ABSTRACT
The study aimed to investigate the role of lncRNA FENDRR in proliferation and angiogenesis of human retinal endothelial cells (HRECs). HRECs were cultured in high-glucose medium to mimic diabetic retinopathy (DR) model. We overexpressed or knocked down FENDRR in HRECs to evaluate the effect of FENDRR expression on cell proliferation, migration, and capillary morphogenesis of HRECs under either normal glucose or high glucose condition. Results showed that VEGF and FENDRR expression were increased in blood from DR patients compared with the control subjects. Furthermore, high glucose treatment upregulated expression of VEGF and FENDRR secreted from HRECs, in a dose- and time-dependent manner. Importantly, FENDRR overexpression significantly promoted the high-glucose-induced proliferation, migration, capillary morphogenesis, and VEGF expression in HRECs. In contrast, FENDRR knockdown exerted the opposite effects. In conclusion, lncRNA FENDRR promotes the high-glucose-induced proliferation and angiogenesis of HRECs and may serve as a potential target for anti-angiogenic therapy for DR.
Graphical abstract
Effect of FENDRR expression on the high-glucose-induced HRECs proliferation, migration, and capillary morphogenesis.
Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes and can eventually cause vision loss [Citation1]. Retinal neovascularization has been identified as a key risk factor for severe visual deterioration in patients with DR [Citation2]. Therefore, the control of neovascularization induced by high glucose is essential for the prevention of DR development.
Long non-coding RNAs (lncRNAs) are a class of ncRNAs that are greater than 200 nucleotides in length and play diverse roles in various biological processes [Citation3,Citation4]. Accumulating studies have demonstrated that lncRNAs may be aberrantly expressed and play important roles in DR [Citation5,Citation6]. For instance, Zhang et al. [Citation7] have recently suggested that lncRNA MIAT (myocardial infarction associated transcript) acts as a biomarker in DR by absorbing miR-29b and regulating cell apoptosis of rat retinal Muller cells (rMC-1) cells. Sun et al. [Citation8] have indicated that lncRNA HOTTIP (HOXA distal transcript antisense RNA) is upregulated in the retina of diabetic rats and improves DR. FENDRR (FOXF1 adjacent non-coding developmental regulatory RNA, also known as FOXF1-AS1), located on chromosome 3q13.31, is a novel lncRNA with 3099-bp [Citation9]. Previous studies have highlighted the importance of FENDRR in cancer, such as breast cancer [Citation10], prostate cancer [Citation11], and gastric cancer [Citation12]. However, the role of FENDRR in DR remains unknown.
FENDRR is divergently transcribed from the promoter of FOXF1 (Forkhead Box transcription factor F1) and co-expressed with FOXF1 [Citation9]. FENDRR regulates its neighboring gene FOXF1 by interacting with and recruiting the polycomb repressive complex 2 (PRC2) to the FOXF1 promoter [Citation13]. Researchers have also reported that FENDRR overexpression significantly increased, whereas FENDRR knockdown decreased FOXF1 expression [Citation14,Citation15]. FOXF1 is required for the formation of the embryonic vasculature via regulation of endothelial genes critical for vascular endothelial growth factor (VEGF) signaling [Citation16]. VEGF can stimulate angiogenesis via induction of the migration and sprouting of endothelial cells and may thereby act as an important mediator for DR development [Citation2,Citation17].
Based on aforementioned findings, we speculated that FENDRR may have potential roles in regulating VEGF expression and angiogenesis in DR. To address this, HRECs were cultured in high-glucose medium to mimic DR model. We overexpressed or knocked down FENDRR in HRECs to evaluate the effect of FENDRR expression on cell proliferation, migration, and capillary morphogenesis, and VEGF expression in HRECs under either normal glucose (5 mM) or high glucose (25 mM) condition.
Materials and methods
Ethics statement and sample collection
The clinical study was approved by the ethics committees of Nanjing First Hospital, Nanjing Medical University (Nanjing, China). Informed written consent was obtained from all participants. Patients with DR in the Nanjing First Hospital were enrolled in this study (n = 15), and healthy subjects who underwent physical examination during the same period in this hospital were selected as the normal control group (n = 10). Serum samples were obtained from the patients and were immediately stored at −70°C until analyzed.
