ABSTRACT
CENPU (centromere protein U), a centromere component essential for mitosis, relates with some cancers progression. However, it is not well illustrated in lung adenocarcinoma (LAC). Here, we aimed to investigate the potential effect of CENPU on LAC progression and prognosis. In this experiment, expression level of CENPU and association between its expression and LAC patients’ clinicopathological characteristics and prognosis were analyzed. The proliferation, migration and invasive abilities of LAC cells were determined by CCK-8, colony formation, transwell assays. Western blot was used to detect PI3K/AKT signaling key proteins. We found CENPU level was overexpressed in LAC tissues on comparing normal tissues. Moreover, CENPU overexpression correlated with clinicopathological variables and predicted an independent prognostic indicator in LAC patients. Functionally, CENPU downregulation significantly inhibited LAC cell proliferation, migration and invasion in, which was possibly mediated by PI3K/AKT pathway inactivation. Our findings insinuate targeting CENPU may be a potential therapeutic strategy for LAC.
Graphical Abstract
CENPU overexpression relates with LAC prognosis and CENPU silencing represses LAC cell proliferation and migration.
Lung adenocarcinoma (LAC), known as the familiar common histological subtype of non-small-cell lung cancer (NSCLC), which is the first cause of cancer-related deaths worldwide [Citation1–Citation3]. Although lung cancer mortality rate has been decreasing owing to advances made in the treatment of LAC, the prognosis of advanced lung adenocarcinoma is very poor because of its invasion and metastasis [Citation4,Citation5]. Therefore, it is of utmost importance to have a better understanding of the molecular mechanism of invasion and migration of LAC and find a more sensitive and novel target for predicting the prognosis.
CENPU, also dominated as KLIP1/MLF1IP/CENPU50/PBIP1 (hereafter referred to as CENPU), encodes centromere protein U which is a centromere component essential for mitosis and localizes in both the nucleus and the cytoplasm [Citation6,Citation7]. The growth rate and the time for these cells to complete mitosis of CENPU-deficient cells are observed to be slower and longer than that of wild-type cells [Citation6]. CENPU is abundantly expressed in human erythroid progenitor cells and deficiency of it inhibits normal erythroid proliferation and CENPU is involved in the pathogenesis of polycythemia vera [Citation8], suggesting it mediates the progression of erythropoietic disorder.
It has been demonstrated that CENPU plays a carcinogenesis action in several malignancies. Jean M. et al has documented that ectopic overexpression of CENPU increase the aggressiveness of two human prostate cancer cell lines [Citation9]. Another report has suggested that elevated CENPU is found in a number of human and rat g glioblastoma cell lines, consistently, CENPU is highly expressed in rat glioblastoma tumor model [Citation10]. Upregulated expression of CENPU is identified in luminal breast cancer tissue and high-level of it is positively correlated with progression and prognosis of luminal breast cancer [Citation11]. CENPU could significantly promote prostate cancer cell proliferation and colony formation and significantly inhibit apoptosis [Citation12]. CENPU is also reported to be overexpression in human bladder cancer and elevation of it is associated with bladder tumor progression and reduced survival [Citation13]. Nevertheless, little is known about whether and how CENPU is involved in the progression and prognosis of LAC.
Accordingly, the purpose of the present study was to investigate the potential function of CENPU in progression and prognosis of LAC, thus to gain a novel biological target for LAC treatment.
Materials and methods
Biostatistics mining of database
Public data of CENPU gene expression of for LAC patients and normal patients collected from 5 cohorts, which were obtained from Oncomine database. For the survival analysis of CENPU, we analyzed the 484 LAC patients’ clinical information obtained from The Cancer Genome Atlas (TCGA) database.
