ABSTRACT
Replication protein A (RPA) is an essential component of DNA metabolic processes. RPA binds to single-stranded DNA (ssDNA) and interacts with multiple DNA-binding proteins. In this study, we showed that two DNA polymerases, PolB and PolD, from the hyperthermophilic archaeon Thermococcus kodakarensis interact directly with RPA in vitro. RPA was expected to play a role in resolving the secondary structure, which may stop the DNA synthesis reaction, in the template ssDNA. Our in vitro DNA synthesis assay showed that the pausing was resolved by RPA for both PolB and PolD. These results supported the fact that RPA interacts with DNA polymerases as a member of the replisome and is involved in the normal progression of DNA replication forks.
Graphical Abstract
A model for the function of RPA in the progression of T. kodakarensis DNA replication.
DNA replication is a highly regulated event, in which multiple DNA-binding proteins function together to synthesize nascent DNA strands according to the template DNA sequences. The bacterial single-stranded binding protein (SSB) and the eukaryotic and archaeal replication protein A (RPA) are hub proteins in the DNA transaction machinery critical in protecting transiently formed single-stranded DNA (ssDNA) during the process to unwind double-stranded DNA (dsDNA), detect DNA damage, and recruit repair proteins [Citation1,Citation2]. Although there is little amino acid sequence similarity between SSB and RPA, these DNA-binding proteins have structurally similar domains [Citation3–Citation5], called the oligonucleotide/oligosaccharide-binding (OB)-fold domains [Citation6]. The common structure in SSB and RPA suggests that the mechanism of single-stranded nucleic acid binding is conserved in living organisms. The subunit composition of SSB proteins varies over different domains of life. In Bacteria, Escherichia coli SSB is a homotetramer of a 20-kDa protein with one OB-fold domain [Citation7], but the SSBs from Deinococcus radiodurans and Thermus aquaticus have been reported to utilize the tetrameric binding mode by combining two SSB homodimers [Citation8]. In contrast, the eukaryotic RPA is a stable heterotrimer [Citation9], composed of 70-, 32-, and 14-kDa proteins. RPA70 contains two tandem repeats of an OB-fold domain, which are responsible for a major interaction with ssDNA in its central region. The N- and C-terminal regions of RPA70 mediate interactions with many cellular or viral proteins, in addition to RPA32 [Citation10,Citation11]. The middle subunit, RPA32, contains an OB-fold domain in the central region [Citation5,Citation12,Citation13], and the C-terminal region of RPA32 interacts with other RPA subunits and various cellular proteins [Citation10,Citation11,Citation14,Citation15]. The smallest subunit, RPA14, also contains an OB-fold [Citation5].
Bacterial and eukaryotic organisms contain one type of SSB or RPA, respectively. In contrast, archaeal organisms have various RPAs, composed of different organizations of OB-fold domains. RPAs from thermophilic methanogen Methanocaldococcus jannaschii and Methanothermobacter thermoautotrophicus have been reported as the first archaeal ssDNA-binding proteins, containing four or five repeated OB-fold domains [Citation16–Citation18]. The M. thermoautotrophicus RPA interacts with the cognate family B DNA polymerase (PolB, described below) during DNA replication [Citation18]. While euryarchaeal organisms have a eukaryotic-type RPA homolog, the crenarchaeal SSB proteins appear to be much more similar to the bacterial proteins, with a single OB-fold domain and a flexible C-terminal tail [Citation19]. We characterized RPA from the hyperthermophilic archaeaon, Pyrococcus furiosus (PfuRPA), earlier [Citation20]. Similar to the eukaryotic RPA in subunit organization, PfuRPA forms a complex consisting of three distinct subunits: RPA41, RPA32, and RPA14. The methanogenic archaeon, Methanosarcina acetivorans, also possesses three different RPAs, but they do not interact with each other and appear to function as homodimers [Citation21]. Each of the three RPAs, as well as their combinations, clearly stimulates the primer extension activity of M. acetivorans PolB. Structural analyses of the architectures of SSB and RPA suggested that they are composed of different combinations of the OB-fold domain. The distributions of SSB and RPA homologs in all archaeal genomes are reviewed in [Citation22].
Archaeal DNA polymerases are also diversified and classified by amino acid sequence similarity, and seven families, A, B, C, D, E, X, and Y, are now recognized widely [Citation23–Citation25]. Euryarchaeal genomes encode a family D DNA polymerase (PolD), which was originally discovered in P. furiosus without any sequence homology, by screening for DNA polymerase activity in the cell extract [Citation26,Citation27]. PolD consists of two proteins, named DP1 and DP2. P. furiosus PolD exhibits efficient strand-extension activity and strong proofreading activity. Bioinformatics analysis also indicated that eukaryotic DNA polymerases have a highly complex relationship with their archaeal ancestors, including contributions of proteins and domains from both PolB and PolD [Citation28]. It is critical to elucidate whether PolB and PolD work together at the replication fork to synthesize the leading and lagging strands, respectively. During lagging-strand synthesis, short Okazaki fragments must be processed together by the removal of RNA primers at the 5′ end of each fragment, filling the gap, and ligating the DNA fragments together. In Archaea, Okazaki fragments are short (100–150 nucleotides), and thus, over 14,000 Okazaki fragments are synthesized during Pyrococcus and Sulfolobus genome replications [Citation29]. In the Okazaki fragment maturation in Thermococcus species 9°N, PolB may synthesize the lagging-strand in its entirety [Citation30]. However, PolB extends RNA primers poorly and lacks interactions with core replisome components [Citation31,Citation32]. A genetic study of the halophilic archaeon, Halobacterium sp. NRC-1, showed that both PolB and PolD are essential for viability [Citation33]. However, in the hyperthermophilic archaeon, Thermococcus kodakarensis, PolB is not required for the normal replication [Citation34]. On the other hand, PolB is suggested to play a role in gap filling and strand displacement (SD) synthesis during Okazaki fragment maturation, and PolD may catalyze lagging-strand synthesis [Citation32,Citation35–Citation37]. The crenarchaeal species lack PolD, but possess at least one additional active PolB, suggesting that the two distinct PolBs specialize in the leading- and lagging-strand replication, as is the case in eukaryotes.
Based on the knowledge described above, it is still unknown how the archaeal RPAs function in replication fork progression. In this study, we prepared the RPA proteins and DNA polymerases from T. kodakarensis to homogeneity and characterized them in vitro. We found that the T. kodakarensis RPA binds directly to DNA polymerases and functions to help DNA synthesis reaction in vitro.
