ABSTRACT
Phenethyl isothiocyanate (PEITC) is an aromatic isothiocyanate present in cruciferous vegetables. Several studies have shown that isothiocyanates regulate various intracellular signaling pathways, and thereby show anti-inflammatory and detoxifying activities. However, little is known about the effects of PEITC on glucose metabolism. In this study, we examined whether PEITC promotes glucose utilization in mouse skeletal muscle cells, C2C12 myotubes. PEITC induced glucose uptake, glucose transporter 4 (Glut4) translocation to the plasma membrane, and activation of Akt and ERK in C2C12 cells. Inhibition of Akt suppressed PEITC-induced Glut4 translocation and glucose uptake, whereas ERK inhibition did not. Furthermore, PEITC increased phosphorylation of ErbB2 and ErbB3. Treatment with a pan-ErbB inhibitor reduced Akt activation and the subsequent glucose uptake induced by PEITC. These results indicate that PEITC promotes glucose utilization through the ErbB/Akt pathway in C2C12 myotubes. PEITC may therefore serve as a dietary constituent with beneficial effects on the carbohydrate metabolism.
Abbreviations: PEITC: phenethyl isothiocyanate; Glut4: glucose transporter 4; PI3K: phosphatidylinositide 3-kinase; Nrf2: erythroid−2-related factor; ARE: antioxidant response element; HO−1: heme oxygenase−1; NRG: neuregulin
GRAPHICAL ABSTRACT
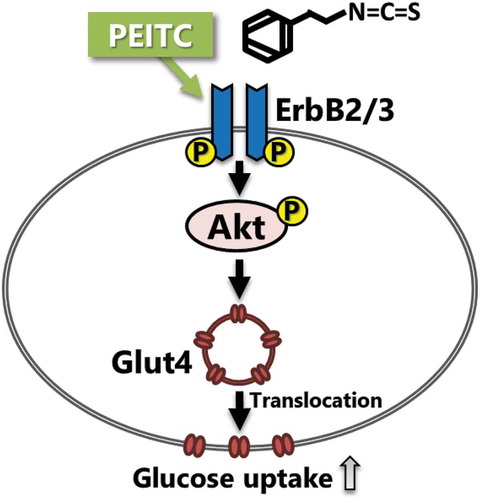
Phenethyl isothiocyanate (PEITC), a dietary isothiocyanate in cruciferous vegetables, induces glucose uptake in C2C12 myotubes through activation of the ErbB/Akt pathway.
Diabetes is one of the most common metabolic diseases, characterized by insulin resistance in peripheral tissues with a failure of insulin secretion. An appropriate control of blood glucose levels is important to prevent the development of this disease. Approximately 70% of postprandial blood glucose clearance occurs in skeletal muscle because it corresponds to nearly 40% of human body mass [Citation1,Citation2]. In diabetic subjects, glucose uptake in skeletal muscle is impaired [Citation3]. Thus, skeletal muscle plays an important role in maintaining whole body glucose homeostasis.
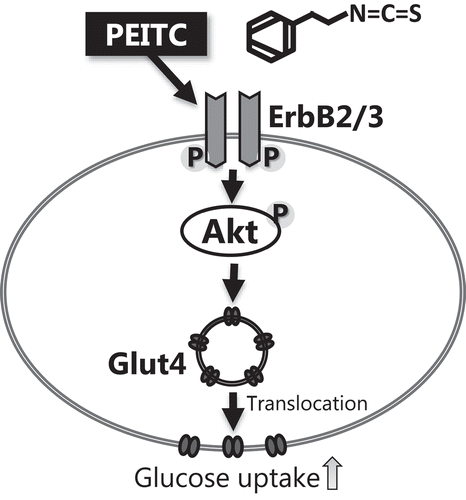
Figure 6. Possible mechanism of PEITC-induced glucose uptake in C2C12 cells.
Our results suggest that PEITC, a dietary isothiocyanate in cruciferous vegetables, induces glucose uptake in C2C12 myotubes through activation of the ErbB/Akt pathway.
Glucose uptake is carried out through glucose transporters, which are membrane proteins that facilitate glucose transport through the plasma membrane. Glucose transporter 4 (Glut4) is a major glucose transporter in skeletal muscle and is regulated by the phosphatidylinositide 3-kinase (PI3K)/Akt pathway downstream of insulin signaling [Citation4]. Because the global prevalence of diabetes has been increasing, there has been much interest in the utilization of natural compounds and food components for the prevention of diabetes and in investigating their mechanisms against the disease [Citation5,Citation6]. Numerous studies revealed that activation of intracellular signaling pathways regulating glucose metabolism, such as the PI3K/Akt pathway, by food components is beneficial for proper glycemic control [Citation7,Citation8].