Cell cultures
Human LAC cell lines (A549 and Calu3) and normal human bronchial epithelial cell BEAS-2B were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were culture in RPMI 1640 medium (Thermo Fisher Scientific Inc., Waltham, MA) containing 10% fetal bovine serum (FBS) and 0.1% streptomycin-penicillin (Thermo Fisher Scientific Inc.) atmosphere at 37°C in a humidified incubator with 5% CO2.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA extraction of tissues and cells were used the Trizol Reagent (Thermo Fisher Scientific Inc.) following the manufacturer’s instructions. Then, the cDNA was synthesized and qRT-PCR was carried out using cDNA Reverse Transcription kit and StepOne Real-Time PCR system (Applied Biosysems, CA, USA) in an ABI Prism 7900HT instrument (Applied Biosysems) according to the supplier’s recommendations, respectively. The used primers used were as follows. CENPU: F: 5ʹ- GGAGC TTCCA GTCGC TCG −3ʹ, R: 5ʹ- TTCTT TGAAC GTCTT GCGCC −3ʹ; GAPDH: F: 5ʹ-GGAGC GAGAT CCCTC CAAAA T −3ʹ, R: 5ʹ-GGCTG TTGTC ATACT TCTCA TGG-3ʹ. All values were normalized to the level of GAPDH. The relative mRNA expression of CENPU was calculated using 2−ΔΔCt comparative method. All experiments were performed in triplicate.
SiRNA transfection
Small interfering RNAs (siRNAs) targeting CENPU (si-CENPU#1: 5ʹ- AGCUG GUCAA AAGUG CAAG-3ʹ; si-CENPU#2: 5ʹ- GUGCA AGCCU AUUGA CGUG-3ʹ) and negative control siRNA (NC-siRNA, si-NC) were purchased from Thermo Fisher Scientific Inc.. siRNAs were transfected into lung cell lines using Lipofectamine 2000 (Thermo Fisher Scientific Inc.) in accordance with the manufacturer’s introductions. The efficacy of transfection was measured by testing CENPU mRNA and protein using qRT-PCR and western blot assays. The experiments were carried out three times.
Cell counting −8 (CCK-8) and cell colony formation assay
Cells were collected at 24 h, 48 h, 72 h and 96 h after transfection. For cell viability detection using CCK-8 assay, 10 μL CCK-8 was added into the test wells and cells were additionally cultured at 37°C for 2 h. A microplate reader was used to obtain the absorbance at 450 nm. The absorbance with optical density (OD) was measured at a wavelength of 450 nm (OD450) with a microplate reader (Bio-Rad, Hercules, CA, USA). For performing the cell colony formation assay, cells (300 cells/well) were seeded in 6-well plates and were incubated at 37°C for two weeks. The culture medium was replaced with fresh culture medium every three days. After washing twice with PBS, colonies were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 20 min. Then, the visible colonies were counted and images were taken using a high-resolution camera. Experiments were repeated three times.
Cell migration and invasion assays
Cells migration was measured by wound-healing assay. When the cells reached 90% confluence, an identical and straight wound was made through the cells using a sterile 200 μL plastic pipette tip. Then, the medium was removed and the remaining cells were washed with phosphate-buffered saline (PBS) twice, and maintained in a serum-free medium for 24 h. Images were taken using a microscope (Olympus Corporation) at 0, 24 h after scratching, respectively. All procedures were conducted in triplicate.
Invasion of LAC cells were assessed in vitro using a Boyden chamber Transwell system (Corning, Tewksbury, MA, USA). 24 hours after transfection, Matrigel (BD Bioscience, San Jose, USA) was added to the filter and incubated at 37°C for 1 h. Cells were seeded in Transwell plates, then the upper chamber was plated with 1 × 105 cells in 100 µL serum-free DMEM and the lower chamber was filled with 600 µL DMEM with 10% FBS. After 24 h of incubation at 37°C, the cells on the upper surface of the filter were removed using cotton swabs. The migrated cells that remained on the bottom surface were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet. The cells were counted in five randomly chosen fields under a microscope. Experiments were performed in triplicate.
Western blot analysis
Cells were harvested 48 h after transfection and cell lysate was used for total protein extraction. Total cells proteins were extracted using radioimmunoprecipitation (RIPA) lysis buffer (Beyotime Inc., Shanghai, China) and proteins concentrations were tested using BCA protein assay kit (Beyotime). Proteins were then seperated into an 8%-12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% skimmed milk in 1 × TBST for 1.5 h. Anti-CENPU, phosphatidylinositol-3-kinase (PI3K) PI3K, phosphorylated- (p-)PI3K, protein kinase B (AKT), p-AKT and GAPDH (1:1000) primary antibodies were added to the solution, respectively. Membranes were incubated at 4°C overnight. Secondary antibodies were then added and membranes were incubated at room temperature for 2 h. Bands were visualized using enhanced chemiluminescence (ECL) kit (Beyotime) and the Bio-Rad Gel Doc XR þ system (Bio-Rad, Hercules, CA, USA). Expression levels of proteins were measured using Quantity One software (Bio-Rad Laboratories). GAPDH was used as an internal control. Each experiment was repeated three times.