Materials and methods
Preparation of T. kodakarensis proteins from recombinant E. coli
The recombinant RPA complex (TkoRPA) encoded by TK_RS09810–TK_RS09820, TkoPolB from TK_RS00010, TkoPolD from TK_RS09520 (DP1) and TK_RS09525 (DP2), TkoMCM (Mcm3) from TK_RS08085, TkoGINS from TK_RS02640 (Gins51) and TK_RS08080 (Gins23), and TkoGAN from TK_RS06185 were prepared according to procedures described previously [Citation38–Citation40]. The genes encoding RPA41 (TK_RS09820), RPA14 (TK_RS09815), RPA32 (TK_RS09810), RadA (TK_RS09505), and Primase (PriS, TK_RS08960; PriL, TK_RS08965) were amplified from T. kodakarensis genomic DNA, using the primers listed in the supplementary information, Table S1. Each fragment was amplified by Pfu DNA polymerase (Agilent), digested by the corresponding restriction enzymes (New England Biolabs), and ligated by T4 DNA ligase (New England Biolabs) into the corresponding sites of the pET-21a (RPA41, RPA14, RPA32, RadA) and pET-Duet-1 (PriS and PriL) expression vectors (Novagen). The resultant plasmids were designated as pET-TkoRPA41, pET-TkoRPA14, pET-TkoRPA32, pET-TkoRadA, and pET-TkoPrimase, respectively. The nucleotide sequences were confirmed by sequencing using CEQ2000XL (Beckman Coulter). The expression and purification of TkoRPA41, TkoRPA14, TkoRPA32, TkoRadA, and TkoPrimase in T. kodakarensis were performed in a manner similar to those from P. furiosus [Citation20,Citation41]. Briefly, TkoRPA41, TkoRPA14, TkoRPA32, TkoRadA, and TkoPrimase were overproduced in E. coli BL21-CodonPlus (DE3)-RIL cells (Agilent). The proteins were purified by heat treatment at 80°C for 20 min and sequential chromatography on HiTrap Phenyl HP and HiTrap Q HP columns for TkoRPA41, TkoRPA14, and TkoRPA32; HiTrap Phenyl HP, HiTrap Heparin HP, and HiTrap Q HP columns for TkoRadA; and HiTrap Butyl-S FF, HiTrap Heparin HP, and Mono Q HR 5/5 columns for TkoPrimase. The RPA complex was isolated by a gel filtration chromatography using a Superdex200 3.2/30 column with a buffer containing 50 mM Tris–HCl, pH 8.0; 1 mM dithiothreitol (DTT); and 1 M NaCl. The above-mentioned columns were purchased from GE Healthcare. The purified proteins were quantified by measuring the absorbance at 280 nm, with theoretical extinction coefficients of 38,850, 6,990, 32,890, 14,440, and 107,150 M−1cm−1 for TkoRPA41, TkoRPA14, TkoRPA32, TkoRadA, and TkoPrimase, respectively, based on tryptophan and tyrosine contents [Citation42].
Cell cultivation
T. kodakarensis KOD1 cells were cultivated under anaerobic conditions at 85°C for 5 h (exponential growth phase, A600 = 0.3) in nutrient-rich medium (ASW-YT-Pr), in basically the same manner as previously described [Citation39,Citation43]. ASW-YT-Pr medium was composed of 0.8 × artificial seawater, 5.0 g/L yeast extract (Difco), 5.0 g/L tryptone (Difco), and 5.0 g/L sodium pyruvate (nacalai tesque). The harvested T. kodakarensis cells were suspended and disrupted in lysis buffer (50 mM Tris–HCl, pH 8.0, 50 mM NaCl, 0.5 mM DTT, and 0.1 mM EDTA) by sonication.
Detection of TkoRPA32 in the recombinant TkoRPA complex and cells
The cell extracts (1.6 × 105, 8 × 105, 4 × 106, 2 × 107 cells) and recombinant TkoRPA complex (20 fmol) were loaded to SDS-17.5% PAGE, and proteins on the gel were electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) using a Trans-Blot Turbo Transfer System (Bio-Rad), and reacted with the anti-PfuRPA32 antiserum, prepared by recombinant PfuRPA32 [Citation20]. Anti-Rabbit IgG HRP (Rabbit TrueBlot, Rockland Immunochemicals, Inc.) was used as the secondary antibody. The proteins were visualized by an enhanced chemiluminescence system (Millipore), and images were obtained and quantified with an LAS-3000 image analyzer (Fujifilm).
Native PAGE analysis of TkoRPA proteins
TkoRPA41 (20 pmol for CBB staining, 40 fmol for western blot analysis), TkoRPA14 (40 pmol for CBB staining, 80 fmol for western blot analysis), TkoRPA complex (10 pmol for CBB staining, 20 fmol for western blot analysis) were fractioned by 5% native PAGE in 1 × TBE buffer (89 mM Tris, 89 mM boric acid, and 2.5 mM EDTA, pH 8.3), followed by CBB staining or western blot analysis.
DNA-binding assays for TkoRPA proteins
DNA substrates were obtained and prepared as described before [Citation39]. Various concentrations (2, 5, 10, 50, 200, and 1,000 nM for RPA complex and RPA41 as the complex and the monomer, respectively; 10, 100, and 1,000 nM for RPA14 as the monomer) of the proteins were incubated with 5 nM 5′-Cy5-labeled ssDNA (45 nt, 5′-CGAACTGCCTGGAATCCTGACGACATGTAGCGAACGATCACCTCA-3′) and dsDNA (45 bp, 5′-Cy5-labeled ssDNA annealed with its complementary strand) at 50 ̊C for 15 min in a reaction solution (25 mM Tris–HCl, pH 8.0; 125 ng/µL BSA; 1 mM DTT; and 5 mM MgCl2). The protein–DNA complexes were fractionated by 6% native PAGE in a buffer containing 4 mM Tris–acetate, 0.1 mM EDTA, and visualized with a Typhoon Trio+ imager (GE Healthcare).
EMSA for the interaction between DNA-bound TkoRPA complex to TkoPolB or TkoPolD
TkoRPA complex was incubated with 10 nM 5′-Cy5-labeled ssDNA (same as in DNA binding assays) at 50 ̊C for 15 min in a reaction solution (25 mM Tris–HCl, pH 8.0; 125 ng/µL BSA; 1 mM DTT; and 5 mM MgCl2), and then added TkoPolB or TkoPolD, followed by further incubation at 50°C for 10 min. The protein–DNA complexes were fractionated by 1% AGE in 0.5 × TBE buffer, and visualized with a Typhoon Trio+ imager.