Phenethyl isothiocyanate (PEITC) is an aromatic compound present in cruciferous vegetables such as watercress and is found as gluconasturtiin, a PEITC glucosinolate, that is generated after cutting or ingesting the vegetable [Citation9]. PEITC has been shown to possess cellular defense activity by up-regulating phase 2 detoxifying and anti-oxidative enzymes through a nuclear factor erythroid−2-related factor (Nrf2) pathway [Citation9,Citation10]. Induction of anti-oxidative activity by PEITC through Nrf2 activation may protect against oxidative damage associated with diabetes [Citation10,Citation11]. In addition, PEITC and other isothiocyanates such as sulforaphane and allyl isothiocyanate can regulate several intracellular signaling pathways such as PI3K/Akt and extracellular signal-regulated kinases (ERK) [Citation12–Citation14]. Sulforaphane has been shown to induce the activation of the PI3K/Akt pathway in cardiomyocytes, and thereby increases both Nrf2 binding to a nuclear cis-acting enhancer sequence, the antioxidant response element (ARE) and activation of Nrf2 [Citation13]. Treatment with allyl isothiocyanate has been shown to restore fatty acid-induced, impaired insulin-dependent Akt activation and glucose uptake in C2C12 myotubes [Citation14]. These findings led us to speculate that isothiocyanate may regulate glucose uptake through Akt activation. However, it is unclear whether PEITC activates or modulates the PI3K/Akt pathway, thereby contributing to regulate glucose metabolism in skeletal muscle.
In the current study, we examined the effects of PEITC on glucose uptake in mouse C2C12 cells, a skeletal muscle model cell line, and whether intracellular signaling mediated by Akt and ERK are involved in these effects. We show that activation of Akt underlies induction of glucose uptake by PEITC in C2C12 myotubes.
Materials and methods
Reagents
Dulbecco’s modified Eagles’ medium (DMEM, low glucose) and fetal bovine serum (FBS) were purchased from Sigma (St. Louis, MO, USA), horse serum (HS) from Gibco (Grand Island, NY, USA), and PEITC from LKT laboratories (St. Paul, MN, USA). Anti-Akt, anti-pAkt (Thr308), anti-phospho-p44/42 Map kinase (Thr202/Tyr204), anti-Glut4 (1F8), anti-pan-cadherin, anti-Nrf2 (D1Z9C), anti-ErbB2 (D8F12), anti-pErbB2 (Tyr1221/1222), anti-ErbB3 (D22C5), and anti-pErbB3 (Tyr1289) antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA), anti-p-Akt1/2/3 (Ser473) and anti-lamin B antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA), anti-ERK antibody from BD Transduction Laboratories (Franklin Lakes, NJ, USA), anti-HO−1 antibody from Enzo Life Sciences (Plymouth Meeting, PA, USA), and anti-β-actin antibody (AC−15) from Novus Biologicals (Littleton, CO, USA). All other reagents were of analytical grade.
Cell culture and differentiation
Mouse C2C12 myoblasts were cultured in DMEM containing 10% (v/v) FBS and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) in a humidified atmosphere of 5% CO2 at 37ºC until 80−90% confluency. Fusion and differentiation were induced by replacing the medium with DMEM containing 2% (v/v) HS, and then fusion and differentiation were allowed to proceed for 4−5 days. The medium was changed every day during this procedure.
Measurement of glucose uptake
C2C12 myoblasts were seeded into 24-well culture plates (1.0 × 105 cells/well). C2C12 myotubes were washed once with phosphate-buffered saline (PBS) and starved in serum-free DMEM containing 0.1% (w/v) bovine serum albumin (BSA) for 18 h. After serum starvation, cells were washed once with PBS and then incubated with PEITC (0, 20 µM) in phenol red and glucose-free DMEM (Sigma) containing 11 mM glucose for 6 h. Glucose concentrations in the medium were determined using a glucose assay kit (Glucose CII-Test, Wako, Osaka, Japan), and the amount of glucose uptake was calculated from the differences in glucose concentrations in the medium before and after incubation.
