ABSTRACT
It has been reported that lncRNA POU3F3 was upregulated in esophageal squamous-cell carcinomas, indicating its role as an oncogene in this disease. However, the mechanism of its function and its involvement in other malignancies is unknown. In the present study we found that expression levels of lncRNA POU3F3 were higher in tumor tissues than in adjacent healthy tissues of triple negative breast cancer (TNBC) patients and were significantly and inversely correlated with levels of cleaved caspase 9 only in tumor tissues. In addition, plasma levels of lncRNA POU3F3 were higher in TNBC patients than in healthy controls and were significantly and inversely correlated with levels of cleaved caspase 9 only in TNBC patients. In addition, treatment of exogenous Cleaved Caspase-9 significantly attenuated the effects of lncRNA POU3F3 overexpression on cancer cell proliferation and apoptosis. lncRNA POU3F3 may promote proliferation and inhibit apoptosis of cancer cells in triple-negative breast cancer.
Graphical Abstract
LncRNA POU3F3 overexpression promoted cell proliferation and inhibited cell apoptosis possibly through the interaction with cleaved caspase 9
Breast cancer is a major medical condition in females worldwide. In the United States, breast cancer causes more than 200,000 new cases and causes about 40,000 deaths every year [Citation1]. It is generally believed that 1 out of 8 females will develop breast cancer during their life time [Citation2]. At present, low early diagnosis rate is still a big challenge for the survival of breast cancer patients [Citation3]. In developing countries such as China, only 20% breast cancer patients were diagnosed at stage I [Citation4]. As a major type of breast cancer, triple-negative breast cancer (TNBC) is characterized by the lack of ER, PR and HER2 [Citation5]. Patients with TNBC usually show poor survival due to the extreme aggressive nature and the lack of effective treatment therapies [Citation6].
Caspase-9 as a member of caspases family plays pivotal roles in apoptosis and cytokine signaling [Citation7]. Caspase-9 participates in cancer development by regulating cancer cell apoptosis through the interactions with multiple signaling molecules [Citation8,Citation9]. At present, caspase-9 is considered as a promising therapeutic target for cancer treatment [Citation10]. It has been reported that the activity of caspase-9 can be regulated by long non-coding RNAs (lncRNAs) [Citation11,Citation12], which is a group of non-coding RNAs composed of more than 200 nucleotides and participates in cancer biology [Citation13]. Regulation of lncRNA expression is considered as a promising therapeutic target for cancer treatment, while function of most lncRNAs remain unclear, which limits their clinical applications [Citation13]. It has been reported that lncRNA POU3F3 was upregulated in esophageal squamous-cell carcinomas [Citation14], indicating its role as an oncogene in this disease. In the present study we showed that lncRNA POU3F3 may promote proliferation and inhibit apoptosis of cancer cells in triple-negative breast cancer by inactivating caspase 9.
Materials and methods
Specimen collection and cell lines
Plasma specimens and tumor tissue and adjacent healthy tissue (within 2 cm around the tissues) were collected from 56 patients with TNBC who were admitted by People’s hospital of Quzhou City from May 2011 to May 2013. Inclusion criteria: 1) patients diagnosed through pathological biopsies; 2) patients willing to donate specimens; 3) patients signed informed consent; 4) patients completed the treatment and 5-year follow-up. Exclusion criteria: 1) patients who were diagnosed with multiple diseases; 2) patients failed to complete treatment and/or follow-up; 3) patients died of other causes during follow-up. The age range of those patients was 28 to 64 years and the mean age was 45.1 ± 5.3 years. There were 12 cases in stage I, 14 in stage II, 13 in stage III and 17 in stage IV. Plasma specimens were also collected from 38 healthy females (control group) who received systemic physiological examinations at People’s hospital of Quzhou City during the same time period. Age range of control group was 26–65 years and the mean age was 46.6 ± 4.8 years. Ethics Committee of People’s hospital of Quzhou City approved this study. All participants signed informed consent. See for basic clinical data of 2 groups of participants.
Table 1. Basic clinical data of 2 groups of participants.
Cells of human TNBC cell lines BT-20 and MDA-MB-231 were bought from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultivated with ATCC-formulated Eagle’s Minimum Essential Medium (Catalog No. 30–2003) with fetal bovine serum added to a final concentration of 10%.