Primer extension analysis of TkoPolB and TkoPolD
The primer extension activities of TkoPolB and TkoPolD at various concentrations (0, 40, 200, and 800 nM) of TkoRPA complex or (0, 200, 800, and 2000 nM) of TkoRPA41 were detected directly using alkaline agarose gel electrophoresis. As the template primer substrate for one reaction, 2 nM of 5′-Cy5-labeled primer (5′-TCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTC-3′) was annealed to 1 nM (7.2 µM in nucleotide) of M13 mp18 ssDNA by heating the mixture in a solution containing 25 mM Tris–HCl, pH 8.0, and 50 mM NaCl at 98°C for 3 min, followed by the gradual cooling of the mixture to room temperature (the template was regarded as 1 nM primed M13 ssDNA template). The DNA polymerization reaction was performed at 55°C for 4, 8, 16, and 24 min in the reaction mixture (30 µl) containing 1 × Cloned Pfu DNA polymerase buffer (Agilent), 1 nM primed M13 ssDNA template, 1 mM DTT, 0.1 mM of each dNTP, various concentrations of TkoRPA, and the polymerases (1 nM of TkoPolB or 2 nM of TkoPolD). Twelve microliters of the reaction mix was added to 3 µL of stop solution (300 mM NaOH, 6 mM EDTA, 18% Ficoll, and 0.2% OrangeG). The reaction products (8 µL) were analyzed on a 1.5% alkaline agarose gel in 50 mM NaOH and 1 mM EDTA, and then detected using a Typhoon Trio+ imager.
Detection and analysis of protein–protein interactions by native PAGE analysis
Purified TkoPolD (25 pmol), TkoPrimase (25 pmol), TkoPolB (25 pmol), TkoRadA (24 pmol), TkoMCM (9 pmol), TkoGINS (27 pmol), and TkoGAN (27 pmol) were incubated at 50°C for 5 min with 10 pmol of TkoRPA complex in a 10-µL solution containing 25 mM Tris–HCl, pH 8.0; 100 mM NaCl; 0.1% Triton X-100; 1 mM DTT; and 10% glycerol. The protein–protein complexes were fractionated by 5% native PAGE in 1 × TBE buffer, followed by CBB staining.
Results
Purification of recombinant TkoRPA proteins
The genes encoding RPA41, RPA14, and RPA32 are tandemly arranged in the T. kodakarensis genome and seem to be an operon ()), as observed in P. furiosus [Citation20]. As shown in the Supplementary information, Figure S1, TkoRPA and PfuRPA showed high sequence similarities (percent identity matrixes of RPA32, 41, and 14, created by CLUSTAL 2.1 [Citation44], were 73.68%, 66.12%, and 61.36%, respectively). The recombinant RPA41 and RPA14 were successfully overproduced as soluble proteins by cultivating E. coli cells bearing the expression plasmids, pET-TkoRPA41 and pET-TkoRPA14, respectively, as described in the Materials and Methods. However, the production of soluble TkoRPA32 was not successful, probably because TkoRPA32 alone is unstable without forming complex with RPA41 and RPA14.
Figure 1. Preparation of RPA complex from Thermococcus kodakarensis (TkoRPA). (a) Schematic map of the gene organization at the operon of rpa41-rpa14-rpa32 in the T. kodakarensis genome. (b) Purified recombinant TkoRPA14 (0.5 μg, lane 1), TkoRPA41 (0.5 µg, lane 2), and TkoRPA complex (2 μg, lane 4) proteins were subjected to SDS −17.5% PAGE followed by CBB staining. Protein size markers were run in lane 3, and their sizes are indicated on the left of the gels. (c) Recombinant RPA complex (20 fmol, lane 1, indicating “r” at the top of the panel) and the cell extracts (1.6 × 105, 8 × 105, 4 × 106, and 2 × 107 cells in lanes 2–5) were loaded to SDS-17.5% PAGE, followed by western blot analysis. The anti-PfuRPA32 antiserum was used to detect TkoRPA32 in the TkoRPA complex or in the cells. Asterisk (*) may indicate the degradation product of TkoRPA32 in the cell. (d) The properties of the purified TkoRPA41 (lane 1), TkoRPA14 (lane 2), and TkoRPA complex (lane 3) in 8% native PAGE run in TBE followed by CBB staining (left of the panel) and western blot analysis (right of the panel), using anti-PfuRPA32 antiserum.
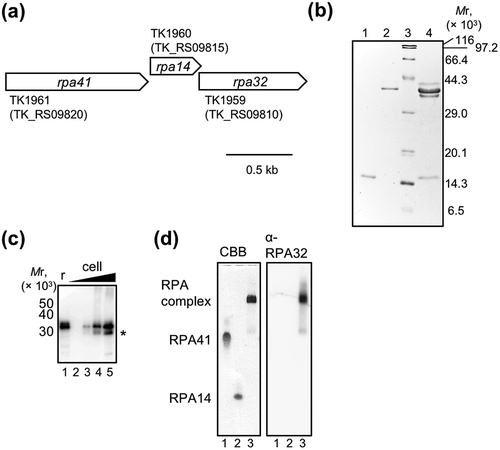
The relative molecular mass of TkoRPA32, calculated from the deduced amino acid sequence, is 31,259.6, but its CBB-stained band appeared at the position corresponding to a higher molecular weight (near that of TkoRPA41 in )). A similar phenomenon was observed in RPA32 from P. furiosus [Citation20]. These unexpected electrophoretic mobilities of RPA32 were probably due to the existence of intrinsically disordered regions (IDRs) in RPA32. In DisEMBL (http://dis.embl.de/), Rem 465 suggested that TkoRPA32 actually contains an IDR at the C-terminus (189–208 residues) (Supplementary information, Figure S2) [Citation45,Citation46]. The human RPA32 also has a dynamically disordered region, although it is in the N-terminal region [Citation47].
As shown in ), we detected the presence of TkoRPA32 in the recombinant TkoRPA complex and T. kodakarensis cells by western blot analysis using anti-PfuRPA32 antiserum prepared by purified P. furiosus RPA32. We also found the single bands of TkoRPA41, TkoRPA14, and TkoRPA complex at different positions in a native PAGE, indicating these proteins are stable and each exists a single state in solution ()). Western blot analysis of this gel using the anti-RPA32 antiserum showed that the positive band of TkoRPA32 was detected only in the lane of the TkoRPA complex.