Analysis of cellular signaling by Western blotting
C2C12 myoblasts were seeded onto 60 mm dishes (9.0 × 105 cells/dish). After 18 h of serum starvation, differentiated C2C12 myotubes were incubated with PEITC (0−20 µM) for 180 min in DMEM containing 0.1% BSA. Inhibitors of Akt (Akt 1/2 kinase inhibitor, 1.5 µM, Sigma), ERK (PD98059, 25 µM), or ErbB receptor tyrosine-kinase (CI−1033, 2.5 µM) were added 30 min before PEITC treatment. The medium was removed and the cells were lysed in a lysis buffer solution (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% (w/v) SDS, 1% (v/v) Triton-X, 1% (w/v) deoxycholic acid, 0.5 mM Na3VO4, and 5 mM EDTA). Cell extracts were prepared by centrifugation for 10 min at 20,000 × g at 4ºC to remove cell debris. Protein concentrations were measured as described previously [Citation10]. Equal amounts of proteins were separated on SDS-PAGE and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The membrane was immersed in blocking buffer (5% BSA in 20 mM Tris-HCl (pH 7.6), 137 mM NaCl, 1 mM EDTA, and 0.1% Tween 20 [TBS-T]) or 1% skim milk at room temperature for 1 h, incubated overnight at 4°C with primary antibodies, and then incubated with the appropriate secondary antibody, i.e., anti-rabbit or anti-mouse IgG, conjugated with horseradish peroxidase at room temperature for 1 h. Immunoreactive signals were detected using ECL Western Blotting Substrate (GE Healthcare, Tokyo, Japan) and a luminoimage analyzer (ImageQuant LAS 4000, GE Healthcare) and then analyzed via software (NIH Image).
Measurement of Glut4 translocation
The plasma membrane fraction was prepared according to a previously described method [Citation15]. The amount of Glut4 in the obtained fraction was determined using Western blotting with anti-Glut4 antibody.
Measurement of Nrf2 nuclear translocation
The amount of Nrf2 in the nuclear fraction was determined using Western blotting with anti-Nrf2 antibody as previously described [Citation10]. The nuclear fraction was prepared from cells treated with PEITC for 180 min.
Statistical analysis
Data are expressed as the mean ± SE. For comparison of groups with comparable variance, one-way ANOVA and Tukey’s post hoc tests were carried out (GraphPad Instat Software ver. 3.0a, GraphPad Software, Inc., San Diego, CA, USA). Pairwise comparisons were performed using unpaired t-tests, and two-tailed p values were calculated. The p values below 0.05 were considered significant.
Results
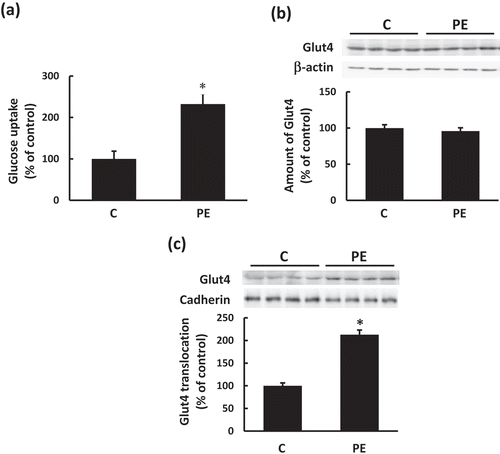
PEITC induces glucose uptake and translocation of Glut4 to the plasma membrane in C2C12 myotubes
We first investigated whether PEITC induces glucose uptake in C2C12 myotubes. As shown in ), the amount of consumption of glucose from medium increased when cells were treated with PEITC for 6 h. To elucidate the mechanism by which PEITC induces an increase in glucose consumption, we determined the amount of Glut4 expression and its translocation to the plasma membrane. As shown in ), consistent with the observed rate of glucose consumption, treatment with PEITC (20 µM) induced translocation of Glut4 to the plasma membrane, although PEITC did not affect the total Glut4 protein levels in the whole cell lysate ()). These results suggest that PEITC stimulates translocation of Glut4 to the plasma membrane and induces glucose uptake in C2C12 myotubes.
Figure 1. Effects of phenethyl isothiocyanate (PEITC) on the glucose uptake, Glut4 protein levels, and translocation of Glut4 protein to the plasma membrane.