Detection of serum VEGF level
The serum VEGF level was measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Cell culture
Human retinal endothelial cells (HRECs) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). HRECs were maintained in high-glucose Dulbecco’s modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 100 µg/mL streptomycin, and 100 U/mL penicillin (all from Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA), at 37°C in a humidified atmosphere containing 5% CO2. They were grown to confluence and incubated with the designated concentration of D-glucose (5, 10, 15, 20, 25, 30 mM; Sigma-Aldrich; St Louis, MO, USA) for the designated time (0, 6, 12, 24, 48 h). Cells were harvested for RNA and protein isolation.
RT-qPCR analysis
Total RNA was extracted from blood from DR patients or HRECs using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.), following the manufacturer’s instructions. Total RNA was transcribed into cDNA using standard HiFiScript cDNA Synthesis Kit (CWBIO, Beijing, China). RT-qPCR was performed on a StepOne Real-time PCR system (Applied Biosystem; Thermo Fisher Scientific, Inc.). The primers were listed as follows: FENDRR forward, 5ʹ-TTCATCGGCTGCGTATTCG-3ʹ and reverse, 5ʹ-TTGCCTTCTAGTCGCCTCC-3ʹ; VEGF forward, 5ʹ- AAGGAGGAGGGCAGAATCAT-3ʹ and reverse, 5ʹ-ATCTGCATGGTGATGTTGGA-3ʹ; β-actin (reference gene) forward, 5ʹ-CATGTACGTTGCTATCCAGGC-3ʹ and reverse, 5ʹ-CATGTACGTTGCTATCCAGGC-3ʹ. The relative expression of the target genes was analyzed using the 2−ΔΔCq method [Citation18] and normalized to β-actin.
Western blot analysis
HRECs were lysed in SDS lysis buffer containing protease inhibitor (Sigma-Aldrich) according to the manufacturer’s instructions. The protein concentration was measured using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA). Proteins were then separated by 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad). After being blocked with 5% nonfat dry milk, the membrane was then incubated with the primary antibody against VEGF (Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C overnight, and incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Blots were developed using an enhanced chemiluminescence kit (ECL kit, Pierce Biotechnology, IL) and band intensity was quantified with Quantity One software. GAPDH served as the loading control.
Plasmid construction and cell transfection
To overexpress FENDRR in HRECs, the synthetic FENDRR sequence was subcloned into the pcDNA3.1 vector (Invitrogen), generating a pcDNA3.1-FENDRR vector. An empty pcDNA3.1 vector served as a control. To knock down FENDRR, FENDRR siRNA (si-FENDRR) and negative control siRNA (si-NC) were purchased from Genepharm (Shanghai, China). The sequence of si-FENDRR was 5ʹ-GGAGGGAATTAGAAGCGTT-3ʹ, and the seque-nce of si-NC was 5ʹ-TTCTCCGAACGTGTCACGT-3ʹ. HRECs were transfected with these plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 48 h post-transfection, cells were harvested and the overexpression and knockdown efficiency was determined by RT-qPCR. After that, cells were subjected to CCK-8 assay, Transwell migration assay, and tube formation assay.
CCK-8 assay
The Cell Counting Kit-8 (CCK-8) assay was performed to evaluate the effect of FENDRR expression on high-glucose-induced proliferation of HRECs. HRECs that had been transfected with the indicated plasmids were seeded into a 96-well plate at a density of 3 × 103 cells/well and allowed to adhere for 24 h, after which cells were cultured in serum-free media for starvation for 24 h. Then, HRECs were cultured with either 5 or 25 mM glucose for 48 h, and the proliferative activity of HRECs was measured by a CCK-8 kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Briefly, CCK-8 (10 μL) were added to each well followed by incubation for an additional 2.5 h. The optical density (OD) at a wavelength of 450 nm was detected using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The experiment was performed at least three times with similar results
Transwell migration assay
Transwell migration assay was performed to evaluate the effect of FENDRR expression on the high-glucose-induced migration of HRECs according to a previous study [Citation2], with some alterations. The bottom sides of 24-well Transwell inserts (8-µm pore size) were coated with 2 µg/mL of fibronectin at 4°C overnight. The following day, HRECs that had been transfected with the designated plasmids before being cultured under 5 or 25 mM glucose conditions for 48 h were seeded at a density of 1 × 105 cells/ml in the upper chamber of inserts with serum-free medium. After being cultured with different media for 12 h, HRECs on the upper side of the insert were scraped off gently using a moist cotton-tipped swab, and HRECs on the bottom side of the insert were washed with PBS, fixed with 4% paraformaldehyde and then stained with haematoxylin and eosin. The number of cells migrated to the bottom side of the insert was determined by counting five random microscope fields.