DNA-binding properties of TkoRPAs
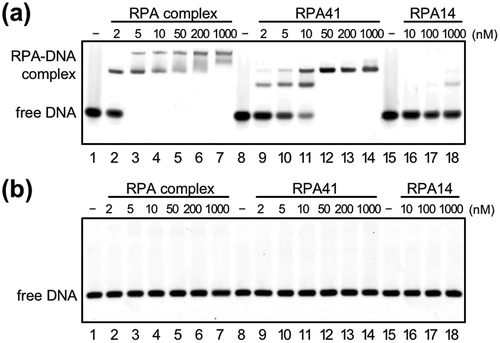
To investigate whether TkoPRA binds to ssDNA, a gel retardation assay was performed using Cy5-labeled 45-nt ssDNA and dsDNA. As shown in , the TkoRPA complex specifically bound ssDNA. The all of the DNA probe (5 nM) were shifted by binding of TkoRPA complex (5 nM) (), lane 3). RPA41 alone also bound ssDNA, with less affinity to the three-subunit complex but free DNA disappeared by binding of 50 nM TkoRPA41 to 5 nM DNA (), lane 12). The ssDNA-binding affinity of the TkoRPA complex seems to be about ten times higher than that of TkoRPA41 alone (), comparing lanes 2–4 to 9–11). Two shifted DNA bands were observed in a concentration-dependent manner from both the TkoRPA complex and TkoRPA41, suggesting that one and two molecules of RPAs bound to the ssDNA, and two stable conformations existed for the DNA-RPA complex. TkoRPA14 hardly bound ssDNA or dsDNA (,b), lanes 15–18). These data clearly showed that the complex formation of the three TkoRPA subunits is important for efficient ssDNA-binding. In general, RPA/SSB proteins form several types of DNA-binding modes using multiple OB-fold domains in vitro. Deinococcus/Thermus SSBs utilize the tetrameric functional binding mode [Citation8]. Human RPA binds to ssDNA in at least three different modes [Citation9,Citation21,Citation48], whereas two transitions in human RPA architecture were observed combining small-angle X-ray and neutron scattering [Citation47]. Human mitochondrial SSB (HmtSSB) also formed a two-binding mode [Citation49]. On the other hand, the crenarchaeal SSB, which has a bacteria-like domain structure with a single OB-fold domain, followed by a flexible C-terminal tail that is not involved in DNA-binding, coats ssDNA with a stoichiometry of approximately five nt DNA per SSB molecule [Citation50].
Figure 2. DNA-binding properties of TkoRPA.
Electrophoretic mobility shift assay (EMSA) of TkoRPA complex, TkoRPA41, and TkoRPA14 were performed with 5 nM Cy5-labeled ssDNA (a) and dsDNA (b). DNA and various concentrations of TkoRPAs were incubated at 50°C for 15 min. The band assignments are indicated on the side of the panels.
Effects of RPA on PolB and PolD polymerase activities
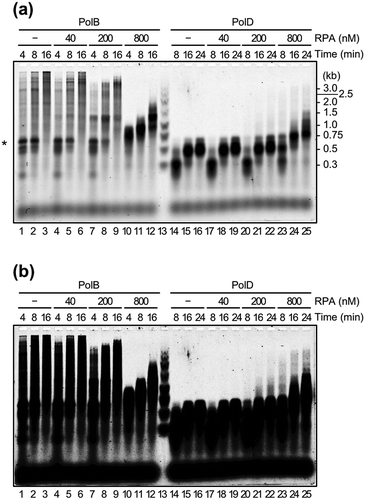
DNA synthesis assays for the two DNA polymerases, PolB and PolD, from T. kodakarensis were carried out using M13 ssDNA as template DNA, which contains sequences tend to form a secondary structure. A 5′-labeled primer was extended on the M13 ssDNA. As shown in (lanes 1, 2, 14, and 15), accumulation of the products with 0.6–0.7 kb, which is probably from pausing at specific site, was observed from both PolB and PolD reactions (indicated by asterisk). The pausing was relieved by high concentration (800 nM) of TkoRPA for both PolB and PolD (, lanes 10–12 for PolB and 22–25 for PolD). In the case of PolB, the extension was partially processed with or without RPA, probably due to the resolution of the secondary structure of the DNA template by the intrinsic SD activity of PolB. High concentration of TkoRPA (800 nM) relieved the pausing, but the products became shorter, suggesting that ssDNA-bound RPA inhibited the processivity of PolB (lanes 10–12). On the other hand, the DNA synthesis reaction by PolD was strictly stopped at the pausing site with or without RPA. However, the pausing was also relieved with TkoRPA at 800 nM (lanes 23–25). PolD stopped at around 0.3 kb via the 8-min reaction with or without RPA (lanes 14, 17, and 20). These data suggested that there may be another pausing site for PolD. The pausing was also relieved with TkoRPA at 800 nM (comparing lanes 14 and 23). The relief of pausing was also found by using TkoRPA41 (Supplementary information, Figure S3(a)), although the inhibitions of processivity of PolB by TkoRPA41 was not observed even if containing high amount of TkoRPA41 (2000 nM). However, as shown in Supplementary information, Figure S3(b), we did not detect the stable complex between RPA41 and PolB nor PolD, indicating that RPA41 also can resolve the secondary structure of DNA, but the complex formation of RPA is necessary to stable interaction to DNA polymerases, and the DNA-TkoRPA complex is more stable than that of TkoRPA41 in vitro.
Figure 3. Effects of TkoRPA complex on DNA synthesis reactions by TkoPolB and TkoPolD.
(a) The primer extension reactions were performed with M13mp18 ssDNA as the template, in the presence or absence of TkoRPA complex. The reaction products from a 5′-Cy5-labeled primer were fractionated by 1.5% alkaline agarose gel electrophoresis and visualized with the Typhoon Trio+ imager. Lane 13 shows the size marker DNAs and the sizes indicated on the right of the panel. The DNA synthesis arrested point was indicated by asterisk(*) on the left. (b) The same gel electrophoresis with different contrast of the panel (a) is shown.
Physical interactions of RPA and other replication and repair proteins
In several replication systems, DNA polymerases have been shown to interact directly with their cognate RPA/SSBs [Citation51–Citation53]. We tested whether TkoPolB, TkoPolD, and other proteins interact with TkoRPA by native PAGE analysis, followed by western blot analysis. As shown in ), the protein bands of TkoPolB and TkoPolD shifted in the presence of TkoRPA (lanes 2–3 and 6–7), indicating that both TkoPolB and TkoPolD directly form a complex with TkoRPA in vitro. The amount of proteins in were overloaded for the western blot analysis. Therefore, we loaded reduced amounts of the proteins separately for the western analysis. As shown in Figure S4, pmol of TkoRPA complex and 2 pmol of TkoPolB or TkoPolD were incubated and loaded on 5% native PAGE, followed by western blot analysis using anti-PfuRPA32 antiserum. The positive signals of RPA were detected in the positions shifted by DNA polymerases in lanes 1, 3, and 5. The distinct amount of RPA was not shifted in this experiment as compared with the case of CBB staining. It can be explained by the lower protein concentrations for the western blot experiment. This result further supported that TkoRPA bound PolB and PolD in solution. Our finding was consistent with the interaction observed between RPA and PolB in M. thermoautotrophicus [Citation54]. In the case of Pyrococcus abyssi, PolD, but not PolB, bound RPA-coated ssDNA from the reported SPR analysis [Citation55]. We also found the interactions between RPA and PolB or PolD on Cy5-labeled ssDNA by gel shift assay. As shown in ), there are smear bands of DNA (lanes 7–8 and 9–10). The smear bands imply the equilibrium state of TkoRPA complex and TkoPolB or TkoPolD on DNA.