(a) C2C12 myotube cells were treated with PEITC (0, 20 µM) for 6 h. Differences in glucose concentrations in the medium before and after incubation are shown as glucose uptake. (b) Cells were treated with 20 µM PEITC for 180 min in serum-free DMEM. The amount of Glut4 protein in whole cell lysates were determined using Western blotting as described in Materials and methods. Expression levels were normalized by that of β-actin. (c) After 20 µM PEITC treatment for 180 min, the cells were lysed and plasma membrane fractions were prepared. Glut4 proteins in the plasma membrane were detected using Western blotting. Immunoblotting with anti-cadherin antibody was performed as preparation control for membrane fractions. Values are shown as mean ± SE (n=4). Values with an asterisk are significantly different from each other (p < 0.05).
PEITC stimulates Akt and ERK pathways in C2C12 myotubes
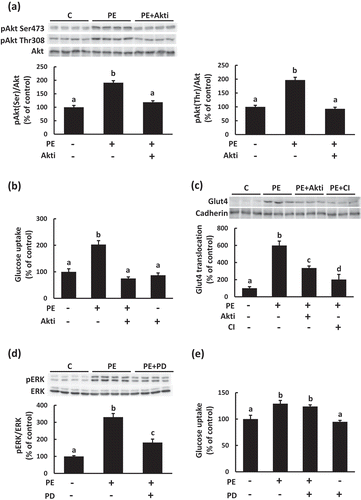
To assess whether PEITC activates intracellular signals which regulates glucose uptake in C2C12 cells, we analyzed the effects of PEITC on the phosphorylation of Akt and ERK, which has been reported to regulate glucose uptake in skeletal muscle cells [Citation16–Citation18]. As shown in , phosphorylation of Akt and ERK increased in a dose-dependent manner when the differentiated cells were incubated with PEITC (0−20 µM) for 180 min. We then examined whether activation of Akt and ERK are involved in the regulation of PEITC-induced glucose uptake in C2C12 myotubes. When C2C12 cells were treated with the Akt1/2 kinase inhibitor (Akti, 1.5 µM) or with PD98059 (PD, 25 µM), phosphorylation of Akt or ERK, respectively, stimulated by PEITC was suppressed (). Treatment with Akti suppressed PEITC-induced glucose uptake and Glut4 translocation to the plasma membrane (), whereas PEITC-induced glucose uptake was not affected by treatment with the ERK inhibitor ()). These results indicate that activation of Akt plays an important role in PEITC-induced Glut4 translocation and glucose uptake, and that the ERK pathway is not involved in the effects of PEITC.
Figure 2. Effects of PEITC on Akt and ERK activation.
After 18 h of serum starvation, C2C12 myotubes were stimulated with PEITC (0−20 µM) for 180 min in serum-free DMEM containing 0.1% BSA. Cell lysates were prepared as described in Materials and methods. Equal amounts of protein were analyzed using Western blotting with anti-phospho Akt Ser473, anti-phospho Akt Thr308, and anti-Akt (b), and anti-phospho p44/42 MAP kinase Thr202/Tyr204 and anti-ERK (c) antibodies. Representative results are shown in (a). Values are shown as mean ± SE (n = 4). Significant differences between values are indicated by different superscript characters (p < 0.05).
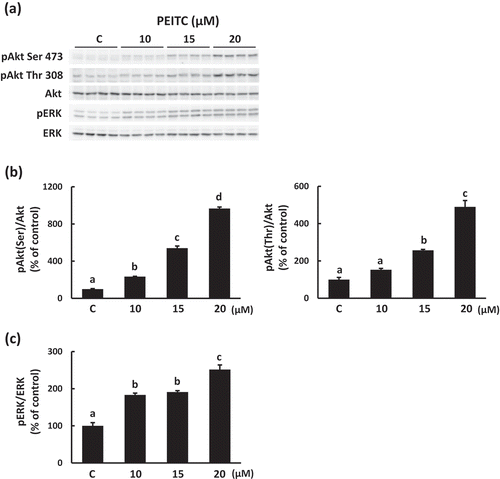
Figure 3. Effects of Akt and ERK inhibitors on PEITC-induced glucose uptake.
After pretreatment with the Akt inhibitor (Akt 1/2 kinase inhibitor, 1.5 µM, Akti) or ERK inhibitor (PD98059, 25 µM, PD) for 30 min, the cells were treated with 20 µM PEITC for 180 min in serum-free DMEM containing 0.1% BSA. To analyze Glut4 proteins levels in the plasma membrane, the cells were treated with 20 µM PEITC for 6 h after pretreatment with the Akt inhibitor or ErbB inhibitor (CI−1033, 5.0 µM, CI) for 30 min. Phosphorylation levels of Akt (a) and ERK (d) and Glut4 proteins levels in the plasma membrane (c) were detected using Western blotting with specific antibodies. To assess the effects of Akt and ERK inhibitors on glucose uptake induced by PEITC, the cells were treated with 20 µM PEITC for 6 h in the absence or presence of the inhibitor of Akt (b) or ERK (e), and then glucose uptake was determined as described in Materials and methods. Values are shown as mean ± SE (n= 3−4 for Western blotting, n= 3−6 for glucose uptake assays). Significant differences between values are indicated with different superscript characters (p < 0.05).
