ABSTRACT
Soy sauce – a fermented food made from soybeans and wheat – is considered a healthy seasoning, but little scientific evidence is available to support this. In this study, physiological effects of soy sauce were analyzed using Caenorhabditis elegans. When soy sauce was fed to C. elegans together with Escherichia coli OP50, fat accumulation decreased, and resistance to oxidative stress by H2O2 was greatly increased in the nematodes. qRT-PCR revealed that mRNA expression of oxidative stress tolerance genes, including sod, ctl, and gpx, was markedly increased in soy sauce-fed nematodes. Worms ingesting soy sauce showed high mitochondrial membrane potential and reactive oxygen species (ROS) and low intracellular ROS, suggesting that soy sauce induced mitohormesis and decreased cytoplasmic ROS. Therefore, soy sauce ingestion affects the mitochondria and may alter the fat metabolism in C. elegans. Furthermore, the increase in oxidative stress tolerance is mediated through p38 MAPK pathway.
Graphical Abstract

Soy sauce reduced fat accumulation and changed ROS level in nematode. Furthermore, soy sauce increased the oxidative stress tolerance of nematode through p38 MAPK pathway.
Soy sauce is a traditional fermented seasoning used in Japan and consumed globally [Citation1]. Soy sauce is made by adding salt to soybean and wheat and fermenting the mixture with Aspergillus oryzae. This is called moromi. By the enzymatic activity of Aspergillus, soybean protein is digested into amino acids, and wheat starch is decomposed into sugar. These amino acids and sugar content determine the taste of soy sauce. Soy sauce is classified into two types according to the manufacturing process. One type is non-heat-treated soy sauce, termed ki-jyoyu. Non-heat-treated soy sauce is made by filtration of moromi. The other type is a heat-treated soy sauce known as hiire-shoyu, which is made by heating ki-jyoyu. So far, a number of physiological effects of soy sauce, such as antioxidant, antiallergic, and antitumor effects, have been reported [Citation2–Citation6], but the detailed mechanism of action has yet to be clarified. This study aimed to clarify the physiological action of soy sauce using nematodes as a model organism.
Nematodes (Caenorhabditis elegans) can easily be raised in the laboratory using Escherichia coli as their food source [Citation7]. They are widely used for biological analyses requiring model organisms because many nematode genes are homologous to those of higher animals, and their structures, such as the digestive and nervous systems and muscles, are basic. Many previous studies have investigated stress tolerance and anti-aging using nematodes [Citation8,Citation9] because the life cycle of nematodes is very short, allowing repetition of many experiments in a short period of time. Furthermore, nematode sensitivity to bioactive substances is very high, and the phenotypic changes in response are remarkable. For these reasons, nematodes are effective as a primary evaluation system for physiologically functional substances, and the results obtained can be applied to higher animals in the future.
In this study, the physiological effects on nematodes fed soy sauce were analyzed. In particular, analysis was carried out focusing on oxidative stress tolerance. Oxidative stress arises from a number of factors and is closely related to health including cell damage and disease induction [Citation10,Citation11]. Therefore, from this study, conclusions can be drawn regarding the physiological effect of soy sauce on health using nematodes as a model.
Materials & methods
Nematodes
The following nematode strains were used in this study: N2 Bristol (wild type) and sek-1 (AU1) were obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN, USA); skn-1 (tm4241), pmk-1 (tm676) and nsy-1 (tm850) were obtained from the National BioResource Project (NBRP, Tokyo Women’s Medical University, Japan). Nematodes were cultured on nematode growth medium (NGM) plates spread with E. coli (OP50) at 20°C [Citation7].
Soy sauce
Two different types of soy sauce used in this study were purchased from a grocery store (soy sauce A and B). Soy sauce A, known as hiire-shoyu, was heat-treated soy sauce, and soy sauce B, known as ki-jyoyu, was a non-heat-treated soy sauce. Each contained 16% to 17% sodium chloride and 3% to 4% ethanol. We prepared a solution containing 16% sodium chloride (NaCl, Kanto Chemical Co., Inc., Tokyo, Japan) and 3% ethanol (Kanto Chemical) in double-distilled water (dDW) as a control. For each assay, we prepared plates as follows:
OP plate: OP50 and dDW (4: 1) were spread on a NGM plate.