Figure 4. TkoRPA complex interacts directly with TkoPolD, TkoPolB, and various proteins.
(a) EMSA of TkoRPA complex and various proteins were performed in 5% native PAGE followed by CBB staining. Purified TkoPolD, TkoPrimase, TkoPolB, TkoRadA, TkoMCM, TkoGINS, and TkoGAN were incubated with TkoRPA complex at 50°C for 5 min. (b) EMSA of TkoRPA complex (10 pmol), TkoPolB (2, 20 pmol), and TkoPolD (2, 20 pmol) to 10 nM Cy5 labeled ssDNA were performed in 1% AGE in 0.5 × TBE. −, +, and ++ stand for 0, 2, and 20 pmol of polymerases, respectively.
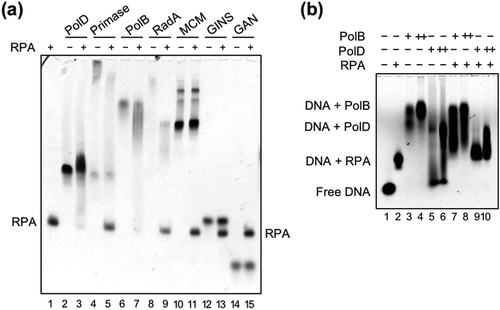
We also examined whether TkoRPA forms a stable complex with other proteins in the replisome in vitro using purified recombinant proteins. The interaction between Cdc45 and RPA in human [Citation56] and between RecJ and SSB in some bacteria have been reported [Citation57–Citation59]. A detailed sequence analysis revealed that the eukaryotic Cdc45 protein possesses a DHH phosphodiesterase domain, which is also present in bacterial RecJs [Citation60,Citation61]. The bacterial RecJ protein has a 5′–3′ ssDNA-specific exonuclease activity to function in DNA repair pathways, including homologous recombination, base excision repair, and mismatch repair in bacterial cells [Citation62–Citation64]. In contrast, no nuclease activity has been detected for eukaryotic Cdc45 so far. The T. kodakarensis genome encodes two different genes encoding Cdc45/RecJ-like proteins, GAN and HAN. They may work in the replication fork and play a role in progression and repair, respectively [Citation39,Citation43]. We proposed that GAN forms a replicative helicase complex with MCM and GINS in T. kodakarensis cells, similar to the eukaryotic CMG complex [Citation39]. Therefore, interactions of RPA with GAN, MCM, and GINS were investigated to detect the connection between RPA and helicase complex. However, no stable complex with TkoRPA complex was detected from TkoGAN, TkoMCM, or TkoGINS (lanes 10–15). In the case of Primase and RadA, the CBB staining profile of TkoPrimase and TkoRadA changed with the presence or absence of TkoRPA complex (lanes 4–5 and 8–9), suggesting the physical interactions of these proteins with TkoRPA complex, although the interactions were not so rigid.
Discussion
We showed that T. kodakarensis RPA has specific binding ability to ssDNA in vitro and positively affected the primer extension reaction of PolB and PolD in T. kodakarensis. We previously detected that PfuRPA specifically bound ssDNA in vitro, in which PfuRPA complex without RPA14 also bound ssDNA with similar affinity as compared with the full complex, but individual subunits did not bind [Citation20]. This is consistent with the observation that TkoRPA14 did not bind ssDNA by itself in this study. However, in contrast to the PfuRPA41, TkoRPA41 bound ssDNA by itself, although there is high sequence similarity among RPAs from Thermococcales. Furthermore, while PfuRPA reportedly non-specifically binds dsDNA [Citation20], dsDNA binding was not observed in TkoRPA (complex, TkoRPA41, and TkoRPA14). It is also possible that the binding mode of TkoRPA is slightly different from that of PfuRPA, although these differences may come from distinct reaction conditions.
TkoRPA seems to function to resolve the secondary structure of the DNA template for the primer extension reaction of PolB and PolD in vitro. This is consistent with findings reported regarding M. acetivorans RPA, which stimulated the extension reaction of PolB [Citation21]. This work is the first report to note that the archaeal PolD reaction is stimulated by RPA. In the case of M. acetivorans, the primer extension reaction by PolB was promoted more clearly by RPA without any disturbance, as compared with our results. The difference may come from the distinct template primer substrates, in addition to the differential properties of the DNA polymerases. In the case of M. thermoautotrophicus, RPA inhibited DNA synthesis activity in a concentration-dependent manner [Citation54]. These differences warrant further investigations. Some mechanisms may be required to remove ssDNA-bound RPA in M. thermoautotrophicus.
The purified human RPA forms specific complexes with the SV40 large T antigen and with DNA polymerase α-Primase [Citation51], and stimulates DNA polymerase activities of Polα and Polδ [Citation65]. In a recent report, human RPA assists Polε in efficient DNA synthesis by resolving secondary structures on the template DNA in vitro [Citation66]. Furthermore, eukaryotic RPA is able to unfold G-quadruplexes (G4s), in addition to duplexes, with the same defined 5′ to 3′ unwinding polarity in vitro, although RPA is not a helicase [Citation67–Citation70]. The ability of human RPA to unfold G4s from 5′ to 3′ is consistent with its important role in unfolding G4 structures on the lagging-strand during telomere replication [Citation71], and a large amount of RPA is needed to unfold the duplex [Citation72]. Notably, yeast RPA stimulates the polymerase activities of Polα [Citation73], and human RPA stimulates the primase activity of PrimPol [Citation74,Citation75], a second human primase harboring DNA polymerase and DNA primase activities involved in translesion DNA synthesis [Citation76].
