ABSTRACT
Sko1 plays a key role in the control of gene expression by osmotic and oxidative stress in yeast. We demonstrate that the decrease in chronological lifespan (CLS) of hog1Δ cells was suppressed by SKO1 deletion. sko1Δ single mutant cells were shown to have a longer CLS, thus implicating Sko1 in the regulation of their CLS.
In the yeast Saccharomyces cerevisiae, Sko1 is a repressor that mediates HOG pathway-dependent regulation in association with Tup1-Cyc8/Ssn6 and functions as a general repressor of the transcription of genes involved in a wide variety of processes, such as the response to osmotic and oxidative stress [Citation1,Citation2]. Hog1 is the MAPK acting in the high osmolarity glycerol (HOG) pathway, which is involved in the response to hyper-osmotic stress, oxidative stress, and modulation of cell-cycle progression [Citation1,Citation3]. Previously, we showed that the growth defect in HOG1 null mutant cells under the condition of high osmolarity was restored by an additional mutation of the tup1-484, sko1-18 or cyc8-389 gene [Citation4]. In these mutants, the expression of the genes under the control of DNA-binding proteins other than Sko1 was apparently normal; however, the Sko1-regulated genes were derepressed under non-stress conditions, suggesting that these mutations appeared to cause a defect specifically in the repression of the Sko1-regulated genes [Citation4]. The sko1Δ mutant was previously reported to affect tolerance to oxidative stress, and the sensitivity of the hog1Δ mutant to hydrogen peroxide was suppressed by the loss of SKO1 [Citation1]. Thus, we examined the growth phenotype of yeast cells with mutated tup1-484, sko1-18 or cyc8-389 in combination with deletion of HOG1. As reported previously [Citation1], loss of function of SKO1 (sko1-18) suppressed the hydrogen peroxide sensitivity of the hog1Δ mutant ()). Mutation of tup1-484 or cyc8-389 also partially suppressed it ()).
Figure 1. Defective SKO1 function suppressed oxidative stress of the hog1Δ strain and extended the chronological lifespan (CLS).
(a) Growth of various strains on solid YPD medium containing the indicated concentrations of hydrogen peroxide. Ten-fold serially diluted cells of W303-1A (wild-type; WT), hog1Δ, hog1Δ tup1-484, hog1Δ sko1-18, and hog1Δ cyc8-389 were spotted onto YPD solid medium containing various concentrations of hydrogen peroxide. The cells were then grown at 25ºC for 3 days before visualization. (b) CLS curves for WT, hog1Δ, hog1Δ tup1-484, hog1Δ sko1-18, and hog1Δ cyc8-389 are shown. Yeast CLS analysis was performed in liquid SDC medium as previously described [Citation12]. Briefly, SDC cultures grown overnight were diluted (2 x 106 cells/ml) in fresh SDC medium and incubated at 28ºC with shaking at 180 rpm. Viability was measured by plating aging cells onto YPD plates and monitoring Colony Forming Units (CFUs) starting from day 3, which was considered to be the initial survival (100%). All data are represented as the average of 3 independent experiments conducted at the same time. At least 2 sets of CLS experiments were performed with similar outcomes. CLS assays were performed with SDC medium [Citation12]. Data represent biological replicates. GraphPad Prism 7 (GraphPad Software) was used for comparison of CLS, and p values were derived from a two-way ANOVA with time and strain used as independent factors. A p value less than p< 0.05 was defined as statistically significant. (c) CLS curves of WT, hog1Δ, and hog1Δ sko1 are shown. Experimental conditions were as described in (b).
