ABSTRACT
The aim of this study was to investigate the effects and underlying mechanisms of calcitonin (CT) on interleukin 1 beta (IL-1β) stimulated human chondrocytes. IL-1β (5 ng/mL) was added into chondrocytes to establish osteoarthritis (OA) model in vitro. Different concentrations of CT (0.1, 0.5, 1, 5, 10 and 50 nM) were used for treating IL-1β stimulated chondrocytes. Cell viability of chondrocytes was measured by cell counting kit-8 (CCK8) method. Western blotting was performed to evaluate the expression of matrix metalloproteinases (MMP-13), tissue inhibitor of metalloproteinases 1 (TIMP-1), p50 and p38. CT inhibited MMP-13 expression and promoted TIMP-1 expression in the IL-1β stimulated human chondrocytes. The CT-mediated alteration of MMP-13/TIMP-1 ratio was partially attributed to the inactivation of the p50- nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway by suppressing p50 in IL-1β stimulated chondrocytes. CT might play a protective role in IL-1β stimulated OA model via p50-NF-κB pathway.
Abbreviations: CT: calcitonin; IL-1β: interleukin-1β; MMP-13: matrix metalloproteinases-13; TIMP-1: tissue inhibitors of metalloproteinases-1.
Graphical Abstract
CT regulates expression of MMP 13/ TIMP-1 via p50-NF-κB pathway.
Osteoarthritis (OA) is the most prevalent chronic joint disease, which is characterized by progressive degradation and destruction of cartilage matrix, bone remodeling, inflammation of synovium and ligaments, causing chronic pain, joint instability, stiffness, swelling, deformities, limited mobility and even disability [Citation1,Citation2]. According to statistics, the prevalence rate of OA in the population over the age of 60 has increased to 50% and the incidence of disability is up to 53%, resulting in serious individual and socioeconomic burdens [Citation3]. The therapeutic strategies for OA, including pharmacological treatments, non-pharmacological therapies and surgeries, are aimed at alleviating pain, reducing inflammation, improving joint function and correcting joint deformities [Citation4,Citation5]. The pharmacological agents can be divided into two categories: symptom modifying osteoarthritis drugs (SMOADs), such as analgesics and non-steroidal anti-inflammatory drugs (NSAIDs); disease modifying osteoarthritis drugs (DMOADs), such as chondroitin sulfate, glucosamine sulfate, bisphosphonates and strontium ranelate [Citation6]. Currently, there are still no effective drugs for reversing or suppressing destructive changes of OA and the studies of therapeutic molecules targeting chondrocytes or inflammatory factors have drawn increasing attentions [Citation7,Citation8].
Although the pathogenesis of OA is not fully elucidated, it is widely accepted that degradation and destruction of type II collagen caused by matrix metalloproteinases (MMPs) have been considered as the main mechanisms of occurrence and development of OA [Citation9]. MMPs are a family of zinc-dependent endopeptidases which can degrade the constituents of extracellular matrix (ECM) and basement membrane, such as aggrecan, collagen, elastin, fibronectin, gelatin and laminin [Citation10]. MMPs consist of more than 20 kinds of metalloproteinase members, including collagenases (MMP-1, -8, -13, -18), gelatinases (MMP-2, -9), stromelysins (MMP-3, -10), matrilysins (MMP-7, -26), membrane-type MMPs (MMP-14, -15, -16, -24, -17, -25), and other types (MMP-12, -19, -20, -21, -23, -27, -28) [Citation11]. Among them, MMP-13 plays an essential role in the pathogenesis of OA via degrading articular cartilage ECMs, especially type II collagen. There are four tissue inhibitors of metalloproteinases (TIMP-1, -2, -3 and -4) which primarily count for specific MMPs inhibition and TMIP-1 can endogenously inhibit degradation of articular cartilage ECMs via tightly binding to MMP-13 and down-regulating MMP-13 activity [Citation12,Citation13]. Moreover, interleukin-1 beta (IL-1β), which is a member of pro-inflammatory cytokines secreted by chondrocytes, can promote MMPs-mediated cartilage degradation via inducing production of cytokines, chemokines, prostanoids, or adipokines and down-regulating the expression of MMPs inhibitors [Citation14]. Therefore, MMP-13/TIMP-1 can become a potential therapeutic target for reversing or suppressing the occurrence and progression of OA.