Statistical analysis
The statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software Inc, LaJolla, CA, USA). Correlation analysis was performed using Chi-squared (χ2) analysis. Kaplan-Meier plot and log-rank test were performed for survival analysis. Univariate and multivariate Cox proportional hazard regression models were performed to identify independent prognostic factors in association with the prognosis of LAC patients. Student’s t-test was used to assess differences between two groups. All values were expressed as means ± standard deviation (SD). P < 0.05 was considered to be statistically significant.
Results
CENPU is over-expressed in LAC tissues and cells
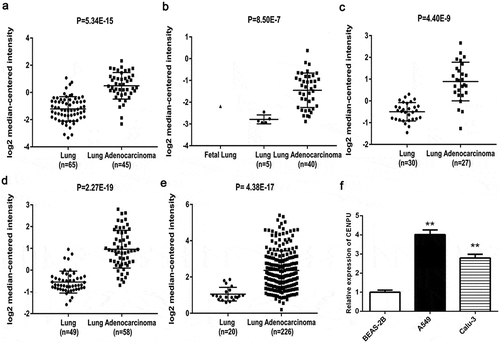
By analyzing data downloaded from the Oncomine datasets, the mRNA level of CENPU in LAC tissues was higher than that in normal lung tissues (), P < 0.01). Moreover, to assess whether CENPU is upregulated in LAC cell, we used qRT-PCR method to measure the level of CENPU in LAC cell lines and normal lung cell. As shown in ), the mRNA expression level of CENPU was evidently elevated in A549 and Calu-3 cells in comparison with normal lung cell BEAS-2E (P < 0.01). A549 cell (expressing higher level of CENPU) was used to perform in the subsequent experiments. All data suggest that CENPU is over-expressed in LAC, indicating CENPU plays a potential oncogene role in LAC progression.
Figure 1. CENPU was upregulated in both LAC clinical tissues and cells.
(a-e), Relative level of CENPU in LAC tissues and normal controls collected from 5 cohorts. (f), Relative mRNA level of CENPU in LAC cells and normal lung cells. The data are presented as the means ± SD from three independent experiments. **P < 0.01.
High CENPU was correlated with clinicopathological features
demonstrated that the correlation between CENPU expression and clinicopathological variables. The results showed that overexpression of CENPU was associated with age (P = 0.032), gender (P = 0.008), Pathologic-Stage (P = 0.025), Pathologic-N (P = 0.001).
Table 1. The relationship between CENPU expression and clinicopathological features of LAC patients.
Overexpression of CENPU was an independent prognostic indicator that correlated with poor survival in LAC patients
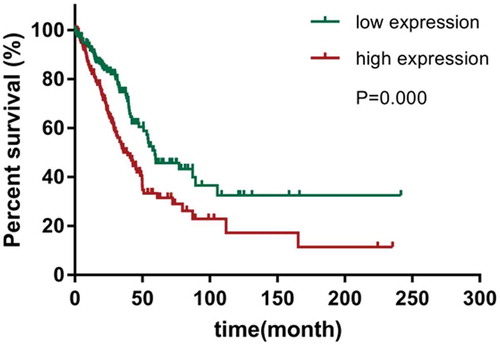
To evaluate the clinical value of CENPU expression in LAC patients survival, Kaplan-Meier plot and log-rank test were performed. As shown in , the results suggested that LAC patients with expressing high CENPU levels had conspicuously lower overall survival (OS) than those expressing low CENPU levels (P = 0.000). In addition, as shown in , univariate Cox regression analysis revealed that CENPU expression, Clinical-stage, Pathologic-T/N/M stage (all P < 0.05) were prognostic factors for poor prognosis of LAC patients. Multivariate Cox regression analysis for all of the significant variables in the univariate analysis further showed that CENPU expression (P = 0.006), node-stage (P = 0.000) were independent prognostic indicator for the OS time of LAC patients.
Table 2. Univariate and multivariate analysis for overall survival in LAC.