NaCl plate: OP50 and sodium chloride solution (4: 1) were spread on a NGM plate.
Soy sauce (SS) plate: OP50 and soy sauce (4: 1) were spread on a NGM plate.
For fat accumulation assay, saline solution and soy sauce were diluted with dDW (10 × and 100 ×) and spread on a NGM plate together with OP50 (OP50: diluted solution = 4: 1).
Nematode synchronization
To collect eggs, adult nematodes were crushed with NaClO solution (10 N NaOH [Wako Pure Chemical Industries, Ltd., Osaka, Japan]: NaClO [Haiter, KAO, Tokyo, Japan] = 1: 10). This procedure was performed to synchronize the growth stages of the nematodes.
Fat accumulation
Synchronized worms were placed on OP, NaCl, and SS plates. Worms were cultured on these plates for 96 hours and fixed with 4% paraformaldehyde (PFA, Wako) at 4°C. Worms were washed, and 500 µL of 5 µg/mL Nile red (Wako) was dropped onto the worms and incubated for 10 min at 4°C. Thereafter, worms were washed twice, and the fluorescence was measured by a BZ8000 microscope (KEYENCE Japan, Osaka, Japan) and analyzed with ImageJ (NIH). The fluorescence level of control worms was set as 100%, and > 25 worms were evaluated for each group.
Oxidative stress tolerance
Synchronized worms were cultured on OP, NaCl, and SS plates for 96 hours and transferred to 0.3% hydrogen peroxide solution (H2O2, Sigma Aldrich Japan, Tokyo, Japan). This was designated as the 0th hour. The survival rate was measured every hour, beginning 2 hours after transfer. H2O2 (400 µL) was dropped in 24-well plates (Techno Plastic Products AG, Trasadingen, Switzerland). The survival rate at the 0th hour was set as 100%, and 24 worms were assessed per group.
Activation of mitochondria
Synchronized worms were cultured on OP, NaCl, and SS plates for 72 hours. In order to quantify the mitochondrial membrane potential and mitochondrial reactive oxygen species (ROS), 400 µL each of 0.5 mg/mL MitoTracker® Orange CMTMRos (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 0.5 mg/mL MitoTracker® Orange CM-H2TMRos (Thermo Fisher Scientific) was dropped on the plates. After 24 hours, worms were washed and fixed by 10% ethanol (Kanto Chemical). The fluorescence was measured by a BZ8000 microscope and analyzed with ImageJ. The fluorescence level of control worms was set as 100%, and > 20 worms per group were analyzed.
Cellular ROS
Synchronized worms were cultured on OP, NaCl, and SS plates for 96 hours. In order to quantify the cellular ROS, 400 µL of 50 µM DCF-DA solution was dropped on the plates. To prepare the DCF-DA solution, DCF-DA (Wako) was diluted with dimethyl sulfoxide (Kanto Chemical). After one hour, worms were washed and fixed by 10% ethanol. The fluorescence was measured by a BZ8000 microscope and analyzed with ImageJ. The fluorescence level of control worms was set as 100%, and > 18 worms per group were analyzed.
Gene expression
Synchronized worms were cultured on OP and SS plates for 96 hours. In order to extract the mRNA, worms were crushed by Power Masher® (Nippi Inc., Tokyo, Japan) and Bio Masher® (Nippi). For cDNA generation, reverse transcriptase (PrimeScript™ RT Reagent Kit with gDNA Eraser, Takara, Shiga, Japan) was used. Thereafter, real-time PCR was conducted by the Thermal Cycler Dice® Real Time System Lite (Takara) with Thunderbird® SYBR® qPCR Mix (TOYOBO, Osaka, Japan). In addition to wild-type N2 worms, the transcription of mRNA in tm4241 and tm850 was also analysed. Actin was used as a reference gene, and each qPCR reaction was performed in triplicate wells. Primers used in this study are shown in .
Table 1. Sequences of primers used in the gene expression analysis.