Calcitonin (CT), secreted by thyroid gland parafollicular cells, can increase calcium/phosphate uptake and has been widely used in treating osteoporosis due to the anti-osteoclastic action and pain relieving effect [Citation15]. Recently, CT is considered to be the ideal treatment for the progression of OA and several preliminary researches suggest that CT treatment provides the protective effects on articular cartilage and subchondral bone via regulating calcium homeostasis, inhibiting bone-resorbing activity of osteoclasts and promoting bone-building activity of osteoblasts [Citation16]. CT treatment has significant chondroprotective protection potential and can be used to prevent and treat degenerative joint diseases. Evidence for expression of calcitonin receptor in articular chondrocytes and chondroprotection of CT may be involved in inhibiting MMP expression [Citation17]. However, the specific mechanism is still not fully understood. The aim of this study is to investigate the inhibitory effects of CT on OA progression and related mechanisms by using IL-1β stimulated human chondrocytes.
Materials and methods
Cell lines and main reagents
CT, recombinant human IL-1β, SN50 and SB203580 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Human chondrocytes were obtained from the Cell Applications (San Diego, CA, USA). They were cryopreserved in their second passage and resuscitated for using in the subsequent experiments at their third passage. Human chondrocytes were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 IU/mL penicillin and 100 µg/mL streptomycin. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Cell viability assay
The effect of CT on the viability of human chondrocytes was assessed by cell counting kit-8 (CCK8, Dojindo, Japan) according to the manufacturers’ instructions. Human chondrocytes were seeded in 96-well plates at a density of 1 × 104 cells/well. They were treated with CT at concentrations of 0.1, 0.5, 1, 5, 10 and 50 nM for 24 h. At the endpoint of treatment, 10 μL CCK8 solutions was added into the medium for 2 h and the optical density (OD) values of 450 nm were measured by using an enzyme-labeled meter (Thermo Fisher Scientific, Waltham, MA, USA).
Western blotting
Prior to the treatment, the human chondrocytes (1 × 105 cells/cm2) were starved for 24 h in DMEM without fetal bovine serum. Subsequently, the cells were pretreated with different concentrations of CT (0.1, 0.5, 1, 5, 10 and 50 nM) 24 h followed by adding IL-1β (5 ng/mL) into culture medium for 1 h. After treatment, human chondrocytes were washed with phosphate buffered saline and lysed for 30 min on ice by using lysis buffer (pH = 6.8, containing 10% glycerol, 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate, 62.5 mmol/L Tris-HCl) plus 1% protease inhibitor cocktail (Roche Molecular Diagnostics, Pleasanton, CA, USA). Subsequently, phosphorylated protein was extracted using the phosphorylated protein extraction kit (Roche Molecular Diagnostics, Pleasanton, CA, USA). The protein concentration was measured using a BCA protein assay (Thermo Fisher Scientific, Waltham, MA, USA). The protein sample was separated by using 10–12% sodium dodecyl sulphate-polyacrylamide (SDS-PAGE) gel electrophoresis, transferred onto polyvinylidene fluoride membranes, and blocked with 5% skimmed milk in TBS–Tween (0.2% Tween-20) for 2 h at 37°C, then incubated with primary antibodies, including anti-MMP-13 (polyclonal antibody, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-TIMP-1 (polyclonal antibody, 1:500, Santa Cruz Biotechnology, Inc.), anti-cleaved PARP (polyclonal antibody, 1:500, Santa Cruz Biotechnology, Inc.), anti-p50 (monoclonal antibody, 1:1,000, Santa Cruz Biotechnology, Inc.), and β-actin (monoclonal antibody 1:5,000, Santa Cruz Biotechnology, Inc.), overnight at 4°C. Membranes were washed with 1% TBS–Tween, incubated with the horseradish peroxidase (HRP)-labeled secondary antibodies at room temperature for 1 h and exposed with the enhanced chemiluminescence (ECL) chemiluminescent substrate kit (Beyotime Institute of Biotechnology, Shanghai, China). Immunoblots were analyzed with ChemiDoc XRS+ (Bio Rad, Berkeley, CA, USA) and densitometry data were analyzed by calculating the ratio of the gray values of MMP-13 and TIMP-1 to the gray value of β-actin.