Tube formation assay
To evaluate the effect of FENDRR expression on high-glucose-induced HRECs tube formation, the morphogenesis assay on Matrigel was performed as previously described [Citation1]. Briefly, Matrigel (60 μL) was added to a pre-cooled 96-well plate, after which the plate was immediately placed in a humidified atmosphere containing 5% CO2 at 37 C for 30 min to solidify the Matrigel. HRECs that had been transfected with the indicated plasmids were cultured with either 5 or 25 mM glucose. After 48 h of cultivation, cells (1.5 × 104 cells/well) were seeded immediately on the solidified Matrigel. Subsequently, the plates were incubated in a humidified atmosphere containing 5% CO2 and 95% air at 37°C for 8 h to allow the formation of a capillary-like structure. The pictures were photographed, and the number of capillaries formed was qualitatively assessed using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The data are presented as the mean ± standard deviation (SD) from at least three independent experiments. All statistical analyses were performed using SPSS 16.0 software. The differences between the two groups were analyzed using the unpaired Student’s t-test. The differences among multiple groups were analyzed using one-way analysis of variance (ANOVA). p < 0.05 were considered statistically significant.
Results
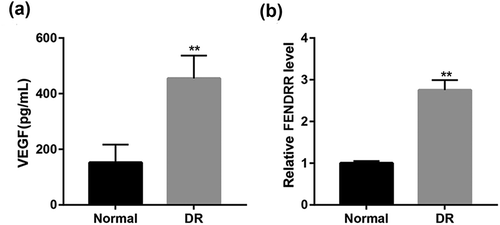
Increased VEGF and FENDRR expression in DR patients
ELISA results showed that the serum VEGF level in DR patients was significantly increased compared with the normal group (). Furthermore, RT-qPCR analysis revealed that relative FENDRR level in blood from DR patients was significantly also upregulated compared with the normal subjects ().
Figure 1. Increased VEGF and FENDRR expression in DR patients. (a) The serum VEGF level in normal subjects and DR patients was detected by ELISA. (b) The relative FENDRR level in blood from normal and DR patients was examined using RT-qPCR. **p < 0.01 vs. the normal group. Student’s t-test was used to analyze the differences between the two groups.
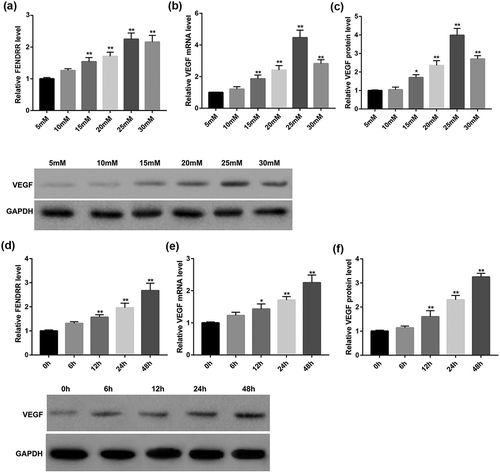
Effect of high glucose on VEGF and FENDRR expression in HRECs
We next explored whether the high glucose level would affect the secretion of VEGF and FENDRR from HRECs. Results of RT-qPCR revealed that D-glucose increased FENDRR expression in HRECs in a dose-dependent manner (). Furthermore, D-glucose dose-dependently increased VEGF expression both mRNA () and protein level () in HRECs. It was observed that 25 mM glucose was the optimal concentration and was used for the following experiments. As shown in , 25 mM glucose increased VEGF and FENDRR expression in HRECs in a time-dependent manner.
Figure 2. D-glucose dose- and time-dependently increased FENDRR and VEGF expression in HRECs. HRECs were incubated with increasing concentrations of D-glucose (5, 10, 15, 20, 25, and 30 mM) for 48 h, then relative FENDRR level (a) and VEGF mRNA level (b) were examined by RT-qPCR, and protein expression of VEGF (c) was evaluated by Western blot. *p < 0.05, **p < 0.01 vs. the 5 mM group. In parallel experiments, HRECs were incubated with D-glucose (25 mM) for 0, 6, 12, 24, or 48 h, then relative FENDRR level (d) and VEGF mRNA level (e) were examined by RT-qPCR, and protein expression of VEGF (f) was evaluated by Western blot. The data are expressed as the mean ± SD from three independent experiments. *p < 0.05, **p < 0.01 vs. the 0 h group. ANOVA followed by Dunnett’s test was used to analyze the differences among multiple groups.