Relevance of Nrf2 pathway activation for PEITC-induced glucose uptake
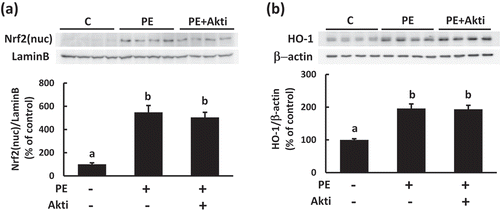
It has recently been reported that Nrf2 in skeletal muscle is involved in the lowering effect of blood glucose levels through alteration of glycogen metabolism [Citation19], and that Akt activation is involved in nuclear translocation and activation of Nrf2 [Citation13,Citation20]. Thus, we next examined the contribution of Nrf2 pathway activation to PEITC-induced glucose uptake in C2C12 cells. As shown in , nuclear translocation of Nrf2 and the expression of the HO−1 protein, which is regulated by Nrf2 [Citation21], were induced in C2C12 cells treated with PEITC, indicating that PEITC stimulates the Nrf2 pathway in C2C12 myotubes. Inhibition of the Akt pathway with Akti affected neither the nuclear translocation of Nrf2 nor the expression of HO−1 induced by PEITC, whereas glucose uptake stimulated by PEITC was dependent on Akt activation. These results suggest that Nrf2 activation is not dependent on Akt activity in the present context, and that Akt activation, but not Nrf2 activation, plays a primary role in PEITC-induced glucose uptake in C2C12 cells.
Figure 4. Effects of PEITC on the activation of the Nrf2 pathway in C2C12 cells.
After pretreatment with the Akt inhibitor (1.5 µM, Akti) for 30 min, the cells were treated with 20 µM PEITC for 180 min. The levels of nuclear translocation of Nrf2 (a) and the amount of HO−1 protein expression (b) were determined using Western blotting with specific antibodies. Lamin B and β-actin were detected as a preparation control for the nuclear fraction and as a reference control for equal protein loading, respectively. Values are shown as mean ± SE (n = 4). Significant differences between values are indicated with different superscript characters (p < 0.05).
Activation of the ErbB pathway is involved in PEITC-induced glucose uptake
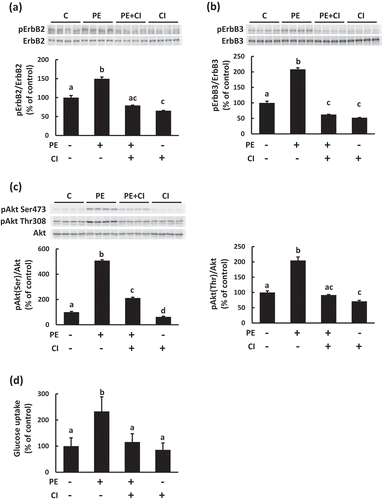
Next, we explored which signal pathway is involved in Akt activation induced by PEITC. Recently, the neuregulin (NRG)-ErbB pathway has been reported to regulate glucose and energy metabolisms [Citation22,Citation23]. NRG belongs to the epidermal growth factor family and exerts several biological activities in skeletal muscle, such as myogenesis and regulation of muscle metabolism through the ErbB family, which comprise NRG receptors [Citation24]. ErbB2 and ErbB3 are expressed in mature skeletal muscle and cultured muscle cells [Citation25]. Furthermore, activation of Akt induced by NRG is observed in C2C12 myotubes [Citation26]. We therefore analyzed whether PEITC induces activation of the ErbB receptor signaling pathway. As shown in , phosphorylation of ErbB2 and ErbB3 was observed in C2C12 myotubes treated with PEITC. Treatment with CI−1033, a pan-ErbB receptor tyrosine-kinase inhibitor, suppressed phosphorylation of ErbB2, ErbB3 and Akt (), translocation of Glut4 to plasma membrane ()) and glucose uptake ()) induced by PEITC. These results suggest that the ErbB pathway is involved in PEITC-induced glucose uptake ().