Our results indicated that TkoRPA complex interacts physically with both PolB and PolD, consistent with our previous results for P. furiosus RPA [Citation20]. Interaction networks involved in archaeal DNA replication have been reported to date for three hyperthermophilic archaea: Archaeoglobus fulgidus [Citation77], T. kodakarensis [Citation31], and P. abyssi [Citation55]. Neither RPA–PolB nor RPA–PolD was detected from A. fulgidus. The RPA–PolB interaction was suggested in T. kodakarensis cells. The RPA complex in P. abyssi was co-purified with the His-tagged PolD complex [Citation55]. In P. abyssi, PolD and PriS interacted with RPA-bound ssDNA, whereas PolB did not bind. In addition, RPA in P. abyssi stimulated the transcription activity in vitro, indicating that RPA can help the strand-extension reaction of the archaeal RNA polymerase [Citation55].
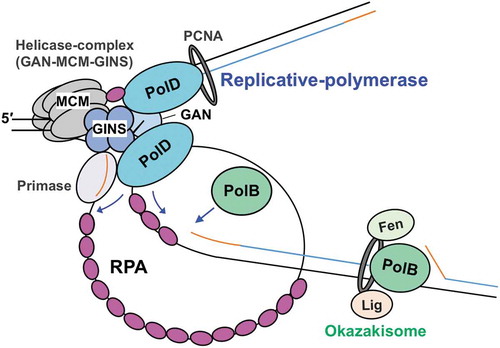
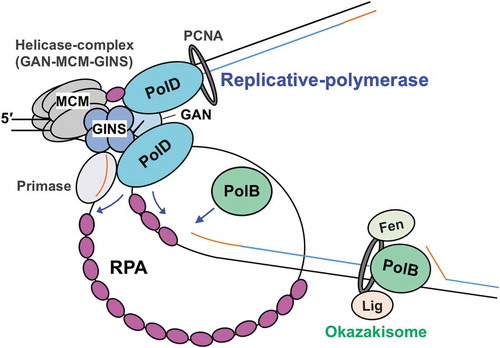
Based on our current knowledge, we developed a replisome structure model (). PolD is connected to the CMG replicative helicase via interaction with GINS [Citation31], and therefore, the main replicative DNA polymerase is probably PolD. However, PolB in Thermococcus has been reported to have SD activity and rapidly fills the gaps left by PolD and probably functions to displace the downstream Okazaki fragments to create a flap structure in vitro [Citation30,Citation78]. The prediction that PolB is required to function in Okazaki fragment maturation is supported by the fact that this maturation did not occur in the reaction using the cell extract from the ΔpolB mutant strain [Citation78]. The replisome structure model includes all the information described here. Further analyses are necessary to elucidate how RPA works for leading- and lagging- strand synthesis.
Figure 5. A model for the function of RPA in the progression of T. kodakarensis DNA replication.
The replicative helicase complex (consisting of GAN, MCM, and GINS) unwinds template DNA, generating ssDNA that is coated by RPA. RPA also contributes to the progression of the replication fork by unfolding the ssDNA secondary structure. Both PolB and PolD can progress DNA synthesis while removing RPA. Template DNA, nascent DNA, and primer are shown as black, blue, and orange lines, respectively.
The concrete function of the IDR of RPA32 remains to be elucidated. It would be important to interact with multiple factors depending on the occasion [Citation79]. An IDR is conserved in the C-terminal region of both bacterial- and archaeal-type SSBs [Citation80,Citation81]. The S. solfataricus SSB has an unusual structure with a single OB-fold domain coupled to a flexible C-terminal tail [Citation82]. SSBs with the mutation in the IDR are defective for SSB•SSB interactions and cooperative ssDNA-binding by SSB [Citation83,Citation84]. It is interesting that HmtSSB shares many physicochemical properties with SSB from E. coli [Citation85], but lacks the disordered C-terminal region. The N-terminus of human RPA32 is also disordered dynamically [Citation47]. Further analysis will expand our understanding of the conservation and diversification of the structure and functions of the SSB/RPA family.
Author contributions
M.N., S.I., and Y.I. conceived the project. M.N. and S.I. designed the experiments. M.N. and T.Y. performed the experiments with help from S.I. M.N. and Y.I. wrote the manuscript, and all authors revised the manuscript before submission.
BBB-180666_final_Supplementary_information.pdf
Download PDF (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Ashton NW, Bolderson E, Cubeddu L, et al. Human single-stranded DNA binding proteins are essential for maintaining genomic stability. BMC Mol Biol. 2013;14:9.
- Dickey TH, Altschuler SE, Wuttke DS. Single-stranded DNA-binding proteins: multiple domains for multiple functions. Structure. 2013;21:1074–1084.
- Raghunathan S, Ricard CS, Lohman TM, et al. Crystal structure of the homo-tetrameric DNA binding domain of Escherichia coli single-stranded DNA-binding protein determined by multiwavelength x-ray diffraction on the selenomethionyl protein at 2.9Å resolution. Proc Natl Acad Sci USA. 1997;94:6652–6657.
- Bochkarev A, Pfuetzner RA, Edwards AM, et al. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385:176–181.
- Bochkarev A, Bochkareva E, Frappier L, et al. The crystal structure of the complex of replication protein A subunits RPA32 and RPA14 reveals a mechanism for single-stranded DNA binding. Embo J. 1999;18:4498–4504.
- Murzin AG. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. Embo J. 1993;12:861–867.
- Sancar A, Williams KR, Chase JW, et al. Sequences of the ssb gene and protein. Proc Natl Acad Sci USA. 1981;78:4274–4278.
- Dabrowski S, Olszewski M, Piatek R, et al. Identification and characterization of single-stranded-DNA-binding proteins from Thermus thermophilus and Thermus aquaticus - new arrangement of binding domains. Microbiology. 2002;148:3307–3315.
- Bochkareva E, Korolev S, Lees-Miller SP, et al. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. Embo J. 2002;21:1855–1863.
- Braun KA, Lao Y, He Z, et al. Role of protein-protein interactions in the function of replication protein A (RPA): RPA modulates the activity of DNA polymerase alpha by multiple mechanisms. Biochemistry. 1997;36:8443–8454.
- Lin YL, Chen C, Keshav KF, et al. Dissection of functional domains of the human DNA replication protein complex replication protein A. J Biol Chem. 1996;271:17190–17198.
- Bochkareva E, Frappier L, Edwards AM, et al. The RPA32 subunit of human replication protein A contains a single-stranded DNA-binding domain. J Biol Chem. 1998;273:3932–3936.
- Philipova D, Mullen JR, Maniar HS, et al. A hierarchy of SSB protomers in replication protein A. Genes Dev. 1996;10:2222–2233.
- Gomes XV, Wold MS. Structural analysis of human replication protein A. Mapping functional domains of the 70-kDa subunit. J Biol Chem. 1995;270:4534–4543.
- Mer G, Bochkarev A, Gupta R, et al. Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA. Cell. 2000;103:449–456.