Enzyme-linked immunosorbent assay (ELISA)
Expression levels of cleaved caspase 9 in tumor tissues, adjacent healthy tissues and plasma were measured using active/cleaved Caspase 9 Assay Kit (Fluorometric) (NBP2-54872-25Assays, NOVUS).
Real-time quantitative PCR (RT-qPCR)
All total RNA extractions were performed using GenElute™ Total RNA Purification Kit (Sigma-Aldrich, St. Louis, MO, USA). Reverse transcriptions were performed using ReadyScript® cDNA Synthesis Mix (Sigma-Aldrich, St. Louis, MO, USA). SYBR® Green Quantitative RT-qPCR Kit (Sigma-Aldrich, St. Louis, MO, USA) was used to prepare all PCR reaction systems. Primers of lncRNA POU3F3 and endogenous control GAPDH were designed and synthesized by GenePharma (Shanghai, China). Sequences of primers used in PCR reactions were: 5ʹ-AATCACTGCAATTGAAGGAAAAA-3ʹ and 5ʹ-CCTTGTTTTCCAACCCTTAGACT-3ʹ for POU3F3; 5ʹ-ATCCCATCACCATCTTCC-3ʹ and 5ʹ-GAGTCCTTCCACGATACCA-3ʹ for GAPDH. All PCR reactions were carried out on Applied Biosystems™ 7500 Real-Time PCR System (Fisher Scientific) through following conditions: 95°C for 55 s, followed by 40 cycles of 95°C for 16 s and 58.5°C for 34 s. 2−ΔΔCt method was used for all data normalizations.
Cell transfection
LncRNA POU3F3 expressing pIRSE2 vectors were designed and prepared by Sangon (Shanghai, China). All transfections were performed using Lipofectamine 3000 Reagent (Thermo Fisher Scientific). The dose of vectors was 15 nM. Cells treated with Lipofectamine 3000 Reagent only were control cells. Cells transfected with empty vectors were negative control cells. Transfection efficiency was checked by qRT-PCR at 24 h after transfection and an overexpression rate above 200% was achieved.
Cell proliferation assay
Cell proliferation abilities were measured using a Cell Counting Kit-8 kit Dojindo (Molecular Technologies, Inc., Rockville, MD, USA) at 24 h after transfection. Cells were harvested to prepare single cell suspensions at a cell density of 5 × 104 cells/mL. Each well of a 96-well plate was filled with 100 μL cell suspension. The plate was kept at 37ºC in a 5% CO2 incubator, and 10 μL of CCK-8 was added 24, 48, 72 and 96 h later. After that, cells were cultivated from additional 4 h and OD values at 450 nm were measured using a microplate reader to represent cell proliferation rates.
Cell apoptosis assay
Cell apoptosis rates were measured using through cell apoptosis assay at 24 h after transfection. Serum-free cell suspensions (5 × 104 cells/mL) were prepared and each well of a 6-well plate as filled with 10 ml cell suspension. Cells were cultivated for 48 h, followed by digestion with 0.25% trypsin. After that, Annexin V-FITC (Dojindo, Japan) and propidium iodide (PI) staining was performed and apoptotic cells were detected through flow cytometry.
Western-blot
All protein extractions were performed using CelLytic™ MEM Protein Extraction Kit (Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations were measured using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). After that, protein samples were denatured and electrophoresis was carried out using 10% SDS-PAGE gel. Gel transfer was then performed and PVDF membranes were blocked in fat-free milk at room temperature for 2 h. Western blot was carried out using conventional conditions. Primary antibodies were rabbit anti-human cleaved caspase 9 (ab2324, 1:1200; Abcam) and rabbit anti-human GAPDH (ab9485, 1: 1200, Abcam). The secondary antibody was goat anti-rabbit IgG-HRP secondary antibody (1:1000, MBS435036, MyBioSource). ECL reagent (Sigma-Aldrich, USA) was used for signal development. Data normalizations were performed using Image J v1.46 software.