Figure 5. Effects of PEITC on the activation of the ErbB pathway in C2C12 cells.
After pretreatment with an ErbB inhibitor (CI−1033, 2.5 µM, CI) for 30 min, the cells were treated with 20 µM PEITC for 180 min in serum-free DMEM containing 0.1% BSA. Phosphorylation levels of ErbB2 (a), ErbB3 (b), and Akt (c) were detected using Western blotting with specific antibodies. To assess the effects of ErbB inhibitor on glucose uptake induced by PEITC, the cells were treated with 20 µM PEITC for 6 h in the absence or presence of the ErbB receptor inhibitor, and then glucose uptake was determined as described in Materials and methods (d). Values are shown as mean ± SE (n= 4 for Western blotting, n= 4−6 for glucose uptake assays). Significant differences between values are indicated with different superscript characters (p < 0.05).
Discussion
Isothiocyanates have been shown to have favorable effects on the regulation of lipid and glucose metabolisms. Dietary supplementation of allyl isothiocyanate or glucoraphane, a precursor of sulforaphane, suppresses body weight gain and hepatic steatosis, and improves impaired glucose tolerance induced by consumption of a high-fat diet in mice [Citation14,Citation27]. On the other hand, the beneficial effects of PEITC on glucose metabolism has not been fully investigated, although it has been reported to have anti-inflammatory and chemopreventive activities against various cancers [Citation9,Citation28,Citation29]. We recently have demonstrated that PEITC shows protective effects against insulin response impaired by oxidative stress through the induction of anti-oxidative activity in 3T3-L1 adipocytes [Citation10]. However, a stimulatory effect on glucose uptake by PEITC alone was not observed. In the present study, we revealed that treatment with PEITC increased Glut4 translocation to the plasma membrane and glucose uptake in C2C12 myotubes.
Activation of Akt regulates Glut4 translocation to the plasma membrane and the subsequent increase in glucose uptake. Sulforaphane stimulates Akt activation and is involved in cytoprotective and anti-oxidative effects through regulation of Nrf2 activity [Citation13,Citation20]. In contrast, few studies have reported that PEITC can activate the Akt pathway. To our knowledge, this is the first report demonstrating that PEITC activates Akt and thereby increases glucose utilization in C2C12 cells.
We found that inhibition of ErbB receptor activity suppressed Akt activation and glucose uptake (), suggesting that the ErbB receptor pathway underlies the effects of PEITC on C2C12 myotubes. ErbB2 and ErbB3 receptors are expressed in mature skeletal muscle and C2 muscle cells, and the physiological ligand NRG stimulates protein synthesis through the PI3K/Akt pathway in C2C12 cells [Citation25,Citation26]. Recently, Pentassuglia et al. have shown that NRG1 induces glucose uptake through the PI3K/Akt pathway in neonatal rat cardiomyocytes [Citation22]. These results support that the ErbB pathway can play a substantial role in regulation of anabolic response and glucose metabolism in muscle cells. Thus, the stimulatory effect of PEITC on glucose uptake in C2C12 cells shown in the present study may mimic part of the regulatory activity on the metabolic response by the NRG-ErbB pathway. However, our results are inconsistent with the previous observation that Akt activation is not essential for NRG stimulation of glucose uptake in cultured muscle cells [Citation30]. This inconsistency may result from differences in mode of action of ErbB activation between PEITC and the natural ligand NRG.
A possible mechanism for the modulation of intracellular signaling pathways by isothiocyanates may be ascribed to the fact that isothiocyanates covalently bind to a cysteine residue in the active site of protein tyrosine phosphatases (PTPases), which inhibits activity [Citation31]. Inhibition of PTPases has been shown to induce activation of Akt and promote glucose uptake in C2C12 myotubes [Citation32,Citation33]. SHP2, a cytoplasmic PTPase, is a negative regulator of ErbB [Citation34], and some isothiocyanates have been shown to inhibit SHP2 [Citation31]. Thus, inhibition of PTPases by PEITC may be involved in the effects of PEITC described in the present study. In addition, PTPases play an important role in glucose metabolism through regulating insulin signaling activity. A recent study has shown that ErbB3 phosphorylates insulin receptor substrate 1, a mediator protein of insulin signaling, in breast cancer cells [Citation35]. Thus, the interplay between insulin signaling and ErbBs might be involved in the biological action by PEITC. Further studies are needed to reveal the mechanism of activation of Akt by PEITC.