Statistical analysis
All data in graphs are generated from at least three independent experiments. They were expressed as the mean ± standard deviation (SD) and analyzed by SPSS 19.0 (IBM Software, Armonk, NY, USA). One-way analysis of variance (ANOVA)-Bonferroni analysis was used for the comparison of mean values of different groups followed by checking Gaussian distributions. A value of P < 0.05 was considered to be statistically significant.
Results
Effect of CT on the proliferation of human chondrocytes
In order to determine the effect of CT on the proliferation of human chondrocytes, the chondrocytes were treated with different concentrations of CT (0.1, 0.5, 1, 5, 10 and 50 nM) along with 5 ng/mL of IL-1β and the cell viability were measured by CCK8 assay. After CT treatment at a dose of 0.1 nM to 50 nM, there were no significant differences in cell viability of human chondrocytes between groups (, P > 0.05).
Figure 1. Compared to non IL-1β stimulated human chondrocytes, CT has no significant effects on cell viability at concentrations (0.1, 0.5, 1, 5, 10 and 50 nM) after 24 h treatment in IL-1β stimulated human chondrocytes.
The values were presented as mean ± SD and there were no significant differences between each groups (P > 0.05). CT: calcitonin; IL-11β: interleukin-11β.
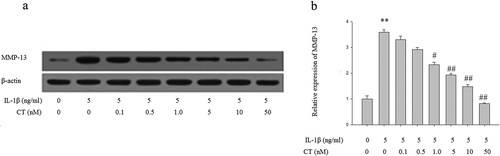
Effect of CT on expression of MMP-13 in IL-1β stimulated human chondrocytes
To investigate the effect of CT on MMP-13 expression in IL-1β stimulated human chondrocytes, the cells were pretreated with different concentrations of CT for 24 h and then 5 ng/mL IL-1β was added to the medium for 1 h. Subsequently, the expression of MMP-13 protein was analyzed by Western blotting (). The relative quantitation results of MMP-13 showed that IL-1β stimulated MMP-13 expression was suppressed by pretreatment of CT in a dose-dependent manner and the MMP-13 level significantly decreased with CT pretreatment at the doses of 1, 5, 10 and 50 nM (, P < 0.05).
Figure 2. CT inhibits expression of MMP-13 in IL-1β stimulated human chondrocytes.
(a) Western blotting analysis of MMP-13; (b) Relative quantitation of MMP-13 expression; ** P < 0.01 compared with the control group (** vs. *); # P < 0.05 compared with the IL-1β group (# vs. **); ##P < 0.01 compared with the IL-1β group (## vs.**); CT: calcitonin; IL-1β: interleukin-1β; MMP-13: matrix metalloproteinases-13.
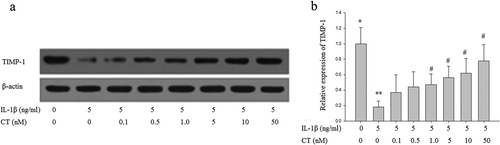
Effect of CT on expression of TIMP-1 in IL-1β stimulated human chondrocytes
To explore the role of CT on the expression of TIMP-1 in IL-1β stimulated human chondrocytes, chondrocytes were pretreated with different concentrations of CT 24 h then 5ng/mL IL-1β was added to the medium for 1 h. Subsequently, the expression of TIMP-1 protein was analyzed by Western blotting (). The relative quantitation of TIMP-1 expression results showed that IL-1β inhibited TIMP-1 expression was increased by pretreatment of CT in a dose-dependent manner and the TIMP-1 level significantly increased with CT pretreatment at the doses of 1, 5, 10 and 50 nM (, P < 0.05).
Figure 3. CT increases expression of TIMP-1 in IL-1β stimulated human chondrocytes. (a) Western blotting analysis of TIMP-1; (b) Relative quantitation of TIMP-1 expression; ** P < 0.01 compared with the control group (** vs. *); # P < 0.05 compared with the IL-1β group (# vs. **); CT: calcitonin; IL-1β: interleukin-1β; TIMP-1: tissue inhibitors of metalloproteinases-1.