Statistical processing
Data were shown as the mean ± SEM and analyzed with Tukey’s test. The survival rate was analyzed with the log-rank test. Graphs were generated using Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and Microsoft PowerPoint (Microsoft Corp.). P-values of < 0.05 indicated statistically significant differences.
Results
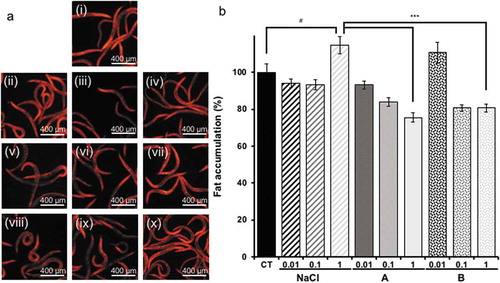
According to a previous study, accumulation of fat increases with high salt intake [Citation12]. On the other hand, soybean protein reduced the body fat of obese rats [Citation13]. Soy sauce contains both salt and soy protein. To examine whether salt and soy protein affect fat accumulation, Nile red staining was used to analyze fat accumulation in nematodes fed soy sauce. In nematodes fed a high dose of sodium chloride, body fat increased, while the fat accumulation in nematodes fed soy sauce A decreased in a dose-dependent manner (). Although the effect of soy sauce B was not dose dependent, soy sauce B intake decreased the fat accumulation in nematodes. This indicated that soy sauce intake decreases the fat accumulation in worms. However, the same amount of sodium chloride included in soy sauce increased the fat accumulation in worms.
Figure 1. Fat accumulation.
(a) Fat stained with Nile red: (i) OP plate, (ii) undiluted saline solution, (iii) saline solution diluted 10×, (iv) saline solution diluted 100×, (v) undiluted soy sauce A, (vi) soy sauce A diluted 10×, (vii) soy sauce A diluted 100×, (viii) undiluted soy sauce B, (ix) soy sauce B diluted 10×, (x) soy sauce B diluted 100×. Scale bars indicate 400 μm. (b) Analysis results of A. Only the significant difference between the control and experimental samples is shown. Data are shown as the mean ± SEM, and significant differences between the control and experimental samples were analyzed with Tukey’s test. #P < 0.05 vs. control group and ***P < 0.005 vs. NaCl group.
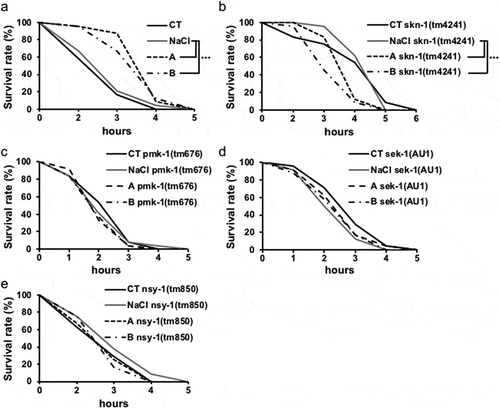
Previous studies have revealed that soy sauce increased antioxidant activity [Citation3]. To examine the physiological mechanism of soy sauce in detail, oxidative stress tolerance of nematodes was analyzed. In order to clarify the signaling pathway of oxidative stress tolerance, skn-1, pmk-1, sek-1 and nsy-1 mutants were used. Soy sauce increased the survival rate of wild-type N2 following hydrogen peroxide stress ()) but did not increase the survival rates of tm4241, tm676, AU1 and tm850 (). This suggested that soy sauce increases oxidative stress tolerance via skn-1, pmk-1, sek-1 and nsy-1.
Figure 2. Oxidative stress tolerance.
Two hours after transferring the nematodes to H2O2, the survival rate was measured hourly. (a) The survival rate of wild-type N2 worms. (b), (c), (d) and (e) tm4241, tm676, AU1 and tm850 worms, respectively. The survival rate of the 0th hour was set as 100%. Data are shown as the mean ± SEM and analyzed with the log-rank test. The significant differences between the control and experimental samples are shown. ***P < 0.005 vs. NaCl group.