Isothiocyanates are known as natural activators of the Nrf2 pathway. Activation of Nrf2 regulates clusters of genes associated with lipid and carbohydrate metabolisms [Citation36]. Uruno et al. have reported that skeletal muscle-specific activation of Nrf2 increases energy expenditure and improves glucose tolerance in genetic Keap1 knockdown mice [Citation19]. In addition, evidence has been accumulating that Nrf2 plays a role in maintaining mitochondrial homeostasis and structural integrity [Citation37]. Ahn et al. demonstrated that mitochondrial DNA content and oxygen consumption rate are elevated in C2C12 cells treated with allyl isothiocyanate [Citation14]. Sulforaphane increases glucose utilization with enhanced expression of Nrf2 and the transcriptional regulators of mitochondrial biogenesis peroxisome proliferator activated receptor gamma coactivator 1 alpha and nuclear respiratory factor 1, in 3T3-L1 adipocytes [Citation38]. Furthermore, several kinases regulate Nrf2 activity through phosphorylation of specific sites favoring the release of Nrf2 from the inhibitory regulator Keap1 [Citation39], and sulforaphane induces Akt activity involved in the activation of the Nrf2 pathway [Citation13,Citation20]. In the present study, we revealed that PEITC induces glucose uptake through activation of Akt. Thus, we examined whether activation of the Nrf2 pathway is involved in glucose uptake induced by PEITC. However, inhibition of Akt did not affect translocation of Nrf2 into nucleus or the expression of HO−1 protein induced by PEITC, whereas glucose uptake by PEITC was suppressed ( and ). It is therefore unlikely that the Nrf2 pathway plays a primary role in glucose uptake induced by PEITC in C2C12 cells. We cannot rule out the possibility that PEITC induces changes in mitochondrial function and subsequently increases glucose utilization. Considering the difference in treatment period, with long term stimulation (24 or 48 h) performed in the reports described above and 3−6 h stimulation in our study, the role of mitochondrial activity modulation by PEITC on glucose uptake may be limited, even if PEITC could activate mitochondrial function [Citation14,Citation38]. In the present study, stimulatory effect of PEITC on glucose uptake was evaluated by glucose consumption for several hours after PEITC treatment, which has the possibility that changes in glucose and energy metabolic activities are involved in it. It might give some influence on the results that the level of glucose uptake was not completely consistent with the translocation level of Glut4 to the plasma membrane when Akt activation induced by PEITC was suppressed by the Akt inhibitor (). Further studies are needed to clarify whether PEITC can regulate mitochondrial function and metabolic activities such as glucose and energy utilizations.
In conclusion, PEITC induces translocation of Glut4 to the plasma membrane and subsequently increases glucose uptake in C2C12 myotubes. The effects of PEITC on glucose utilization was dependent on Akt activation but not on Nrf2 activation. We also found that modulation of the ErbB receptor activity by PEITC underlies the effect of PEITC on glucose uptake in C2C12 cells. PEITC may therefore serve as a dietary constituent with beneficial effects on the carbohydrate metabolism.
Author contributions
Y.I. designed the study, M.C. performed the experiments, M.C., Y.I., and T.N. analyzed the data and discussed results, M.C. and Y.I. wrote the manuscript. All authors read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Katz LD, Glickman MG, Rapoport S, et al. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983;32:675–679.
- Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195.
- DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687.
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflügers Arch. 2004;447:480–489.
- Ogurtsova K, Da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
- Kim S, Go GW, Imm JY. Promotion of glucose uptake in C2C12 myotubes by cereal flavone tricin and its underlying molecular mechanism. J Agric Food Chem. 2017;65:3819–3826.
- Waterman C, Rojas-Silva P, Tumer TB, et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol Nutr Food Res. 2015;59:1013–1024.
- Granato D, Nunes DS, Barba FJ. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci Technol. 2017;62:13–22.
- Telang U, Morris ME. Effect of orally administered phenethyl isothiocyanate on hepatic gene expression in rats. Mol Nutr Food Res. 2010;54:1802–1806.
- Nagami M, Ito Y, Nagasawa T. Phenethyl isothiocyanate protects against H2O2-induced insulin resistance in 3T3-L1 adipocytes. Biosci Biotechnol Biochem. 2017;81:2195–2203.
- Bhakkiyalakshmi E, Sireesh D, Rajaguru P, et al. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res. 2015;91:104–114.
- Xu C, Yuan X, Pan Z, et al. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–1926.