Statistical analysis
Graphpad Prism 6 software was used for all statistical analyses. Experiments were performed in triplicate manner and mean ± standard deviation was calculated. Correlations between levels of lncRNA POU3F3 and cleaved caspase 9 were analyzed by Pearson Correlation Coefficient. According to Youden index, patients were divided into high (n = 30) and low (n = 26) lncRNA POU3F3 expression groups. Kaplan-Meier method was used to plot survival curves for both groups and survival curves were compared by Log-rank test. Diagnostic value of plasma lncRNA POU3F3 for early stage TNBC was evaluated by ROC curve analysis with early stage TNBC patients and true positive cases and healthy females as true negative cases. Comparisons between 2 groups were performed by paired (between tumor tissues and healthy tissue) or unpaired (between patients and healthy controls) t test. Comparisons among multiple groups were performed by one way analysis of variance followed by Tukey test. p < 0.05 was considered to be statistically significant.
Results
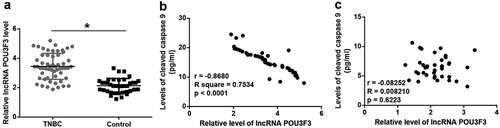
LncRNA POU3F3 level was upregulated in tumor tissues and was significantly and inversely correlated with levels of cleaved caspase 9
RT-qPCR results showed that expression levels of lncRNA POU3F3 were higher in tumor tissues than in adjacent healthy tissues of TNBC patients (), p < 0.05). Pearson Correlation Coefficient analysis revealed a significant and positive correlation between levels of lncRNA POU3F3 and cleaved caspase 9 in tumor tissues (), p < 0.05). However, the correlation between levels of lncRNA POU3F3 and cleaved caspase 9 in adjacent healthy tissues was not significant (), p < 0.05).
Figure 1. LncRNA POU3F3 level was upregulated in tumor tissues and was significantly and inversely correlated with levels of cleaved caspase 9.
Expression levels of lncRNA POU3F3 were higher in tumor tissues than in adjacent healthy tissues of TNBC patients (a) and were significantly and inversely correlated with levels of cleaved caspase 9 in tumor tissues (b) but not in adjacent healthy tissues (c) (*, p < 0.05).
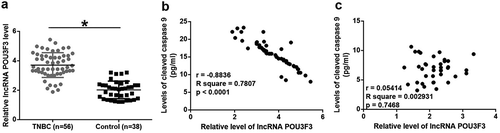
Plasma lncrna POU3F3 level was upregulated in TNBC patients and was significantly and inversely correlated with levels of cleaved caspase 9
RT-qPCR results showed that plasma levels of lncRNA POU3F3 were higher in TNBC patients than in healthy controls (), p < 0.05). Pearson Correlation Coefficient analysis revealed a significant and positive correlation between levels of lncRNA POU3F3 and cleaved caspase 9 in TNBC patients (), p < 0.05). However, the correlation between levels of lncRNA POU3F3 and cleaved caspase 9 in healthy controls was not significant (), p < 0.05).
Figure 2. Plasma lncRNA POU3F3 level was upregulated in TNBC patients and was significantly and inversely correlated with levels of cleaved caspase 9.
Expression levels of lncRNA POU3F3 were higher in TNBC patients than in healthy controls (a) and were significantly and inversely correlated with levels of cleaved caspase 9 in TNBC patients (b) but not in healthy controls (c) (*, p < 0.05).
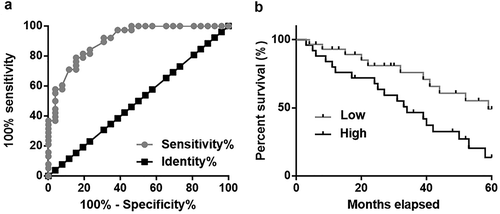
Altered plasma lncRNA POU3F3 level provide guidance for the diagnosis and prognosis of TNBC
Our study included 26 patients at stage I and II, which are early stages of TNBC. Diagnostic value of plasma lncRNA POU3F3 for early stage TNBC was evaluated by ROC curve analysis with early stage TNBC patients and true positive cases and healthy females as true negative cases. As shown in ), area under the curve was 0.9028 (standard error: 0.03768; 95% confidence interval: 0.8290–0.9767; p < 0.0001). According to Youden index, patients were divided into high (n = 30) and low (n = 26) lncRNA POU3F3 expression groups. Kaplan-Meier method was used to plot survival curves for both groups and survival curves were compared by Log-rank test. As shown in ), patients with high level of lncRNA POU3F3 showed significantly lower overall survival rate (p = 0.0133).
Figure 3. Altered plasma lncRNA POU3F3 level provide guidance for the diagnosis and prognosis of TNBC.