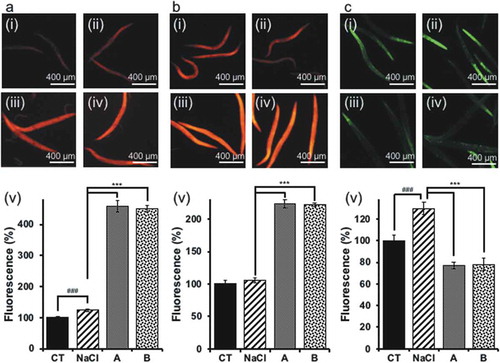
Oxidative stress induces ROS Citation[13]; that is, oxidative stress tolerance and ROS are closely related [Citation14]. High levels of ROS induce many biological effects such as cell damage and diseases [Citation15]. Mitochondria are one of the main sources of ROS. Therefore, mitochondrial membrane potential, mitochondrial ROS, and intracellular ROS were analyzed with MitoTracker® and DCF-DA staining. As a result, soy sauce increased mitochondrial membrane potential ()) and mitochondrial ROS ()). Conversely, soy sauce reduced ROS in the whole cell ()). This suggests that the ROS in the cytoplasm was decreased.
Figure 3. Mitochondrial activity and cellular ROS level.
(a) Mitochondrial membrane potential assay: (i) OP plate, (ii) NaCl plate, (iii) soy sauce A plate, (iv) soy sauce B plate. Scale bars indicate 400 μm. (v) Analysis of data in (i)–(iv). (b) Mitochondrial ROS assay; the conditions are the same as those in a. (c) Cellular ROS assay; the conditions are the same as those in a. The fluorescence of the control was set as 100%. Data are shown as the mean ± SEM and analyzed with Tukey’s test. ###P < 0.005 vs. control group and ***P < 0.005 vs. NaCl group.
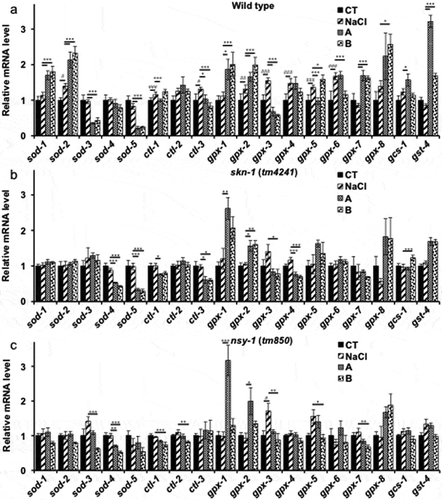
As mentioned, soy sauce increased the oxidative stress tolerance of worms ()). The mRNA levels of genes related to oxidative stress were quantified by real-time PCR. As shown by the results of real-time PCR, soy sauce increased expression levels of multiple sod, ctl, and gpx mRNAs ()). Both A and B soy sauces increased expression levels of sod-1, sod-2, gpx-1, gpx-2, gpx-7, gpx-8 and gst-4 compared with those in the control and NaCl groups. Expression of ctl-2, gpx-6 and gcs-1 was increased only with soy sauce A, and expression of ctl-1 and gpx-5 was increased only with soy sauce B. Conversely, soy sauce reduced the expression of ctl-3, sod-5, and gpx-5 mRNA. Salt intake alone increased ctl-3 and gpx-3 mRNA. To elucidate the relation to p38 MAPK pathway, the mRNA levels in skn-1 (tm4241) and nsy-1 mutants (tm850) were quantified. Soy sauce A and B increased the expression of several genes related to oxidative stress similarly in wild-type N2 worms, though some differences were not significantly (). Especially the expression of sod-1, sod-2, gpx-7 and gst-4 were strongly suppressed.
Figure 4. Gene expression.
The quantities of mRNA are shown as relative values. (a) The mRNA level of wild-type N2 worms. (b) and (c) tm4241 and tm850 worms, respectively. Actin was used as an internal standard. Each cDNA sample was amplified in triplicate wells, independently. Data are shown as the mean ± SEM, and significant differences between the control and experimental samples were analyzed with Tukey’s test. #P < 0.05, ##P < 0.01, and ###P < 0.005 vs. control group; *P < 0.05 and ***P < 0.005 vs. NaCl group.