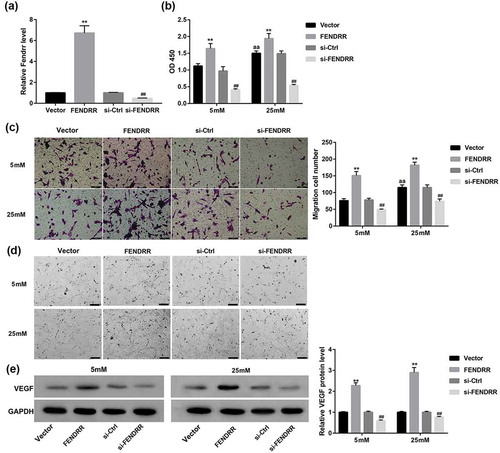
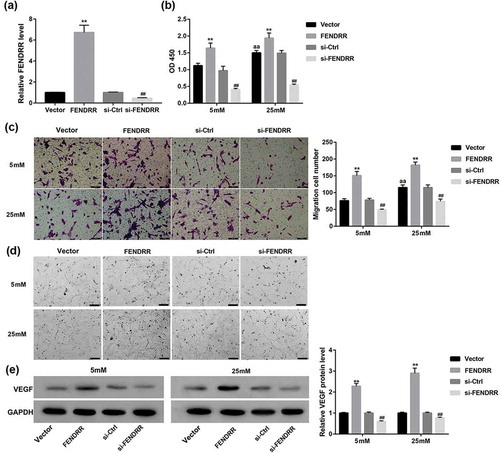
Effect of FENDRR expression on the high-glucose-induced HRECs proliferation and angiogenesis
The abnormal proliferation and angiogenesis of retinal endothelial cells induced by continuous high glucose are typically pathological features of DR [Citation19]. The migration of endothelial cells is the fundamental step of angiogenesis. Furthermore, most endothelial cells including HRECs rapidly form a capillary-like network that recapitulates the late stage of angiogenesis when seeded on Matrigel [Citation2]. To gain further insight into the potential role of FENDRR in the proliferation and angiogenesis of HRECs, we overexpressed or knocked down FENDRR in HRECs to evaluate the effect of FENDRR expression on cell proliferation, migration, and capillary morphogenesis of HRECs under normal or high glucose condition in vitro.
The overexpression and knockdown efficiency of FENDRR in HRECs was determined by RT-qPCR (). It was observed that the proliferative and migrative ability of HRECs were enhanced by 25 mM high glucose compared with the 5 mM glucose group. Furthermore, 25 mM glucose enhanced HRECs to form more capillary-like network compared with the normal 5 mM glucose condition. Importantly, FENDRR overexpression significantly promoted the high-glucose-induced HRECs proliferation (), migration ()), and capillary morphogenesis (). In contrast, FENDRR knockdown significantly inhibited the high-glucose-induced HRECs proliferation (), migration (), and capillary morphogenesis (). Meanwhile, FENDRR overexpression greatly increased the protein expression of VEGF (a pivotal marker of angiogenesis) in HRECs, whereas FENDRR knockdown exerted opposite effects ().
Figure 3. Effect of FENDRR expression on the high-glucose-induced HRECs proliferation, migration, and capillary morphogenesis. (a) The overexpression and knockdown efficiency of FENDRR in HRECs was determined by RT-qPCR. (b) HRECs that had been transfected with the designated plasmids were cultured under 5 or 25 mM glucose condition for 48 h. Then, (b) CCK-8 assay was performed to assess cell proliferation, (c) Transwell migration assay was performed to measure cell migration (scale bar: 100 μm), (d) Matrigel assay was performed to assess tube formation (scale bar: 200 μm), (e) and Western blot was performed to examine the protein expression of VEGF. Photographs are representative of at least three individual experiments. The data are expressed as the mean ± SD from three independent experiments. **p < 0.01 vs. the vector group, ##p < 0.01 vs. the si-Ctrl group, aap<0.01 vs. the vector+5 mM group. ANOVA followed by LSD test was used to analyze he differences among multiple groups.