Knockdown of CENPU inhibited LAC cells proliferation
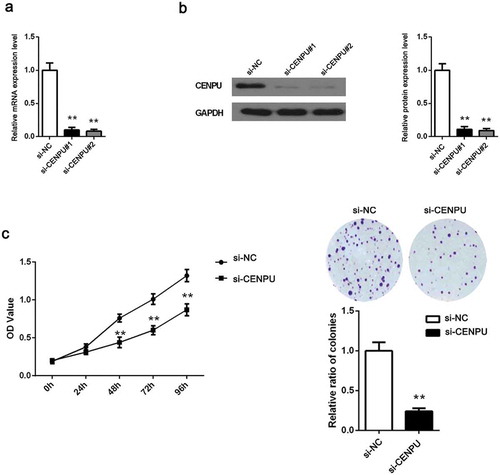
To determine the potential function of CENPU in promoting LAC progression, CENPU was downregulated by transfecting siRNAs harboring RNAi sequence targeting the human CENPU and assessed the effects of CENPU silencing on LAC cell proliferation. Firstly, the transfection efficiency was identified by testing mRNA and protein level of CENPU after transfection of siRNA (si-CENPU#1, si-CENPU#2 and si-NC) using qRT-PCR and western blot assays, respectively. The results suggested that CENPU both in mRNA and protein level was effectively downregulated in si-CENPU#1 and si-CENPU#2 groups compared with si-NC group (), P < 0.01). We chose one of si-CENPU#1 and si-CENPU#2 groups as si-CENPU group to carry out in the following tests. Additionally, the OD values of LAC cell, measured by performing CCK-8 assay, suggested that the proliferation of A549 cell was significantly decreased in si-CENPU group as compared to the si-NC group (), P < 0.01). All results indicates that downregulation of CENPU represses the proliferation of LAC cell.
Figure 3. CENPU silencing inhibited LAC cell proliferation.
(a-b), The expression of CENPU silenced A549 cells were identified by measuring its mRNA and protein level using qRT-PCR and western blot analyses. (c), The OD values of A549 cells were detected by CCK-8 assay. (d), Colony formation assay determined the effect of CENPU knockdown in colony formation ability in A549 cells. The data are presented as the means ± SD from three independent experiments. **P < 0.01.
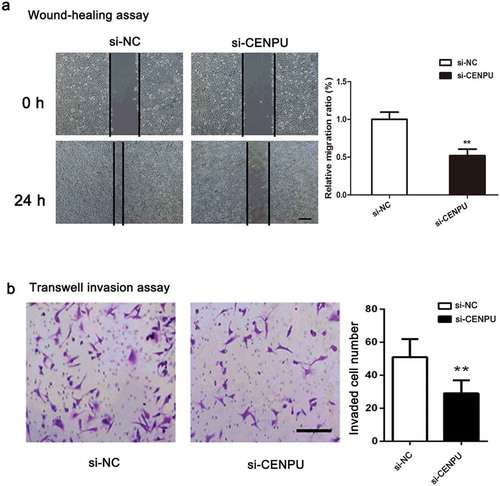
Knockdown of CENPU repressed LAC cells migration and invasion
To further assess the effect of CENPU in migration and invasion capabilities of LAC cell, wound-healing assay and transwell invasion assay were carried out. The result of wound-healing assay showed that wound closures were obviously delayed in si-CENPU group, compared with si-NC group ()). Then, the result of transwell invasion assay showed that the invaded number of A549 cell was significantly decreased in si-CENPU group compared with si-NC group (), P < 0.01). In short, results suggests that downregulation of CENPU suppresses capabilities of LAC cell migration and invasion.
Figure 4. CENPU silencing repressed the migration and invasion of LAC cells.
(a), Cell migration was determined by a wound-healing assay in the A549 cells transfected with the si-SPOCK1 or si-NC, and the migration rate was quantified. (b), Transwell assays of A549 cells after treatment with si-SPOCK1 or si-NC. The data are presented as the means ± SD from three independent experiments. Scale bar 200 µm. Original magnification: ×200. **P < 0.01.
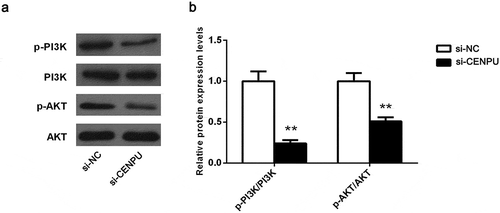
Knockdown of CENPU resulted in an inhibition of PI3K/AKT signaling pathway
Having established that PI3K/AKT signaling is of importance to tumor occurrence and development, in an effort to underlying molecular mechanism of CENPU regulating LAC progression, hallmarks of PI3K/AKT signaling including PI3K, p-PI3K, AKT, p-AKT were evaluate. The protein levels of p-PI3K and p-AKT were reduced in si-CENPU group while no detectable changed in PI3K and AKT, compared with si-NC group (, P < 0.01). Results indicate that CENPU plays an oncogenic role through PI3K/AKT signaling involvement.