- Leoncini E, Malaguti M, Angeloni C, et al. Cruciferous vegetable phytochemical sulforaphane affects phase II enzyme expression and activity in rat cardiomyocytes through modulation of Akt signaling pathway. J Food Sci. 2011;76:H175–H181.
- Ahn J, Lee H, Im SW, et al. Allyl isothiocyanate ameliorates insulin resistance through the regulation of mitochondrial function. J Nutr Biochem. 2014;25:1026–1034.
- Nishiumi S, Ashida H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci Biotechnol Biochem. 2007;71:2343–2346.
- Klip A, Sun Y, Chiu TT, et al. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol. 2014;306:C879–C886.
- Chen HC, Bandyopadhyay G, Sajan MP, et al. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562.
- Sajan MP, Bandyopadhyay G, Miura A, et al. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Am J Physiol Endocrinol Metab. 2010;298:E179–E192.
- Uruno A, Yagishita Y, Katsuoka F, et al. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol. 2016;36:1655–1672.
- Deng C, Tao R, Yu SZ, et al. Sulforaphane protects against 6-hydroxydopamine-induced cytotoxicity by increasing expression of heme oxygenase-1 in a PI3K/Akt-dependent manner. Mol Med Rep. 2012;5:847–851.
- Kang JS, Choi IW, Han MH, et al. The cytoprotective effects of 7,8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts. Int J Mol Med. 2015;36:501–510.
- Pentassuglia L, Heim P, Lebboukh S, et al. Neuregulin-1β promotes glucose uptake via PI3K/Akt in neonatal rat cardiomyocytes. Am J Physiol Endocrinol Metab. 2016;310:E782–E794.
- Cantó C, Pich S, Paz JC, et al. Neuregulins increase mitochondrial oxidative capacity and insulin sensitivity in skeletal muscle cells. Diabetes. 2007;56:2185–2193.
- Gumà A, Martínez-Redondo V, López-Soldado I, et al. Emerging role of neuregulin as a modulator of muscle metabolism. Am J Physiol Endocrinol Meta. 2010;298:E742–E750.
- Jo SA, Zhu X, Marchionni MA, et al. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature. 1995;373:158–161.
- Hellyer NJ, Mantilla CB, Park EW, et al. Neuregulin-dependent protein synthesis in C2C12 myotubes and rat diaphragm muscle. Am J Physiol Cell Physiol. 2006;291:C1056–C1061.
- Nagata N, Xu L, Kohno S, et al. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes. 2017;66:1222–1236.
- Brown KK, Blaikie FH, Smith RA, et al. Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. J Biol Chem. 2009;284:32425–32433.
- Sturm C, Wagner AE. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int J Mol Sci. 2017;18:E1890.
- Cantó C, Suárez E, Lizcano JM, et al. Neuregulin signaling on glucose transport in muscle cells. J Biol Chem. 2004;279:12260–12268.
- Lewis SM, Li Y, Catalano MJ, et al. Inactivation of protein tyrosine phosphatases by dietary isothiocyanates. Bioorg Med Chem Lett. 2015;25:4549–4552.
- Liu X, Takano C, Shimizu T, et al. Inhibition of phosphatidylinositide 3-kinase ameliorates antiproliferation by benzyl isothiocyanate in human colon cancer cells. Biochem Biophys Res Commun. 2017;491:209–216.
- Takada M, Sumi M, Maeda A, et al. Pyrroloquinoline quinone, a novel protein tyrosine phosphatase 1B inhibitor, activates insulin signaling in C2C12 myotubes and improves impaired glucose tolerance in diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2012;428:315–320.
- Tanowitz M, Si J, Yu DH, et al. Regulation of neuregulin-mediated acetylcholine receptor synthesis by protein tyrosine phosphatase SHP2. J Neurosci. 1999;19:9426–9435.
- Knowlden JM, Gee JM, Barrow D, et al. ErbB3 recruitment of insulin receptor substrate 1 modulates insulin-like growth factor receptor signalling in oestrogen receptor-positive breast cancer cell lines. Breast Cancer Res. 2011;13:R93.
- Yates MS, Tran QT, Dolan PM, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031.
- Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188.
- Zhang HQ, Chen SY, Wang AS, et al. Sulforaphane induces adipocyte browning and promotes glucose and lipid utilization. Mol Nutr Food Res. 2016;60:2185–2197.
- Bryan HK, Olayanju A, Goldring CE, et al. The Nrf2 cell defence pathway: keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717.