Discussion
Prior studies have noted the importance of lncRNAs, such as lncRNA MIAT [Citation7], HOTTIP [Citation8], ANRIL (antisense RNA to INK4 locus) [Citation20], MEG3 (maternally expressed gene 3) [Citation21], and MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) [Citation22], in regulating the development of DR. This study provides the first evidence that lncRNA FENDRR expression was increased in both blood from DR patients and high-glucose-induced HRECs compared with the normal group. Furthermore, FENDRR promotes high-glucose-induced proliferation, migration, capillary morphogenesis, and VEGF expression in HRECs.
Researchers have reported that FENDRR knockdown significantly reduced, whereas FENDRR overexpression significantly increased expression of its neighboring gene FOXF1 [Citation14,Citation15]. Ren et al. [Citation16] indicated that deletion of FOXF1 from endothelial cells decreased expression of FENDRR, inhibited VEGF signaling, and reduced endothelial proliferation. VEGF is a secreted angiogenic mitogen and serves as a crucial mediator for the development of DR [Citation2,Citation17]. The blockable of the angiogenesis marker VEGF seems to be one of the strategies to treat DR [Citation2,Citation5]. Thus, we examined VEGF expression in DR patients and high-glucose-induced HRECs. Data in the present study showed that the serum VEGF level was increased in DR patients compared with the control subjects. Moreover, high glucose treatment dose- and time-dependently upregulated expression of VEGF secreted from HRECs. These observations in this study were in accordance with the aforementioned studies [Citation1,Citation17,Citation23].
LncRNAs can induce or reduce protein translation via alternative splicing, turnover, export, and translocation of mRNAs [Citation5]. For example, Thomas et al. [Citation20] have shown that knockdown of lncRNA ANRIL decreases the diabetes-induced upregulation of retinal VEGF expression, and prevents the functional consequences of VEGF and retinal microvascular permeability in DR. Herein, the effect of FENDRR expression on VEGF expression in high-glucose-induced HRECs were then explored. Our results showed that FENDRR overexpression significantly promoted, whereas FENDRR knockdown significantly reduced the high-glucose-induced VEGF expression in HRECs.
The abnormal proliferation and angiogenesis of retinal endothelial cells induced by continuous high glucose are typically pathological features of DR [Citation19]. Several reports have highlighted the inhibitory role of FENDRR in regulating cancer cell proliferation, migration, and invasion [Citation10,Citation12]. For example, Zhang et al. [Citation11] have recently found that FENDRR overexpression inhibits proliferation, invasion, and migration of prostate cancer cells in vitro. Miao et al. [Citation14] have also demonstrated that stable overexpression of FENDRR suppresses lung cancer cell migration and invasion by regulating epithelial-mesenchymal transition. Contrary to the abovementioned literature, a previous study showed that FENDRR overexpression promote proliferation, migration and invasion of osteosarcoma cells [Citation15]. Consistent with the pro-proliferation effect of FENDRR in osteosarcoma cells, the results in this study showed that FENDRR promotes the high-glucose-induced proliferation and migration in HRECs. The reasons for this discrepancy in the role of FENDRR in regulating cell proliferation and migration are unclear but may relate to differences in cell type and cell treatment.
Conclusion
In conclusion, the decreased expressed lncRNA FENDRR exerts a crucial role in the DR progression. The findings in this study demonstrate that FENDRR promotes high-glucose-induced proliferation and, in particular, angiogenesis of HRECs. Thus, inhibition of FENDRR may serve as a potential target for anti-angiogenic therapy for diabetes-related microvascular complications including DR.
Author contribution
Y. Shi designed the study; Y. Shi, C. Chen, Y. Xu, Y. Liu performed the experiments; Y. Shi, H. Zhang, Y. Liu analyzed the data; Y. Shi contributed analytical tools; Y. Shi drafted the manuscript. All authors read and approve the manuscript.
Acknowledgments
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen Z, Liu G, Xiao Y, et al. Adrenomedullin(22-52) suppresses high-glucose-induced migration, proliferation, and tube formation of human retinal endothelial cells. Mol Vis. 2014;20:259–269. PubMed PMID: PMC3945807
- Chen X, Li J, Li M, et al. KH902 suppresses high glucose-induced migration and sprouting of human retinal endothelial cells by blocking VEGF and PIGF. Diabetes Obes Metab. 2013;15(3):224–233.