Figure 5. Downregulation of CENPU suppressed the PI3K/AKT signaling pathway.
(a), Western blot analysis shows changes in the PI3K/AKT signaling pathway components in si-A549 cells. (b), Quantitative analysis of these proteins bands. The data are presented as the means ± SD from three independent experiments. **P < 0.01.
Discussion
In the current study, CENPU was identified to be overexpressed in LAC tissues and cells. CENPU overexpression was closely associated with LAC patients’ clinicopathological implications, including age, gender, Pathologic-Stage and Pathologic-N. Moreover, CENPU overexpression predicated poor OS of LAC patients. Importantly, CENPU was an independent prognostic indicator for OS of LAC patients. Functionally, downregulation of CENPU led to inhibitions in LAC cell proliferation, migration and invasion, which was possibly regulated by PI3K/AKT signaling suppression. Collectively, our finding provided a novel insight of LAC pathogenesis, indicating a potential molecular target for treating LAC in the further clinics.
Growing evidence has shown that CENPU is tightly associated with tumorigenesis in various cancers. CENPU has been documented to be overexpressed in some of cancer tissues and cells, namely, luminal breast cancer tissue, prostate cancer cells and glioblastoma cell lines [Citation10–Citation12]. There is no report about CENPU linkage with LAC pathogenesis up to date. In this study, the relative level of CENPU was elevated in LAC tissues on comparing normal controls by analyzing data downloaded from Oncomine datasets. Consistently, the mRNA level of CENPU in LAC cells was higher than normal lung cells. To determine the associate between CENPU overexpression and LAC patient prognosis, the clinical information was collected from TCGA database. The results revealed that elevation of CENPU was closely associated with poorer OS and it was considered as an independent prognostic marker of LAC patients. Furthermore, in an effort to evaluate the function of CENPU on LAC proliferation, we knocked down this gene using siRNA approach and investigated effect of si-CENPU on LAC cells proliferation. Results obtained from CCK-8 assay showed that OD value in si-CENPU group was significantly reduced compared with si-NC group. Collectively, all these data indicates that CENPU might be an oncogene in LAC.
Invasion and migration are known to be uncontrollable properties of tumorigenesis, barriers for treating cancers and thus exploration emphases for researchers [Citation14,Citation15]. To further determine the oncogenic role of CENPU in LAC, we used the wound-healing and transwell invasion assays to assess LAC cell migration and invasion. Our observations showed that abilities of migration and invasion in LAC cell were reduced in si-CENPU group in comparison with si-NC group. These results further demonstrate the oncogenic action of CENPU in LAC.
An increasing number of researches have reported that abnormality of PI3K/AKT signaling pathway is of significance to carcinogenesis in a variety of cancers [Citation16–Citation19]. Accumulating evidence has demonstrated that PI3K/AKT signaling pathway is implicated in the LAC progression [Citation20–Citation23]. In order to explore the underlying mechanisms by which CENPU regulate LAC cell proliferation, migration and invasion, our attention paid on PI3K/AKT signaling pathway. Western blot analysis showed that expression levels in key molecules of this signaling p-PI3K and p-AKT were downregulated in si-CENPU group in contrast with si-NC group while the levels of PI3K and AKT were stable. In light of the above, PI3K/AKT signaling might be considered as the rational mechanism by which CENPU regulates LAC progression. However, the precise molecular mechanisms need further studies to be elucidated deeply. Despite the biological functions of CENPU on LAC were determined in vitro only a cell line, there are some of validations to be confirmed through more cell lines and even in vivo animal model experiments.
In conclusion, based on our investigations, overexpression of CENPU was an independent prognostic indicator that correlated with poor survival in LAC patients. In addition, knockdown of CENPU inhibited LAC cell proliferation, migration and invasion, which was probably associated with PI3K/AKT signaling participation. Our findings indicate that CENPU is of importance to promoting tumorigenesis in LAC and provide a primary rationale for targeting CENPU as a promising approach in LAC treatment.