ROC curve analysis revealed that altered plasma lncRNA POU3F3 distinguished early stage TNBC patients from healthy controls (a). Survival curve analysis showed that patients with high level of lncRNA POU3F3 had significantly lower overall survival rate (b).
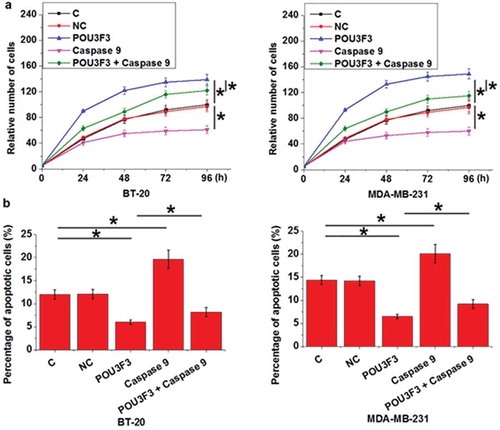
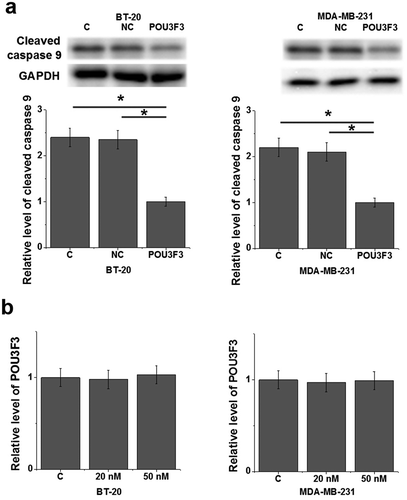
LncRNA POU3F3 overexpression led to reduced levels of cleaved caspase 9 in cells of TNBC cell lines BT-20 and MDA-MB-231
After transfection, compared with control cells (C) and negative control cells (NC), lncRNA POU3F3 overexpression resulted in significantly reduced levels of cleaved caspase 9 in cells of TNBC cell lines BT-20 and MDA-MB-231 (), p < 0.05). In contrast, treatment with Cleaved Caspase-9 (Cell Signaling Technology) at doses of 20 and 50 nM [Citation15] failed to significantly affect lncRNA POU3F3 expression (), p < 0.05).
Figure 4. LncRNA POU3F3 overexpression led to reduced levels of cleaved caspase 9 in cells of TNBC cell lines BT-20 and MDA-MB-231.
LncRNA POU3F3 overexpression resulted in significantly reduced levels of cleaved caspase 9 in cells of TNBC cell lines BT-20 and MDA-MB-231 (a). In contrast, treatment with Cleaved Caspase-9 at doses of 20 and 50 nM failed to significantly affect lncRNA POU3F3 expression (b) (*, p < 0.05).
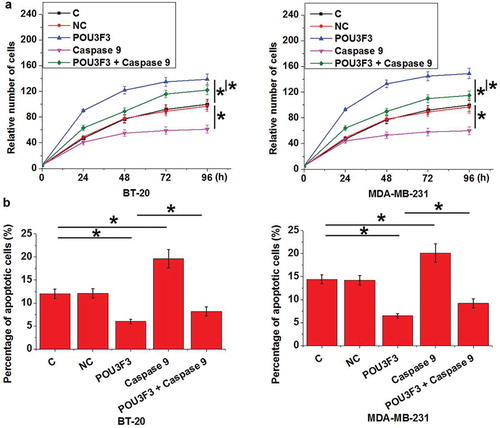
LncRNA POU3F3 overexpression promoted cell proliferation and inhibited cell apoptosis possibly through cleaved caspase 9
Compared with control cells (C) and negative control cells (NC), lncRNA POU3F3 overexpression resulted in promoted cell proliferation ()) and inhibited cell apoptosis ()) of TNBC cell lines BT-20 and MDA-MB-231 (p < 0.05), while treatment with Cleaved Caspase-9 at a dose of 50 nM played an opposite role and significantly attenuated the effects of lncRNA POU3F3 overexpression on cancer cell proliferation and apoptosis (p < 0.05).
Figure 5. LncRNA POU3F3 overexpression promoted cell proliferation and inhibited cell apoptosis possibly through cleaved caspase 9.