![Figure 1. Defective SKO1 function suppressed oxidative stress of the hog1Δ strain and extended the chronological lifespan (CLS).(a) Growth of various strains on solid YPD medium containing the indicated concentrations of hydrogen peroxide. Ten-fold serially diluted cells of W303-1A (wild-type; WT), hog1Δ, hog1Δ tup1-484, hog1Δ sko1-18, and hog1Δ cyc8-389 were spotted onto YPD solid medium containing various concentrations of hydrogen peroxide. The cells were then grown at 25ºC for 3 days before visualization. (b) CLS curves for WT, hog1Δ, hog1Δ tup1-484, hog1Δ sko1-18, and hog1Δ cyc8-389 are shown. Yeast CLS analysis was performed in liquid SDC medium as previously described [Citation12]. Briefly, SDC cultures grown overnight were diluted (2 x 106 cells/ml) in fresh SDC medium and incubated at 28ºC with shaking at 180 rpm. Viability was measured by plating aging cells onto YPD plates and monitoring Colony Forming Units (CFUs) starting from day 3, which was considered to be the initial survival (100%). All data are represented as the average of 3 independent experiments conducted at the same time. At least 2 sets of CLS experiments were performed with similar outcomes. CLS assays were performed with SDC medium [Citation12]. Data represent biological replicates. GraphPad Prism 7 (GraphPad Software) was used for comparison of CLS, and p values were derived from a two-way ANOVA with time and strain used as independent factors. A p value less than p< 0.05 was defined as statistically significant. (c) CLS curves of WT, hog1Δ, and hog1Δ sko1 are shown. Experimental conditions were as described in (b).](/cms/asset/3b841c10-ab60-4d50-a933-fcce20e719f6/tbbb_a_1571901_f0001_c.jpg)
Because lifespan extension is often associated with increased resistance to various stresses, including oxidative stress [Citation5], we measured the chronological lifespan (CLS) by monitoring the survival periods of nondividing yeast cells that had passed the postdiauxic phase [Citation6]. As shown in ), the hog1Δ strain had a shortened CLS in the absence of osmotic stress, suggesting that HOG pathway was required for cell survival under normal conditions (p< 0.0001). To investigate whether the tup1-484, sko1-18 or cyc8-389 mutation could suppress the short-lived of hog1Δ cells, we measured the CLS ()). Notably, hog1Δ tup1-484, hog1Δ sko1-18, and hog1Δ cyc8-389 mutants were found to have a longer CLS compared with that of hog1Δ strains (p< 0.0001). Further, the shortened CLS of hog1Δ was abolished by the deletion of SKO1 (); p< 0.0001). Moreover, we noticed that hog1Δ sko1Δ strains exhibited a CLS significantly longer than the wild-type (WT) (); p< 0.0001), suggesting that the Sko1 protein might play a role in lifespan. Both hog1Δ tup1Δ and hog1Δ cyc8Δ double-deletion cells were highly flocculent (data not shown), thus preventing us from utilizing these double-deletion mutants for further analyses. Further, because our previous data have shown that tup1-484 and cyc8-389 mutants were defective in the repression of Sko1-dependent genes [Citation4], we used the sko1Δ strain in most of the subsequent experiments.
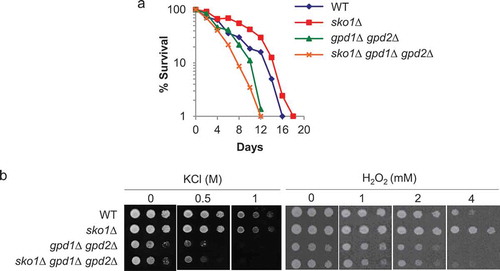
Next, we examined whether SKO1-deletion yeast strains showed an extended CLS. Strikingly, the loss of SKO1 on the WT background significantly extended the CLS (); p< 0.0001), thus implicating transcriptional release from the repression of Sko1-target gene(s) in the increase in the CLS. Previous studies have shown that hyperosmolarity increases the replicative lifespan (RLS) [Citation7], which is defined as the number of daughter cells that mother cells produce before undergoing senescence [Citation8], in budding yeast. In this case, the biosynthesis of glycerol, achieved by adding sorbitol to the growth medium, is required for extending the RLS [Citation7]. To determine if CLS extension by SKO1 deletion strains was mediated by the synthesized glycerol, we tested the CLS by deleting GPD1 and GPD2, encoding glycerol-3-phosphate dehydrogenase. CLS of gpd1Δ gpd2Δ double deletion mutants was comparable with that of the WT cells (); p= 0.0686). The extended CLS of sko1Δ strains was abolished by the deletion of both GPD1 and GPD2 (); p< 0.0001), suggesting that glycerol synthesis appears to be required for CLS extension by sko1Δ under normal conditions. In order to determine further if beneficial effects of sko1Δ were the result of glycerol synthesis, we tested the stress resistance of the sko1Δ gpd1Δ gpd2Δ triple mutants ()). Osmotic (KCl) and hydrogen peroxide (H2O2) stress resistance of sko1Δ cells was eliminated by deleting GPD1 and GPD2, and the stress sensitivity of sko1Δ gpd1Δ gpd2Δ triple mutant strains was similar to that of gpd1Δ gpd2Δ cells ()). Altogether, these data demonstrated that glycerol synthesis might be a key downstream factor that mediated the effects of sko1Δ on CLS extension and stress resistance. Further, we noted that sko1Δ mutants were more resistant to oxidative stress than WT strains; however, this mutation did not give rise to a clear osmotic resistance (), 1.0 M KCl) despite the fact that the sko1Δ mutation suppresses the growth defect of hog1Δ mutant cells under the condition of high osmolarity [Citation1,Citation4]. Thus, our data suggest that there are little beneficial effects of sko1Δ on the WT background for vegetative growth under conditions of osmotic stress.
Figure 2. CLS extension by loss of SKO1 strains is required for the glycerol synthesis.
(a) CLS curves for WT, sko1Δ, gpd1Δ gpd2Δ, and hog1Δ gpd1Δ gpd2Δ are shown. Experimental conditions were as described in . (b) Osmotic (KCl) (left panel) and hydrogen peroxide (right panel) test. Ten-fold serially diluted cells of WT, sko1Δ, gpd1Δ gpd2Δ, and sko1Δ gpd1Δ gpd2Δ strains were spotted onto solid medium containing KCl or hydrogen peroxide at 25ºC and incubated for 3 days.