Author contribution
YYW and JL guided all the experiments, analyzed the data from biostatistics mining of database and wrote the manuscript. ZGW, LBP, RHZ performed the majority of experiments, contributed to analyzing data and generating the figures and gave input on the manuscript. All authors agreed and approved the manuscript to be published.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mazereeuw MV, Withrow DR, Nishri ED, et al. Cancer incidence and survival among metis adults in Canada: results from the Canadian census follow-up cohort (1992-2009). Cmaj. 2018;190:E320–e326.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12.
- Borczuk AC. Prognostic considerations of the new World Health Organization classification of lung adenocarcinoma. Eur Respir Rev. 2016;25:364–371.
- Li J, You W, Zheng D, et al. A comprehensive evaluation of clinicopathologic characteristics, molecular features and prognosis in lung adenocarcinoma with solid component. J Cancer Res Clin Oncol. 2018;144:725–734.
- Minoshima Y, Hori T, Okada M, et al. The constitutive centromere component CENP-50 is required for recovery from spindle damage. Mol Cell Biol. 2005;25:10315–10328.
- Rogon C, Ulbricht A, Hesse M, et al. HSP70-binding protein HSPBP1 regulates chaperone expression at a posttranslational level and is essential for spermatogenesis. Mol Biol Cell. 2014;25:2260–2271.
- Feng G, Zhang T, Liu J, et al. MLF1IP promotes normal erythroid proliferation and is involved in the pathogenesis of polycythemia vera. FEBS Lett. 2017;591:760–773.
- Winter JM, Gildea DE, Andreas JP, et al. Mapping complex traits in a diversity outbred F1 mouse population identifies germline modifiers of metastasis in human prostate cancer. Cell Syst. 2017;4:31–45 e6.
- Hanissian SH, Teng B, Akbar U, et al. Regulation of myeloid leukemia factor-1 interacting protein (MLF1IP) expression in glioblastoma. Brain Res. 2005;1047:56–64.
- Huang DP, Luo RC. MLF1IP is correlated with progression and prognosis in luminal breast cancer. Biochem Biophys Res Commun. 2016;477:923–926.
- Zhang L, Ji G, Shao Y, et al. MLF1 interacting protein: a potential gene therapy target for human prostate cancer? Med Oncol. 2015;32:454.
- Wang S, Liu B, Zhang J, et al. Centromere protein U is a potential target for gene therapy of human bladder cancer. Oncol Rep. 2017;38:735–744.
- Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol. 2013;87:1–11.
- Muller-Tidow C, Diederichs S, Thomas M, et al. Genome-wide screening for prognosis-predicting genes in early-stage non-small-cell lung cancer. Lung Cancer. 2004;45 Suppl 2:S145–50.
- Wu Q, Yang ZF, Wang KJ, et al. AQP8 inhibits colorectal cancer growth and metastasis by down-regulating PI3K/AKT signaling and PCDH7 expression. Am J Cancer Res. 2018;8:266–279.
- Ji C, Guo H, Zhang P, et al. AnnexinA5 promote glioma cell invasion and migration via the PI3K/Akt/NF-kappaB signaling pathway. J Neurooncol. 2018;138:469–478.
- Zhou D, Liu W, Liang S, et al. Apoptin-derived peptide reverses cisplatin resistance in gastric cancer through the PI3K-AKT signaling pathway. Cancer Med. 2018;7:1369–1383.
- Sudhakaran PR, Binu S, Soumya SJ. Effect of sarcosine on endothelial function relevant to angiogenesis. J Cancer Res Ther. 2014;10:603–610.
- Zhu J, Yao J, Huang R, et al. Ghrelin promotes human non-small cell lung cancer A549 cell proliferation through PI3K/Akt/mTOR/P70S6K and ERK signaling pathways. Biochem Biophys Res Commun. 2018;498:616–620.
- Hong SY, Yu FX, Luo Y, et al. Oncogenic activation of the PI3K/Akt pathway promotes cellular glucose uptake by downregulating the expression of thioredoxin-interacting protein. Cell Signal. 2016;28:377–383.
- Lu XX, Cao LY, Chen X, et al. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016:2476842.
- Chen J, Yuan J, Zhou L, et al. Regulation of different components from Ophiopogon japonicus on autophagy in human lung adenocarcinoma A549Cells through PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2017;87:118–126.