- Chedin F, Seitz EM, Kowalczykowski SC. Novel homologs of replication protein A in archaea: implications for the evolution of ssDNA-binding proteins. Trends Biochem Sci. 1998;23:273–277.
- Kelly TJ, Simancek P, Brush GS. Identification and characterization of a single-stranded DNA-binding protein from the archaeon Methanococcus jannaschii. Proc Natl Acad Sci USA. 1998;95:14634–14639.
- Kelman Z, Pietrokovski S, Hurwitz J. Isolation and characterization of a split B-type DNA polymerase from the archaeon Methanobacterium thermoautotrophicum DeltaH. J Biol Chem. 1999;274:28751–28761.
- Kerr ID, Wadsworth RI, Cubeddu L, et al. Insights into ssDNA recognition by the OB fold from a structural and thermodynamic study of Sulfolobus SSB protein. Embo J. 2003;22:2561–2570.
- Komori K, Ishino Y. Replication protein A in Pyrococcus furiosus is involved in homologous DNA recombination. J Biol Chem. 2001;276:25654–25660.
- Robbins JB, Murphy MC, White BA, et al. Functional analysis of multiple single-stranded DNA-binding proteins from Methanosarcina acetivorans and their effects on DNA synthesis by DNA polymerase BI. J Biol Chem. 2004;279:6315–6326.
- Raymann K, Forterre P, Brochier-Armanet C, et al. Global phylogenomic analysis disentangles the complex evolutionary history of DNA replication in archaea. Genome Biol Evol. 2014;6:192–212.
- Ishino Y, Ishino S. Rapid progress of DNA replication studies in Archaea, the third domain of life. Sci China Life Sci. 2012;55:386–403.
- Lujan SA, Williams JS, Kunkel TA. DNA polymerases divide the labor of genome replication. Trends Cell Biol. 2016;26:640–654.
- Ishino S, Ishino Y. DNA polymerases as useful reagents for biotechnology - the history of developmental research in the field. Front Microbiol. 2014;5:465.
- Uemori T, Sato Y, Kato I, et al. A novel DNA polymerase in the hyperthermophilic archaeon, Pyrococcus furiosus: gene cloning, expression, and characterization. Genes Cells. 1997;2:499–512.
- Cann IKO, Komori K, Toh H, et al. A heterodimeric DNA polymerase: evidence that members of Euryarchaeota possess a distinct DNA polymerase. Proc Natl Acad Sci U S A. 1998;95:14250–14255.
- Makarova KS, Krupovic M, Koonin EV. Evolution of replicative DNA polymerases in archaea and their contributions to the eukaryotic replication machinery. Front Microbiol. 2014;5:354.
- Matsunaga F, Norais C, Forterre P, et al. Identification of short ‘eukaryotic’ Okazaki fragments synthesized from a prokaryotic replication origin. EMBO Rep. 2003;4:154–158.
- Greenough L, Kelman Z, Gardner AF. The roles of family B and D DNA polymerases in Thermococcus species 9°N Okazaki fragment maturation. J Biol Chem. 2015;290:12514–12522.
- Li Z, Santangelo TJ, Cubonova L, et al. Affinity purification of an archaeal DNA replication protein network. MBio. 2010;1.
- Henneke G, Flament D, Hubscher U, et al. The hyperthermophilic euryarchaeota Pyrococcus abyssi likely requires the two DNA polymerases D and B for DNA replication. J Mol Biol. 2005;350:53–64.
- Berquist BR, DasSarma P, DasSarma S. Essential and non-essential DNA replication genes in the model halophilic Archaeon, Halobacterium sp. NRC-1. BMC Genet. 2007;8:31.
- Cubonova L, Richardson T, Burkhart BW, et al. Archaeal DNA polymerase D but not DNA polymerase B is required for genome replication in Thermococcus kodakarensis. J Bacteriol. 2013;195:2322–2328.
- Ishino S, Ishino Y. Comprehensive search for DNA polymerase in the hyperthermophilic archaeon, Pyrococcus furiosus. Nucleosides Nucleotides Nucleic Acids. 2006;25:681–691.
- Rouillon C, Henneke G, Flament D, et al. DNA polymerase switching on homotrimeric PCNA at the replication fork of the euryarchaea Pyrococcus abyssi. J Mol Biol. 2007;369:343–355.
- Henneke G. In vitro reconstitution of RNA primer removal in Archaea reveals the existence of two pathways. Biochem J. 2012;447:271–280.
- Kuba Y, Ishino S, Yamagami T, et al. Comparative analyses of the two proliferating cell nuclear antigens from the hyperthermophilic archaeon, Thermococcus kodakarensis. Genes Cells. 2012;17:923–937.
- Nagata M, Ishino S, Yamagami T, et al. The Cdc45/RecJ-like protein forms a complex with GINS and MCM, and is important for DNA replication in Thermococcus kodakarensis. Nucleic Acids Res. 2017;45:10693–10705.
- Oyama T, Ishino S, Shirai T, et al. Atomic structure of an archaeal GAN suggests its dual roles as an exonuclease in DNA repair and a CMG component in DNA replication. Nucleic Acids Res. 2016;44:9505–9517.
- Komori K, Miyata T, DiRuggiero J, et al. Both RadA and RadB are involved in homologous recombination in Pyrococcus furiosus. J Biol Chem. 2000;275:33782–33790.
- Wilkins MR, Gasteiger E, Bairoch A, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552.
- Nagata M, Ishino S, Yamagami T, et al. Possible function of the second RecJ-like protein in stalled replication fork repair by interacting with Hef. Sci Rep. 2017;7:16949.
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948.
- Radivojac P, Iakoucheva LM, Oldfield CJ, et al. Intrinsic disorder and functional proteomics. Biophys J. 2007;92:1439–1456.
- Linding R, Jensen LJ, Diella F, et al. Protein disorder prediction: implications for structural proteomics. Structure. 2003;11:1453–1459.
- Brosey CA, Yan C, Tsutakawa SE, et al. A new structural framework for integrating replication protein A into DNA processing machinery. Nucleic Acids Res. 2013;41:2313–2327.
- Bochkareva E, Belegu V, Korolev S, et al. Structure of the major single-stranded DNA-binding domain of replication protein A suggests a dynamic mechanism for DNA binding. Embo J. 2001;20:612–618.
- Morin JA, Cerron F, Jarillo J, et al. DNA synthesis determines the binding mode of the human mitochondrial single-stranded DNA-binding protein. Nucleic Acids Res. 2017;45:7237–7248.
- Wadsworth RI, White MF. Identification and properties of the crenarchaeal single-stranded DNA binding protein from Sulfolobus solfataricus. Nucleic Acids Res. 2001;29:914–920.