- Yang X, Song JH, Cheng Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014 Jun;63(6):881–890. PubMed PMID: 24000294; PubMed Central PMCID: PMCPmc4612639. eng.
- Li CP, Wang SH, Wang WQ, et al. Long noncoding RNA-Sox2OT knockdown alleviates diabetes mellitus-induced retinal ganglion cell (RGC) injury. Cell Mol Neurobiol. 2017 Mar;37(2):361–369. 10.1007/s10571-016-0380-1. PubMed PMID: 27193103; eng
- Gong Q, Su G. Roles of miRNAs and long noncoding RNAs in the progression of diabetic retinopathy. Biosci Rep. 2017 Dec 22;37(6). PubMed PMID: 29074557; PubMed Central PMCID: PMCPmc5705777. eng. DOI: 10.1042/bsr20171157
- Jae N, Dimmeler S. Long noncoding RNAs in diabetic retinopathy. Circ Res. 2015 Mar 27;116(7):1104–1106. PubMed PMID: 25814678; eng.
- Zhang J, Chen M, Chen J, et al. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Biosci Rep. 2017 Apr 30;37(2): pii: BSR20170036. PubMed PMID: 28246353; PubMed Central PMCID: PMCPmc5408653. eng
- Sun Y, Liu Y. LncRNA HOTTIP improves diabetic retinopathy by regulating the p38-MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22(10):2941–2948.
- Grote P, Wittler L, Hendrix D, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013 Jan 28;24(2):206–214. PubMed PMID: 23369715; PubMed Central PMCID: PMCPmc4149175. eng.
- Li Y, Zhang W, Liu P, et al. Long non-coding RNA FENDRR inhibits cell proliferation and is associated with good prognosis in breast cancer. Onco Targets Ther. 2018;11:1403–1412.
- Zhang G, Han G, Zhang X, et al. Long non-coding RNA FENDRR reduces prostate cancer malignancy by competitively binding miR-18a-5p with RUNX1. Biomarkers. 2018;23(5):435–445.
- Xu T, Huang M, Xia R, et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol. 2014;7:63.
- Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014; 5(4). PubMed PMID: 25483404; PubMed Central PMCID: PMCPmc4581346. eng. DOI:10.4161/21541272.2014.944014
- Miao L, Huang Z, Zengli Z, et al. Loss of long noncoding RNA FOXF1-AS1 regulates epithelial-mesenchymal transition, stemness and metastasis of non-small cell lung cancer cells. Oncotarget. 2016 Oct 18;7(42):68339–68349. PubMed PMID: 27577075; PubMed Central PMCID: PMCPmc5356559. eng.
- Kun-Peng Z, Chun-Lin Z, Xiao-Long M. Antisense lncRNA FOXF1-AS1 promotes migration and invasion of osteosarcoma cells through the FOXF1/MMP-2/-9 pathway. Int J Biol Sci. 2017;139:1180–1191. PubMed PMID: PMC5666333.
- Ren X, Ustiyan V, Pradhan A, et al. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ Res. 2014 Sep 26;115(8):709–720. PubMed PMID: 25091710; PubMed Central PMCID: PMCPmc4810682. eng.
- Wu Y, Zhang Q, Zhang R. Kaempferol targets estrogen-related receptor alpha and suppresses the angiogenesis of human retinal endothelial cells under high glucose conditions. Exp Ther Med. 2017 Dec;14(6):5576–5582. PubMed PMID: 29285095; PubMed Central PMCID: PMCPmc5740587. eng.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408.
- Qiu F, Tong H, Wang Y, et al. Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci Biotechnol Biochem. 2018 Aug;82(8):1366–1376. PubMed PMID: 29658404; eng.
- Thomas AA, Feng B, Chakrabarti S. ANRIL: a regulator of VEGF in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):470–480. PubMed PMID: 28122089; eng.
- Qiu GZ, Tian W, Fu HT, et al. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem Biophys Res Commun. 2016 Feb 26;471(1):135–141. PubMed PMID: 26845358; eng.
- Liu JY, Yao J, Li XM, et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5(10):e1506. PubMed PMID: PMC4649539.
- Ozturk BT, Bozkurt B, Kerimoglu H, et al. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vis. 2009;15:1906–1914. PubMed PMID: PMC2751798