Discussion
This study aimed to elucidate the physiological effects of soy sauce. We found that soy sauce decreased the fat accumulation in nematodes (). Soy sauce contains sodium chloride, which was determined by a previous study to increase fat accumulation [Citation16]. Similarly, in nematodes fed a high concentration of salt, the amount of fat accumulation increased (). Furthermore, except for those fed 0.01% of soy sauce B, nematodes fed soy sauce had less body fat than that of nematodes fed saline with a similar salt concentration. Interestingly, increasing the concentration of soy sauce decreased the accumulation of fat. For nematodes fed soy sauce B, fat reduction was noticeable at high concentrations as with soy sauce A, although this effect was not dependent on concentration. While the reason for this is not clear, several prior studies have associated soy protein with fat reduction [Citation17].
Oxidative stress is closely related to health status [Citation13]. Active oxygen in the body damages cells and DNA, causing aging phenomena and various diseases [Citation15]. Indeed, reducing oxidative stress prevents disease and leads to longevity [Citation18]. In this study, soy sauce increased the oxidative stress tolerance of nematodes ()). Furthermore, it was induced via skn-1, pmk-1, sek-1 and nsy-1 (). NSY-1, PMK-1 and SEK-1 are included in the p38 MAPK pathway [Citation19], which is related to oxidative stress tolerance [Citation19]. The signal from p38 MAPK pathway induces the activation of SKN-1 [Citation19]. Accordingly, soy sauce might raise the oxidative stress tolerance of nematodes via the p38 MAPK pathway and SKN-1. Moreover, activation of the p38 MAPK pathway induced pathogen resistance [Citation20]. Therefore, soy sauce intake might be capable of upregulating pathogen resistance.
ROS is considered the cause of many diseases such as metabolic syndrome [Citation21,Citation22]. In this study, mitochondrial membrane potential and mitochondrial ROS were enhanced following soy sauce ingestion (). This result suggests the possibility of mitochondrial hormesis (mitohormesis) induction [Citation23]. Consequently, some genes related to oxidative stress tolerance were upregulated ()), which could promote reduction of cytoplasmic ROS ()) and enhance the stress tolerance of worms ()). Furthermore this might induce transcription of glutathione S-transferase 4 (GST-4) and it worked as an antioxidant enzyme () [Citation24].
Nematodes have 5 sod, 8 gpx, and 3 ctl genes. Superoxide dismutase (SOD) is one of the main scavengers of ROS, which converts superoxide anion (O2−) to H2O2 [Citation25]. Thereafter, H2O2 is converted to water by catalase (CTL) and glutathione peroxidase (GPX). GPX also facilitates phospholipid hydroperoxide transformation to water and the corresponding alcohol using glutathione [Citation26]. Although there were some differences between the two soy sauces, both increased expression of sod-1, sod-2, gpx-1, gpx-2, gpx-7, gpx-8 and gst-4 mRNA. Conversely, the expression of sod-3, sod-5, and gpx-3 were significantly reduced. SOD-1 and SOD-2 are major SODs that function in the normal developmental stage [Citation11], while SOD-3 and SOD-5 function mainly in the tolerant larval stage [Citation11]. Therefore, soy sauce ingestion possibly affected the development of C. elegans and prevented the dauer larval stage. Intestine is the primary tissue expressing gpx-1, gpx-2, gpx-6, and gpx-7 in C. elegans [Citation26]. The upregulation of these genes could reduce oxidative stress in the intestine. gcs-1 and gst-4 also have an ability to metabolize glutathione and known as antioxidative enzymes [Citation24]. These are the target genes of SKN-1 [Citation10]. Although gcs-1 was upregulated with only soy sauce A, gst-4 was upregulated notably with both soy sauces ()). This result also supported soy sauce activated SKN-1 via p38 MAPK pathway. In order to clarify this mechanism we conducted qPCR with skn-1 (tm4241) and nsy-1 (tm850) mutants. Soy sauce increased the expression of several anti-stress genes similarly in wild-type N2 worms and particularly suppressed the upregulation of sod-1, sod-2, gpx-7 and gst-4.The fact sod-1, sod-2 and gst-4 are the one of the target genes of SKN-1 supported soy sauce activated SKN-1 via p38 MAPK pathway as well [Citation10,Citation27].