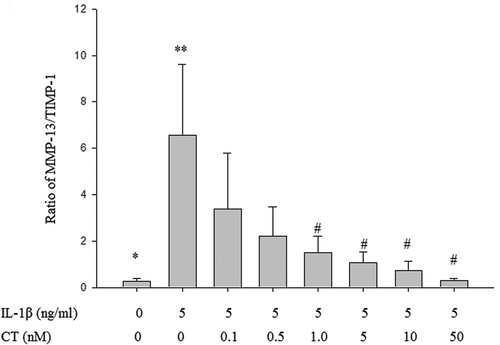
As shown in , the ratio of MMP-13/TIMP-1 in the IL-1β stimulated group was significantly higher than in the control group (** vs. *, P < 0.05). The ratio of MMP-13/TIMP-1 was decreased with CT pretreatment in a dose-dependent manner and the ratios of MMP-13/TIMP-1 at the CT doses of 1, 5, 10 and 50 nM were significantly decreased compared to non CT pretreated cells (## vs. **, , P < 0.05).
Figure 4. Effect of CT on the ratio of MMP-13/TIMP-1 in IL-1β stimulated human chondrocytes.
The ratio of MMP-13/TIMP-1 in the IL-1β stimulated group was significantly higher than in the control group (** vs. *, P < 0.05). The ratio of MMP-13/TIMP-1 at the CT doses of 1, 5, 10 and 50 nM were significantly decreased compared to non CT pretreated cells (## vs. **, , P < 0.05). CT: calcitonin; IL-1β: interleukin-1β; MMP-13: matrix metalloproteinases-13; TIMP-1: tissue inhibitors of metalloproteinases-1.
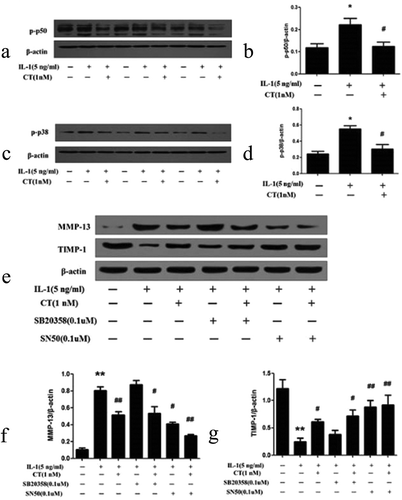
CT regulates expression of MMP 13/TIMP-1 via p50-NF-κB pathway
To unveil the mechanisms on the effect of CT on expression of MMP 13 and TIMP-1, human chondrocytes were pretreated with CT (1 nM) for 24 h and stimulated with IL-1β (5 ng/mL) for 1 h. The expression levels of p50 and p38 in the pretreated cells were examined by western blotting assay. As shown in , the expression level of p50 stimulated by IL-1β was significantly suppressed after pretreatment with 1 nM of CT (P < 0.05). As well, the p38 expression stimulated by IL-1β was significantly declined after pretreatment of 1nM CT (,, P < 0.05). Subsequently, human chondrocytes were pretreated with the P50 nuclear factor kappa-light-chain-enhancer of activated B cells (NF- κB) inhibitor SN50 (0.1 μM) and the mitogen-activated protein kinase (MAPK) inhibitor SB203580 (0.1 μM) for 1 h, followed by culturing with IL-1β (5 ng/mL) for 1 h.
Figure 5. CT regulates expression of MMP 13/TIMP-1 via p50-NF-κB pathway.
(a-b) Western blotting analysis and relative quantitation of p50 expression in IL-1β stimulated human chondrocytes; (c-d) Western blotting analysis and relative quantitation of p38 expression in IL-1β stimulated human chondrocytes; (e-g) Western blotting analysis and relative quantitation of MMP-13 and TIMP-1 expression in IL-1β stimulated human chondrocytes pretreated with SN50 (0.1 μM) or SB203580 (0.1 μM). (e) and (f) showed IL-1β significantly stimulated MMP-13 expression (*) compared to the control group (^) (P < 0.01), CT inhibited IL-1β stimulated high expression of MMP-13 ($) compared to the IL-1β stimulated cells (*) (P < 0.01). After adding SB203580, the MMP-13 expression unchanged (IL-1β-SB203580 treated cells ** vs. IL-1β treated cells * and IL-1β-CT-SB203580 treated cells $$ vs. IL-1β-CT treated cells $, P > 0.05). After adding SN50, IL-1β stimulated the high expression of MMP-13 was significantly decreased (IL-1β-SN50 treated cells # vs. IL-1β treated cells *, P < 0.05), and the expression level of MMP-13 was further significantly decreased (IL-1β-CT-SN50 treated cells ## vs. IL-1β-CT treated cells $, P < 0.05). (e) and (g) showed IL-1β significantly inhibited the expression of TIMP-1 (IL-1β treated cells * vs. the control cells ^, P < 0.01), CT enhanced the expression of TIMP-1 inhibited by IL-1β (IL-1β-CT treated cells # vs. IL-1β treated cells *, P < 0.05). SB203580 increased the expression of TIMP-1 inhibited by IL-1β, but no statistical difference (IL-1β-SB203580 treated cells $ vs. IL-1β treated cells * and IL-1β-CT-SB203580 treated cells $$ vs. IL-1β-CT-SN50 treated cells #, P > 0.05). The expression of TIMP-1 inhibited by IL-1β increased significantly after use of SN50 (IL-1β-SN50 treated cells # vs. IL-1β-treated cells * and IL-1β-CT-SN50 treated cells ** vs. IL-1β-SN50 treated cells #, P < 0.01). CT regulates expression of MMP 13/TIMP-1 via p50-NF-κB pathway.