Upon osmotic stress, Sko1 is inactivated in a HOG pathway-dependent manner [Citation2]. Since hyperosmolarity increases CLS [Citation9], we tested whether CLS extension of sko1Δ strains was associated with high osmolarity. Consistent with a previous report [Citation9], we found that treatment with hyperosmolarity (sorbitol) appeared to prolong the CLS of the WT (; p< 0.0001). We also noted that hyperosmolarity significantly increased the CLS of sko1Δ cells during early and middle period of the lifespan (until 20 days) compared with that of WT strains under osmotic-stressed conditions (; p< 0.0001). These data showed that CLS extension by sko1Δ or by osmotic stress acted through bifurcate pathways.
Figure 3. CLS extension by sko1Δ is distinct from hyperosmolarity-promoted longevity.
CLS curves for WT and sko1Δ in the presence or absence of hyperosmolarity (0.75 M sorbitol) are shown. Experimental conditions were as described in .
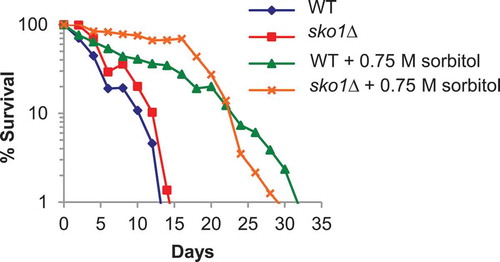
The extended CLS of the sko1Δ mutants was reported previously, as shown by yeast genome-wide screening [Citation10]. In that study genetic interactions were assessed via competition-based fitness measurements in liquid cultures, with the addition of fluorescent markers for tracking cell viability and the use of robotic manipulation to inoculate and measure cell growth [Citation10]. However, the extended CLS of the sko1Δ mutants has not been validated yet by using the standard CLS method. Here we have presented evidence that the loss of SKO1 led to enhanced longevity under non-stressed conditions, suggesting that the beneficial effects of sko1Δ resulted in adaptation to oxidative stress that may be encountered during chronological aging. Although hyperosmolarity derepresses Sko1-regulated genes [Citation1], the extended CLS upon hyperosmolarity was further lengthened by SKO1 deletion, suggesting that sustained expression of Sko1-regulated genes in the sko1Δ strains might have enhanced the protective effects against aging. What is the candidate gene(s) derepressed in sko1Δ mutants and involved in the extension of CLS? One candidate is GPD1 and/or GPD2, although these genes have not been identified as Sko1-regulated ones [Citation1]. GRE2, AHP1, SFA1, GLR1, and YML131w are genes down-regulated by SKO1; and they have been implicated in protection from oxidative stress [Citation1], thus making them other candidates. Identification of the actual effector mechanisms by which loss of SKO1 protects against chronological aging is an important future challenge. During ethanol fermentation, yeast cells encounter various stresses including osmotic, oxidative, and ethanol stresses [Citation11]. Thus, our findings might be useful in breeding to produce strains with tolerance to these fermentation-associated stresses.
Author contribution
M.M. conceived the study and designed the experiments. K.M., S.M., and K.Y. performed the experiments and analyzed the data. M.K., K.K., and D.H. discussed the results and contributed to improvement of the manuscript. M.M. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rep M, Proft M, Remize F, et al. The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol Microbiol. 2001;40:1067–1083.
- Proft M, Struhl K. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol Cell. 2002;9:1307–1317.
- Saito H, Posas F. Response to hyperosmotic stress. Genetics. 2012;192:289–318.
- Kobayashi Y, Inai T, Mizunuma M, et al. Identification of Tup1 and Cyc8 mutations defective in the responses to osmotic stress. Biochem Biophys Res Commun. 2008;368:50–55.
- Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326.
- Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81.
- Kaeberlein M, Andalis AA, Fink GR, et al. High osmolarity extends life span in Saccharomyces cerevisiae by a mechanism related to calorie restriction. Mol Cell Biol. 2002;22:8056–8066.
- Kennedy BK, Austriaco NR, Zhang J, et al. Mutation in the silencing gene SIR4 can delay aging in S cerevisiae. Cell. 1995;80:485–496.
- Smith DL, McClure JM, Matecic M, et al. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–662.
- Garay E, Campos SE, González de la Cruz J, et al. High resolution profiling of stationary phase survival reveals yeast longevity factors and their genetic interactions. PLoS Genet. 2013;10:E1004168.
- Gibson BR, Lawrence SJ, Leclaire JP, et al. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev. 2007;31:535–569.
- Ogawa T, Tsubakiyama R, Kanai M, et al. Stimulating S-adenosyl-l-methionine synthesis extends lifespan via activation of AMPK. Proc Natl Acad Sci U S A. 2016;113:11913–11918.