- Dornreiter I, Erdile LF, Gilbert IU, et al. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. Embo J. 1992;11:769–776.
- Molineux IJ, Gefter ML. Properties of the Escherichia coli in DNA binding (unwinding) protein: interaction with DNA polymerase and DNA. Proc Natl Acad Sci USA. 1974;71:3858–3862.
- Alberts BM, Barry J, Bedinger P, et al. Studies on DNA replication in the bacteriophage T4 in vitro system. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):655–668.
- Kelman Z, Lee JK, Hurwitz J. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum DeltaH contains DNA helicase activity. Proc Natl Acad Sci USA. 1999;96:14783–14788.
- Pluchon PF, Fouqueau T, Creze C, et al. An extended network of genomic maintenance in the archaeon Pyrococcus abyssi highlights unexpected associations between eucaryotic homologs. PLoS One. 2013;8:e79707.
- Szambowska A, Tessmer I, Prus P, et al. Cdc45-induced loading of human RPA onto single-stranded DNA. Nucleic Acids Res. 2017;45:3217–3230.
- Han ES, Cooper DL, Persky NS, et al. RecJ exonuclease: substrates, products and interaction with SSB. Nucleic Acids Res. 2006;34:1084–1091.
- Jiao J, Wang L, Xia W, et al. Function and biochemical characterization of RecJ in Deinococcus radiodurans. DNA Repair (Amst). 2012;11:349–356.
- Sharma R, Rao DN. Orchestration of Haemophilus influenzae RecJ exonuclease by interaction with single-stranded DNA-binding protein. J Mol Biol. 2009;385:1375–1396.
- Sanchez-Pulido L, Ponting CP. Cdc45: the missing RecJ ortholog in eukaryotes? Bioinformatics. 2011;27:1885–1888.
- Makarova KS, Koonin EV, Kelman Z. The CMG (CDC45/RecJ, MCM, GINS) complex is a conserved component of the DNA replication system in all archaea and eukaryotes. Biol Direct. 2012;7:7.
- Persky NS, Lovett ST. Mechanisms of recombination: lessons from E. coli. Crit Rev Biochem Mol Biol. 2008;43:347–370.
- Dianov G, Lindahl T. Reconstitution of the DNA base excision-repair pathway. Curr Biol. 1994;4:1069–1076.
- Burdett V, Baitinger C, Viswanathan M, et al. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc Natl Acad Sci USA. 2001;98:6765–6770.
- Lin YL, Shivji MK, Chen C, et al. The evolutionarily conserved zinc finger motif in the largest subunit of human replication protein a is required for DNA replication and mismatch repair but not for nucleotide excision repair. J Biol Chem. 1998;273:1453–1461.
- Fujisawa R, Ohashi E, Hirota K, et al. Human CTF18-RFC clamp-loader complexed with non-synthesising DNA polymerase epsilon efficiently loads the PCNA sliding clamp. Nucleic Acids Res. 2017;45:4550–4563.
- Salas TR, Petruseva I, Lavrik O, et al. Human replication protein A unfolds telomeric G-quadruplexes. Nucleic Acids Res. 2006;34:4857–4865.
- Safa L, Gueddouda NM, Thiebaut F, et al. 5ʹ to 3ʹ unfolding directionality of DNA secondary structures by replication protein A: G-quadruplexes and duplexes. J Biol Chem. 2016;291:21246–21256.
- Lancrey A, Safa L, Chatain J, et al. The binding efficiency of RPA to telomeric G-strands folded into contiguous G-quadruplexes is independent of the number of G4 units. Biochimie. 2017;146:68–72.
- Safa L, Delagoutte E, Petruseva I, et al. Binding polarity of RPA to telomeric sequences and influence of G-quadruplex stability. Biochimie. 2014;103:80–88.
- Audry J, Maestroni L, Delagoutte E, et al. RPA prevents G-rich structure formation at lagging-strand telomeres to allow maintenance of chromosome ends. Embo J. 2015;34:1942–1958.
- Lavrik OI, Kolpashchikov DM, Weisshart K, et al. RPA subunit arrangement near the 3ʹ-end of the primer is modulated by the length of the template strand and cooperative protein interactions. Nucleic Acids Res. 1999;27:4235–4240.
- Taylor MRG, Yeeles JTP. The initial response of a eukaryotic replisome to DNA damage. Mol Cell. 2018;70:1067–1080 e12.
- Guilliam TA, Brissett NC, Ehlinger A, et al. Molecular basis for PrimPol recruitment to replication forks by RPA. Nat Commun. 2017;8:15222.
- Martinez-Jimenez MI, Lahera A, Blanco L. Human PrimPol activity is enhanced by RPA. Sci Rep. 2017;7:783.
- Garcia-Gomez S, Reyes A, Martinez-Jimenez MI, et al. PrimPol, an archaic primase/polymerase operating in human cells. Mol Cell. 2013;52:541–553.
- Motz M, Kober I, Girardot C, et al. Elucidation of an archaeal replication protein network to generate enhanced PCR enzymes. J Biol Chem. 2002;277:16179–16188.
- Heider MR, Burkhart BW, Santangelo TJ, et al. Defining the RNaseH2 enzyme-initiated ribonucleotide excision repair pathway in Archaea. J Biol Chem. 2017;292:8835–8845.
- Bianco PR, Pottinger S, Tan HY, et al. The IDL of E. coli SSB links ssDNA and protein binding by mediating protein-protein interactions. Protein Sci. 2017;26:227–241.
- Shereda RD, Kozlov AG, Lohman TM, et al. SSB as an organizer/mobilizer of genome maintenance complexes. Crit Rev Biochem Mol Biol. 2008;43:289–318.
- Lohman TM, Ferrari ME. Escherichia coli single-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527–570.
- Morten MJ, Gamsjaeger R, Cubeddu L, et al. High-affinity RNA binding by a hyperthermophilic single-stranded DNA-binding protein. Extremophiles. 2017;21:369–379.
- Kozlov AG, Weiland E, Mittal A, et al. Intrinsically disordered C-terminal tails of E. coli single-stranded DNA binding protein regulate cooperative binding to single-stranded DNA. J Mol Biol. 2015;427:763–774.
- Tan HY, Wilczek LA, Pottinger S, et al. The intrinsically disordered linker of E. coli SSB is critical for the release from single-stranded DNA. Protein Sci. 2017;26:700–717.
- Qian Y, Johnson KA. The human mitochondrial single-stranded DNA-binding protein displays distinct kinetics and thermodynamics of DNA binding and exchange. J Biol Chem. 2017;292:13068–13084.