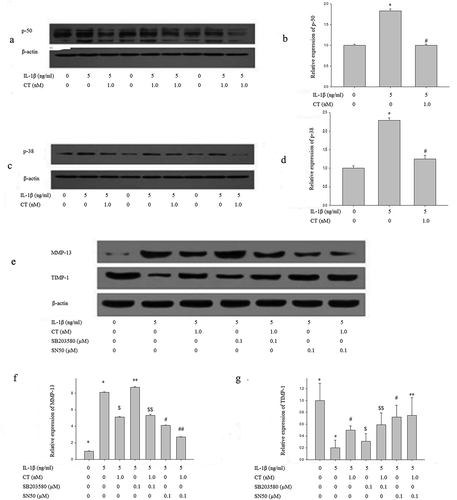
For MMP-13 expression (,), IL-1β significantly stimulated MMP-13 expression when compared to the control group (* vs. ^, P < 0.01)), and CT inhibited IL-1β stimulated high expression of MMP-13 ($ vs. *, P < 0.01). After adding SB203580 (**), IL-1β stimulated high MMP-13 expression was not affected (IL-1β-SB203580 treated cells ** vs. IL-1β-treated cells * and IL-1β-CT-SB203580 treated cells $$ vs. IL-1β-CT treated cells $, P > 0.05). After adding SN50, IL-1β stimulated the high expression of MMP-13 was significantly decreased when compared to SN50 treated group (IL-1β-SN50 treated cells # vs. IL-1β treated cells *, P < 0.05). This inhibition was more obvious in CT-pretreated cells (IL-1β-CT-SN50 treated cells ##), and its expression level of MMP-13 was further significantly decreased compared to non-SN50 treated cells (IL-1β-CT treated cells $) (P < 0.05). CT and SN50 appear to have synergistic effects, inhibiting P50 NF-κB pathway and decreasing MMP-13 expression.
For TIMP-1 expression (,), IL-1β significantly inhibited the expression of TIMP-1 when compared to the control group (^) (IL-1β treated cells * vs. THE the control group ^, P < 0.01), and CT enhanced the expression of TIMP-1 inhibited by IL-1β (IL-1β-CT treated cells # vs. IL-1β treated cells *, P < 0.05). After the addition of SB203580, the expression of TIMP-1 inhibited by IL-1β was increased, but there were no statistical differences between IL-1β-SB203580 treated cells $ vs. IL-1β treated cells *, and between IL-1β-CT-SB203580 treated cells $$ and IL-1β-SN50 treated cells # (P > 0.05). However, the expression of TIMP-1 inhibited by IL-1β was increased significantly after addition of SN50 (IL-1β-SN50 treated cells # vs. IL-1β treated cells * and IL-1β-CT-SN50 treated cells ** vs. IL-1β-SN50 # treated cells, P < 0.01).
Discussion
CT is a hormone secreted by the C-cells of the thyroid gland in response to elevations of the plasma calcium level. As calcitonin is a peptide, the traditional method of administration has been parenteral or intranasal. Following the oral administration, time to maximum concentration is about 15 minutes and half-life is between 9 and 15 minutes. Calcitonin receptors have been identified on the surface of articular chondrocytes, and it has been shown to exert a protective effect on cartilage degradationas measured by changes in C-terminal cross-linked telopeptide of type II collagen, which is present in cartilage [Citation17,Citation18].