LncRNA POU3F3 overexpression resulted promoted cell proliferation (a) and inhibited cell apoptosis (b) of TNBC cell lines BT-20 and MDA-MB-231, while treatment with Cleaved Caspase-9 at a dose of 50 nM played an opposite role and significantly attenuated the effects of lncRNA POU3F3 overexpression on cancer cell proliferation and apoptosis (*, p < 0.05).
Discussion
LncRNA POU3F3 overexpression has been observed in esophageal squamous-cell carcinomas and upregulation of lncRNA POU3F3 may serve as a potential diagnostic marker for this disease [Citation14]. Our study systemically investigated the role of lncRNA POU3F3 in TNBC, which is a major type of breast cancer. Our study showed that lncRNA POU3F3 participates in the regulation of cancer cell proliferation and apoptosis in TNBC possibly through caspase 9. We also revealed that lncRNA POU3F3 may have diagnostic and prognostic values for TNBC.
Differentially expressed lncRNAs are key players in the development of TNBC [Citation16,Citation17]. LncRNA POU3F3 is overexpressed in esophageal squamous-cell carcinomas, while its expression pattern in other human disease is unknown. Our study showed that expression levels of lncRNA POU3F3 were higher in tumor tissues than in adjacent healthy tissues of TNBC patients ()). In addition, plasma levels of lncRNA POU3F3 were higher in TNBC patients than in healthy controls ()). Therefore, lncRNA POU3F3 is also likely an oncogene in TNBC.
Treatment of TNBC is still challenging due to the high prevalence rate of cancer metastasis by the time of first diagnosis [Citation3,Citation4]. Therefore, early diagnosis is still a major focus of the treatment of TNBC. In this study ROC curve analysis showed that upregulation of plasma lncRNA POU3F3 effectively distinguished early stage TNBC patients from healthy controls ()). Therefore, detection of plasma lncRNA POU3F3 may assist early diagnosis or screening of TNBC. Our study also showed that patients with higher plasma level of lncRNA POU3F3 at the time of diagnosis had worse overall surival condition than patients with lower plasma level of lncRNA POU3F3 ()). Therefore, circulating lncRNA POU3F3 may also serve as a prognostic biomarker for TNBC. However, more clinical trials are needed to further confirm those conclusions.
Caspase 9 is an initiator of cell apoptosis [Citation18]. Multiple anti-cancer drugs promote cancer cell apoptosis by activating caspase 9 [Citation19,Citation20]. In this study we showed that lncRNA POU3F3 is possibly an upstream inhibitor of caspase 9 in the regualtion of proliferation and apoptosis of TNBC cells ( and ). Our data is supported by a previous study, in which POU3F3 knockdown resulted in increased abundances of cleaved caspase-9 in colorectal cancer cells [Citation21]. Therefore, inhibition of lncRNA POU3F3 may serve as a therapeutic target for TNBC. No significant correlation between expresssion levels of lncRNA POU3F3 and caspase 9 was found in adjacent healthy tissues of TNBC patients ( and ,)) and in plasma of healthy controls ( and ,). Therefore, lncRNA POU3F3 may regulate the activity of caspase 9 through pathological mediators. It has been repoted that POU3F3 promotes TGF-β1 in cancer cancer biology [Citation22], and TGF-β1 inhibits multiple caspases [Citation23]. Therefore, TGF-β1 may be one of the mediators between POU3F3 and caspase-9 in TNBC patients.
In conclusion, lncRNA POU3F3 was upregulated in TNBC. LncRNA POU3F3 may regulate the activity of caspase 9 to participate in the proliferation and apoptosis of cancer cells in TNBC.
Authors’ contributions
Jun did all the experiments, analyzed all data and was a major contributor in writing the manuscript. Xuli, Yong, Lei and Qinghui did some experiment work. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014 Jan-Feb;64(1):52–62. PubMed PMID: 24114568.
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016 Jan-Feb;66(1):31–42. PubMed PMID: 26513636.
- Unger-Saldana K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014 Aug 10;5(3):465–477. PubMed PMID: 25114860; PubMed Central PMCID: PMCPMC4127616.
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014 Jun;15(7):e279–e289. PubMed PMID: 24872111.
- Cetin I, Topcul M. Triple negative breast cancer. Asian Pac J Cancer Prev. 2014;15(6):2427–2431. PubMed PMID: 24761842.