Here, we elucidated some physiological effects of soy sauce on C. elegans. Since C. elegans has many genes homologous to those of higher animals such as human beings, the results of this study may be applied to humans in the future.
Author contributions
TS, DS and KS conceived and designed the study, TS and DS performed experiments, KS provided essential tools and reagents, TS, DS and KS analyzed data, TS and KS wrote the paper. TS and KS made manuscript revisions. KS supervised the study as a principal investigator. All authors read and approved the final manuscript.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research and Education from University of Tsukuba, Japan. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Winarno FG. Fermented vegetable protein and related foods of Southeast Asia with special reference to Indonesia. J Am Oil Chem Soc. 1979;56:363–366.
- Kataoka S. Functional effects of Japanese style fermented soy sauce (shoyu) and its components. J Biosci Bioeng. 2005;100:227–234.
- Li H, Lin L, Feng Y, et al. Enrichment of antioxidants from soy sauce using macroporous resin and identification of 4-ethylguaiacol, catechol, daidzein, and 4-ethylphenol as key small molecule antioxidants in soy sauce. Food Chem. 2018;240:885–892.
- Malarde L, Groussard C, Lefeuvre-Orfila L, et al. Fermented soy permeate reduces cytokine level and oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2015;18:67–75.
- Kobayashi M. Immunological functions of soy sauce: hypoallergenicity and antiallergic activity of soy sauce. J Biosci Bioeng. 2005;100:144–151.
- Wada K, Tsuji M, Tamura T, et al. Soy isoflavone intake and stomach cancer risk in Japan: from the Takayama study. Int J Cancer. 2015;137:885–892.
- Stiernagle T, Maintenance of C. elegans. WormBook, editor. The C. elegans Research Community. WormBook; 2006 [cited 2018 Jun 1]. p. 1–11. DOI:10.1895/wormbook.1.101.1
- Blackwell TK, Steinbaugh MJ, Hourihan JM, et al. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med. 2015;88:290–301.
- Prasad KN, Bondy SC. Evaluation of role of oxidative stress on aging in Caenorhabditis elegans: a brief review. Curr Aging Sci. 2013;6:215–219.
- Inoue H, Hisamoto N, An JH, et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283.
- Back P, Braeckman BP, Matthijssens F. ROS in aging Caenorhabditis elegans: damage or signaling? Oxid Med Cell Longev. 2012;2012:1–14.
- Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension. 2015;66:843–849.
- Zhou D, Lezmi S, Wang H, et al. Fat accumulation in the liver of obese rats is alleviated by soy protein isolate through β-catenin signalling. Obesity (Silver Spring). 2014;22:151–158.
- Hybertson BM, Gao B, Bose SK, et al. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462.
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486.
- Kobayashi M, Magishi N, Matsushita H, et al. Hypolipidemic effect of shoyu polysaccharides from soy sauce in animal and humans. Int J Mol Med. 2008;22:565–570.
- Park SK, Tedesco PM, Johnson TE. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8:258–269.
- Zhao Y, Zhi L, Wu Q, et al. p38 MAPK-SKN-1/Nrf signaling cascade is required for intestinal barrier against graphene oxide toxicity in Caenorhabditis elegans. Nanotoxicology. 2016;10:1469–1479.
- Papp D, Csermely P, Soti C. A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PLoS Pathog. 2012;8:e1002673.
- Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45:410–418.
- Moreno-Arriola E, Cardenas-Rodriguez N, Coballase-Urrutia E, et al. Caenorhabditis elegans: A useful model for studying metabolic disorders in which oxidative stress is a contributing factor. Oxid Med Cell Longev. 2014;2014:705253.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247.
- Detienne G, Van de Walle P, De Haes W, et al. SKN-1-independent transcriptional activation of glutathione S-transferase 4 (GST-4) by EGF signaling. Worm. 2016;5:e1230585.
- Doonan R, McElwee JJ, Matthijssens F, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241.
- Sakamoto T, Maebayashi K, Nakagawa Y, et al. Deletion of the four phospholipid hydroperoxide glutathione peroxidase genes accelerates aging in Caenorhabditis elegans. Genes Cells. 2014;19:778–792.
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893.