In this study, we demonstrated that IL-1β stimulated MMP-13 expression was suppressed and the IL-1β inhibited TIMP-1 expression was increased by pretreatment of CT in vitro. Moreover, our study indicated that the ratio of MMP-13/TIMP-1 was reversed by CT treatment. Furthermore, we found that CT exhibited the inhibitive effect on MMP-13 expression and promoting effect on TIMP-1 expression via p50-NF-κB pathway.
It has been widely recognized that MMP-13 is a primary collagenase in OA and the expression of MMP-13 increases in OA cartilage [Citation19]. Since MMP-13 exhibits 5–10 folds more effectiveness in degrading type II collagen than other MMPs, MMP-13 is the most important therapeutic target of OA [Citation20]. Currently, MMP-13 has been confirmed that specifically degrades type II collagen which is the main component of ECM in cartilage [Citation21]. Liu et al. [Citation22] reported that Ghrelin reduced IL-1β-induced expression of MMP-3/-13 and ameliorated IL-1β-induced degradation of type II collagen and aggrecan, indicating that inhibition of MMP-13 could relieve symptoms and delaying progression of OA. Moreover, TIMP-1 can endogenously inhibit degradation of articular cartilage ECMs via tightly binding to MMP-13 and down-regulating MMP-13 activity [Citation12,Citation13]. Therefore, the imbalance in the activities of MMP-13 and TIMP-1 has been considered as a critical role in OA progression. In this study, we confirmed that CT significantly inhibited MMP-13 expression and promoted TIMP-1 expression in IL-1β stimulated chondrocytes. Similarly, Kobayashi et al [Citation23] reported that IL-1β up-regulated expression level of MMP-13 and promoted the degradation of aggrecan and collagen.
CT, a 32-amino acid polypeptide hormone secreted by thyroid gland parafollicular cells, can increase calcium/phosphate uptake and has been widely used in treating osteoporosis due to the anti-osteoclastic action and pain relieving effect [Citation15]. Recently, CT has been regarded as an ideal treatment against the OA progression. Preliminary study showed that CT treatment provides the protective effects on articular cartilage and subchondral bone via regulating calcium homeostasis, inhibiting bone-resorbing activity of osteoclasts and promoting bone-building activity of osteoblasts [Citation16]. Sondergaard et al. [Citation17] indicated that CT could inhibit the expression of MMPs in the articular cartilage and exert the chondro-protective effect. Moreover, Greco et al. [Citation24] found that CT treatment increased proteoglycan and collagen synthesis in human OA cartilage and subchondral bone. In the present study, we demonstrated that CT treatment potently down-regulates MMP-13 and up-regulates TIMP-1 expression in IL-1β stimulated human chondrocytes.
Previous studies showed that NF-κB signaling pathway and MAPK signaling pathway were involved in the proliferation, differentiation and apoptosis of chondrocytes [Citation25]. IL-1β induces activation of key enzymes in NF-κB and MAPK signaling pathways, including p50, p52, Rel, Rel A, Rel B,p38, c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) [Citation25–Citation27]. Gu et al [Citation28] reported that stimulation of the chondrocytes with IL-1β resulted in a significant upregulation of toll-like receptor 4 (TLR4) and downstream targets of both TLR4/myeloid differentiation primary response 88 (MyD88)-dependent and -independent signaling pathways which were associated with the synthesis of MMP-13 and IL-6. Kim et al. [Citation29] reported that caffeic acid, S-allyl cysteine, and uracil significantly inhibited the degradation of type І procollagen and the expressions of MMPs via NF-κB signaling. In this study, we showed that CT selectively blocked IL-1β induced p50 (NF-κB) activation and CT protection of IL-1β-stimulated chondrocytes may involve inhibition of CT-mediated p50 phosphorylation. Zhang et al. [Citation30] reported that suppressing p38 MAPK activity could ameliorate cartilage degeneration. However, our study did not confirm the inhibitory effect of CT-inhibited p38 expression on the p38-MAPK signaling pathway. Therefore, the mechanism of CT affecting MMP-13 and TIMP-1 requires more extensive research.
In conclusion, in IL-1β-stimulated human chondrocytes, CT inhibits MMP-13 expression and promotes TIMP-1 expression via the p50-NF-κB pathway, which has protective effects on human chondrocytes. CT has the potential to be a new approach to treating OA, delaying or reversing the progression of OA.