- Collignon J, Lousberg L, Schroeder H, et al. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press). 2016;8:93–107. PubMed PMID: 27284266; PubMed Central PMCID: PMCPMC4881925.
- Kuida K. Caspase-9. Int J Biochem Cell Biol. 2000 Feb;32(2):121–124. PubMed PMID: 10687948.
- Thakor P, Subramanian RB, Thakkar SS, et al. Phytol induces ROS mediated apoptosis by induction of caspase 9 and 3 through activation of TRAIL, FAS and TNF receptors and inhibits tumor progression factor glucose 6 phosphate dehydrogenase in lung carcinoma cell line (A549). Biomed Pharmacother. 2017 Aug;92:491–500. PubMed PMID: 28575806.
- Bou-Hanna C, Jarry A, Lode L, et al. Acute cytotoxicity of MIRA-1/NSC19630, a mutant p53-reactivating small molecule, against human normal and cancer cells via a caspase-9-dependent apoptosis. Cancer Lett. 2015 Apr 10;359(2):211–217. PubMed PMID: 25617798.
- Kim B, Srivastava SK, Kim SH. Caspase-9 as a therapeutic target for treating cancer. Expert Opin Ther Targets. 2015 Jan;19(1):113–127. PubMed PMID: 25256701
- Yin DD, Zhang EB, You LH, et al. Downregulation of lncRNA TUG1 affects apoptosis and insulin secretion in mouse pancreatic beta cells. Cell Physiol Biochem. 2015;35(5):1892–1904. PubMed PMID: 25871529.
- Zou Y, Yao S, Chen X, et al. LncRNA OIP5-AS1 regulates radioresistance by targeting DYRK1A through miR-369-3p in colorectal cancer cells. Eur J Cell Biol. 2018 Jun;97(5):369–378. PubMed PMID: 29773344.
- Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012 Jun;9(6):703–719. PubMed PMID: 22664915; PubMed Central PMCID: PMCPMC3495743
- Tong YS, Wang XW, Zhou XL, et al. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015 Jan 21;14:3. PubMed PMID: 25608466; PubMed Central PMCID: PMCPMC4631113.
- Berger AB, Witte MD, Denault JB, et al. Identification of early intermediates of caspase activation using selective inhibitors and activity-based probes. Mol Cell. 2006 Aug;23(4):509–521. PubMed PMID: 16916639.
- Shen X, Xie B, Ma Z, et al. Identification of novel long non-coding RNAs in triple-negative breast cancer. Oncotarget. 2015 Aug 28;6(25):21730–21739. PubMed PMID: 26078338; PubMed Central PMCID: PMCPMC4673299.
- Pickard MR, Williams GT. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res Treat. 2014 Jun;145(2):359–370. PubMed PMID: 24789445
- Li Y, Zhou M, Hu Q, et al. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc Natl Acad Sci USA. 2017 Feb 14;114(7):1542–1547. PubMed PMID: 28143931; PubMed Central PMCID: PMCPMC5320974.
- Srinivas G, Anto RJ, Srinivas P, et al. Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol. 2003 Jul 25;473(2–3):117–125. PubMed PMID: 12892828.
- Peng W, Wu JG, Jiang YB, et al. Antitumor activity of 4-O-(2ʹ’-O-acetyl-6ʹ’-O-p-coumaroyl-beta-D-glucopyranosyl)-p-coumaric acid against lung cancers via mitochondrial-mediated apoptosis. Chem Biol Interact. 2015 May 25;233:8–13. PubMed PMID: 25824411.
- Shan TD, Xu JH, Yu T, et al. Knockdown of linc-POU3F3 suppresses the proliferation, apoptosis, and migration resistance of colorectal cancer[J]. Oncotarget. 2016;7(1):961–975.
- Li W, Wu X, She W. LncRNA POU3F3 promotes cancer cell migration and invasion in nasopharyngeal carcinoma by upregulating TGF-beta1. Biosci Rep. 2019 Jan 2. DOI:10.1042/BSR20181632. PubMed PMID: 30602452.
- Chua CC, Chua BH, Chen Z, et al. TGF-beta1 inhibits multiple caspases induced by TNF-alpha in murine osteoblastic MC3T3-E1 cells. Biochim Biophys Acta. 2002 Dec 16;1593(1):1–8. PubMed PMID: 12431778.
