification. The sample solution was added to a reaction mixture containing 83.3 mM phosphate buffer (pH 7.2), 2.0 M glucose, 2.0 M fructose, 8.0 mg/mL human serum albumin (HSA), and distilled water (6:1:1:1:1, v/v). As a control, the vehicle was added instead of the sample solution. For each blank, glucose or fructose were replaced with distilled water, and the total volume was set to 1,000 µL. The solutions were diluted eight-fold and dispensed into a black microplate in 200 µL portions after incubation of the mixture at 60°C for 40 h. The fluorescences of the samples were measured at excitation and emission wavelengths of 370 and 465 nm, respectively, using a Power Scan HT (DS Pharma Biomedical Co. Ltd., Osaka, Japan). The inhibitory rate was calculated as follows:
where S is the relative intensity of the sample solution, C is the relative intensity of the control solution, and SB and CB are the intensities of the glucose or fructose-omitted blank solutions.
The IC50 values were calculated by probit analysis. shows the inhibitory effects of all tested samples toward the formation of AGEs by glycation of HSA with glucose and fructose. All the tested samples show stronger inhibitory activities than aminoguanidine (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as a positive control.
Table 2. Inhibitory effects on AGE formation in HSA/glucose and fructose, and AGE-derived crosslink-cleaving activities, of proanthocyanidin oligomers from red-kerneled rice hull and of the beard extract.a
Furthermore, we investigated the AGE crosslink-cleaving activity of the same samples according to the method reported by Kato et al. [Citation16]. The tested proanthocyanidins showed weaker activities for AGE crosslink cleaving than that of catechin as a positive control. However, the beard extract exhibited a stronger cleaving activity stronger than catechin, suggesting that the anthocyanins mainly localized in the beards show activities.
In summary, proanthocyanidin (1) was isolated from the hulls of red-kerneled rice and the structure was revealed to contain average octamer composed of catechin-(4α→8)-catechin units. The oligomeric characteristics of proanthocyanidin-rich fractions from several different varieties of red-kerneled rice were found to be almost similar. Two main pigments isolated from the beards of red-kerneled rice were identified based on NMR, MS, and HPLC analyses to be the cyanidins 3-O-glucoside (4) and 3-O-rutinoside (5).
The proanthocyanidin-rich fractions and the beard extract from red-kerneled rice were assessed for inhibitory activities toward AGE formation by glycation of HSA with glucose and fructose, and for AGE crosslink-cleaving activity. The proanthocyanidin oligomers from the hulls showed remarkable inhibitory effects toward AGE formation. The beard extract exhibited AGE-derived crosslink-cleaving activity. Our findings suggest that polyphenols from the hulls and beards of red-kerneled rice might play a role in the prevention of several lifestyle diseases and the progress of age-related diseases.
Author contribution
NG isolated and identified the compounds from red-kerneled rice and wrote the draft of the manuscript. SW extracted the hulls and beards of red-kerneled rice. NK, FB, and NG performed the biological assays. MM conducted circular dichroism (CD) analysis. HI designed the study and supervised manuscript preparation. All authors reviewed and approved the final manuscript.
Acknowledgments
The authors are grateful to Redrice Company, Soja, Okayama, Japan for providing red-kerneled rice. The authors thank the SC-NMR Laboratory of Okayama University for NMR measurements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes
1. Catechin-(4α→2)-phloroglucinol (3): pale brown amorphous powder; 1H-NMR [acetone-d6-D2O (9:1 v/v)]: δ 6.94 (1H, d, J = 1.2 Hz, H-2ʹ), 6.76–6.79 (2H, m, H-5ʹ, 6ʹ), 5.99 (1H, brs, H-8), 5.88 (1H, brs, H-6), 5.84 (2H, s, H-phloroglucinol unit), 4.50 (1H, dd, J = 8.4, 9.0 Hz, H-3), 4.40 (1H, d, J = 8.4 Hz, H-4), 4.35 (1H, d, J = 9.0 Hz, H-2); high resolution ESI-MS m/z 413.0888 [M-H]− (calcd for C21H18O9-H, 413.0878).
References
- Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, et al. Physicochemical and antioxidant properties of red and black rice varieties from Thailand, China and Sri Lanka. FoodSci Technol. 2011;124:132–140.
- Gunaratne A, Wu K, Li D, et al. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013;138:1153–1161.
- Walter M, Marchesan E, Massoni PFS, et al. Antioxidant properties of rice grains with light brown, red and black pericarp colors and the effect of processing. Food Res Int. 2013;50:698–703.
- Oki T, Masuda M, Kobayashi M, et al. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J Agric Food Chem. 2002;50:7524–7529.
- Hu Z, Tang X, Liu J, et al. Effect of parboiling on phytochemical content, antioxidant activity and physicochemical properties of germinated red rice. Food Chem. 2017;214:285–292.
- Limtrakul P, Yodkeeree S, Pitchakarn P, et al. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathway in Raw 264.7 macrophages. Nut Res Pract. 2016;10:251–258.
- Pintha K, Yodkeeree S, Limtrakul P. Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell invasion via the expression control of invasive proteins. Biol Pharm Bull. 2015;38:571–581.
- Bate-Smith EC. Phytochemistry of proanthocyanidins. Phytochemistry. 1975;14:1107–1113.
- Park JC, Ito H, Yoshida T. 1H-NMR assignment of HIV protease inhibitor, procyanidin B3 isolated from Rosa rugosa. Nat Prod Sci. 2003;9:49–51.
- Czochanska Z, Foo LY, Newman RH, et al. Polymeric proanthocyanidins. Stereochemistry, structural units, and molecular weight. J Chem Soc Perkin Trans. 1980;1:2278–2286.
- Ito H, Kobayashi E, Takamatsu Y, et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem Pharm Bull. 2000;48:687–693.
- Foo LY. Procyanidin polymers of douglas fir bark: structure from degradation with phloroglucinol. Phytochemistry. 1989;28:3185–3190.
- Foo LY, Newman R, Waghorn G, et al. Proanthocyanidins from Lotus corniculatus. Phytochemistry. 1996;41:617–624.
- Barrett MW, Klyne W, Scopes PN, et al. Plant procyanidins. Part 6. Chiroptical studies. Part 95. Circular dichroism of procyanidins. J Chem Soc Perkin Trans. 1979;1:2375–2377.
- Ito H, Li P, Koreishi M, et al. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014;152:323–330.
- Kato N, Kawabe S, Ganeko N, et al. Polyphenols from flowers of Magnolia coco and their anti-glycation effects. Biosci Biotech Biochem. 2017;81:1285–1288.