Authors’ Contribution
Xiaodong Bai was carried out the literature research, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation and manuscript preparation; Ai Guo was dedicated to the the entire study and study design; Yadong Li was involved in the definition of intellectual content and manuscript review. All authors have read and approved this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126.
- Blaney Davidson EN, van Caam AP. and van der Kraan PM, Osteoarthritis year in review 2016: biology. Osteoarthritis Cartilage. 2017;25:175–180.
- Tang X, Wang S, Zhan S, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol. 2016;68:648–653.
- Miller RE, Block JA, Malfait AM. What is new in pain modification in osteoarthritis? Rheumatology. 2018;57:99–107.
- Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26:319–325.
- Martel-Pelletier J, Wildi LM, Pelletier JP. Future therapeutics for osteoarthritis. Bone. 2012;51:297–311.
- Wieland HA, Michaelis M, Kirschbaum BJ, et al. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344.
- DiDomenico CD, Lintz M, Bonassar LJ. Molecular transport in articular cartilage - what have we learned from the past 50 years? Nat Rev Rheumatol. 2018.
- Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145.
- Li NG, Tang YP, Duan JA, et al. Matrix metalloproteinase inhibitors: a patent review (2011–2013). Expert Opin Ther Pat. 2014;24:1039–1052.
- Xie XW, Wan RZ, Liu ZP. Recent research advances in selective matrix metalloproteinase-13 inhibitors as anti-osteoarthritis agents. ChemMedChem. 2017;12:1157–1168.
- Winum JY, Scozzafava A, Montero JL, et al. The sulfamide motif in the design of enzyme inhibitors. Expert Opin Ther Pat. 2006;16:27–47.
- Dzwonek J, Rylski M, Kaczmarek L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 2004;567:129–135.
- Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15:375.
- Chesnut CH, Azria M, Silverman S, et al. Salmon calcitonin: a review of current and future therapeutic indications. Osteoporos Int. 2008;19:479–491.
- Karsdal MA, Sondergaard BC, Arnold M, et al. Calcitonin affects both bone and cartilage: a dual action treatment for osteoarthritis? Ann N Y Acad Sci. 2007;1117:181–195.
- Sondergaard BC, Wulf H, Henriksen K, et al. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthr Cartil. 2006;14:759–768.
- Karsdal MA, Tanko LB, Riis BJ, et al. Calcitonin is involved in cartilage homeostasis: is calcitonin a treatment for OA? Osteoarthritis Cartilage. 2006;14:617–624.
- Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733.
- Wu Y, Wu T, Xu B, et al. Oxytocin prevents cartilage matrix destruction via regulating matrix metalloproteinases. Biochem Biophys Res Commun. 2017;486:601–606.
- Liang Y, Duan L, Xiong J, et al. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. 2016;18:105.
- Liu J, Cao L, Gao X, et al. Ghrelin prevents articular cartilage matrix destruction in human chondrocytes. Biomed Pharmacother. 2018;98:651–655.
- Kobayashi M, Squires GR, Mousa A, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135.
- Sondergaard BC, Madsen SH, Segovia-Silvestre T, et al. Investigation of the direct effects of salmon calcitonin on human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2010;11:62.
- Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8:305–313.
- Ji B, Guo W, Ma H, et al. Isoliquiritigenin suppresses IL-1β induced apoptosis and inflammation in chondrocyte-like ATDC5 cells by inhibiting NF-κB and exerts chondroprotective effects on a mouse model of anterior cruciate ligament transection. Int J Mol Med. 2017;40:1709–1718.
- Luo M, Hu L, Li D, et al. MD-2 regulates LPS-induced NLRP3 inflammasome activation and IL-1 beta secretion by a MyD88/NF-κB-dependent pathway in alveolar macrophages cell line. Mol Immunol. 2017;90:1–10.
- Gu H, Jiao Y, Yu X, et al. Resveratrol inhibits the IL-1β-induced expression of MMP-13 and IL-6 in human articular chondrocytes via TLR4/MyD88-dependent and -independent signaling cascades. Int J Mol Med. 2017;39:734–740.
- Kim SR, Jung YR, An HJ, et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS One. 2013;8:e73877.
- Zhou Y, Ming J, Li Y, et al. Surfactant protein D attenuates nitric oxide-stimulated apoptosis in rat chondrocyte by suppressing p38 MAPK signaling. Biochem Biophys Res Commun. 2018;495:526–532.
