ABSTRACT
Here we have explored the effect of neoagarotetraose (NAT) on liver injury caused by intense exercise. Our results showed that NAT treatment obviously decreased liver weight (p < 0.01), improved the liver morphological structure, decreased ALT level (p < 0.05) and endotoxin (LPS) (p < 0.01). In addition, NAT could regulate bile acid profiles in feces and serum of mice, which indicated the potential of liver function, suggesting that NAT was effective to relieve intense exercise-induced liver injury. NAT could regulate the expression of colon genes. NAT tended to alter the microbial composition of mice under intense exercise. We uncovered the network interactions between liver traits and microbial communities in NAT treatment mice. Interestingly, our data indicated that intense exercise-induced liver injury may be related to Clostridiales. In summary, these results demonstrated that NAT relieved liver injury induced by intense exercise may be related to gut microbiota.
GRAPHICAL ABSTRACT
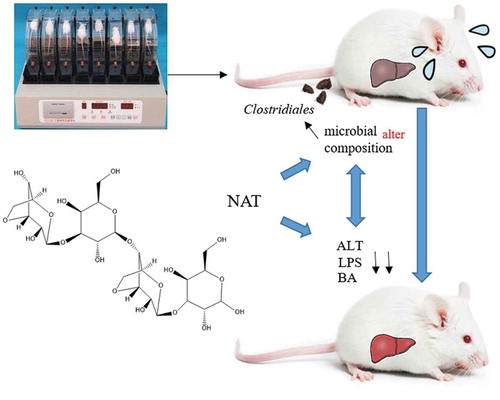
NAT can effectively relieve liver injury induced by intense exercise. We uncovered the network interactions between liver traits and microbial communities in NAT treatment mice.
Recently, the impact of exercise has received sufficient scientific attention. Beneficial effects of aerobic exercise on metabolic health and particularly insulin resistance are well established [Citation1]. In addition, moderate exercise is commonly used as an approach for protecting against or reducing hypertension in the human population [Citation2,Citation3]. Also, brain function and delay neurodegeneration were enhanced by physical exercise [Citation4]. Nevertheless, other studies have investigated the alteration of gut microbiota and increased bacterial translocation following intense exercise [Citation5,Citation6]. Further, intense exercise altered kidney morphology and tissue structure, especially the kidney cortex, and significantly increased the kidney weight [Citation7]. Moreover, high-intensity exhaustive exercise can lead to liver injury and hepatocyte apoptosis whose damage mechanisms had not yet been reported [Citation8].
The role of the gut: liver axis in liver disease is well recognized. The gut and the liver are intimately associated, and there is continuous bidirectional communication through the bile, hormones, inflammatory mediators, and products of digestion and absorption [Citation9]. Because of the anatomical position and its unique vascular system, the liver is susceptible to the exposure to the microbial products from the gut, such as lipopolysaccharide (LPS). LPS is frequently found in patients with cirrhosis, and the degree of LPS is correlated with the degree of liver failure [Citation10]. Studies have indicated that the intestinal microbiota might play an important role in the pathogenesis of liver disease [Citation11]. Chen et al. [Citation12] suggested that increased intestinal pathogenic bacteria facilitate immune-mediated liver injury, which may be due to the activation of natural killer T cells that mediated by intestinal bacterial antigens activated dendritic cells. In addition, the intestinal microbiota and Toll-like receptors (TLRs) promote hepatocellular carcinoma (HCC), a long-term consequence of chronic liver injury, inflammation, and fibrosis [Citation13]. Loomba et al. [Citation14] reveal how a panel of gut microbiome biomarkers can be used as a non-invasive test to accurately diagnose advanced fibrosis in patients with non-alcoholic fatty liver disease. Thus, the intestinal microbiota should be expected to have both direct and indirect effects on liver disease and liver function [Citation15].
We cloned a novel β-agarase gene from A. gilvus WH0801, AgWH50A. And we demonstrated that AgWH50A is a neoagarotetraose (NAT)-forming β-agarase, and is capable of hydrolyzing agar to neoagarotetraose, which has important industrial applications [Citation16]. We had prepared NAT by the enzymatic hydrolysis of agar. To date, NAT has been found to exhibit various biological and physiological functions [Citation17]. Our former research showed that NAT could reshape the microbiota composition, improved gut colonization of anti-inflammatory bacteria and helped to alleviate systemic inflammation caused by antibiotics [Citation18]. Moreover, NAT could protect mice against intense exercise-induced fatigue damage by modulating gut microbial and maintaining gut epithelial integrity [Citation19]. However, as a novel marine oligosaccharide, the effect of NAT on liver injury induced by intense exercise had not yet been reported. Intense exercise can affect gut permeability by decreasing the barrier function of the epithelium. Additionally, intense exercise has been shown to alter gut microbiota. Interestingly, NAT could promote commensal bacteria growth, and preserve intestinal barrier integrity. Indeed, liver injury is associated with dysbiosis, disruption of gut barrier integrity, and leakage of inflammatory bacterial-derived components (i.e., endotoxin) from the gut into the systemic circulation [Citation20]. The purpose of the current study was to test if the prebiotic NAT, an oligosaccharide derived from seaweeds, could protect against liver injury caused by intense exercise.
Materials and methods
NAT preparation
NAT was prepared using enzymatic digestion of low-melting agarose, as previously reported [Citation16]. β-agarases AgWH50A from Agarivorans gilvus WH0801 was used for the preparation of NAT. AgWH50A enzyme and moderate low-melting agarose were mixed and incubated at 35°C for 48 h. The enzyme hydrolysate was heated in boiling water and then concentrated. The concentrate was transferred and then mixed with absolute ethanol, which precipitated agarose that did not participate in the hydrolysis reaction. The supernatant was collected by centrifugation and concentrated repeatedly to the NAT powder without moisture. NAT consists of two sugar units called neoagarobiose, which is made up of 3-O-linked-β-D-galactopyranose, and 4-O-linked 3, 6-anhydro-α-L-galactose, with the D-galactopyranose as the reducing end. The resultant NAT has purity greater than 98% with a molecular weight 630.55 [Citation19].
Animal experiment
Male Balb/c mice (18–20 g, 4 weeks old) were obtained from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). During the experimental period, mice were housed in a room maintained under a 12-h light/dark cycle at 24°C. Mice had free access to freshwater [Citation21]. Mice were fed ad libitum a Maintenance Purified Diet (AIN-93M) throughout the experiment period. After a 7-day acclimation period, mice were randomly assigned to 3 groups with 8 mice each: Untreated group (Normal), intense exercise group (Exercise), and intense exercise plus NAT treatment group (NAT) [Citation22]. The detailed exercise protocol was described in the following section. In the following 16 days, the Normal and Exercise groups were given an oral administration of normal saline at 1 p.m. once a day without fasting. The NAT treatment group of mice were given NAT with a dosage of 150 mg/kg by gavage [Citation23,Citation24]. The Exercise and NAT mice were given identical intense exercise simultaneously, while Normal mice kept still. At the end of the 16 days feeding period and following overnight fasting from 8 p.m. to 8 a.m. the next day, the same time as the animal room was turned off, mice were given inhalation anesthesia with diethyl ether [Citation25] and killed by cervical dislocation. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Ocean University of China (Approved protocol ID SCKK2012-0001).
Exercise protocol
A forced exercise wheel-track treadmill (YLS-10B, Shandong Academy of Medical Sciences, Jinan, China) was used in this study. Animals in both Exercise and NAT groups were subjected to an intensive exercise protocol while the animals in the Normal group were kept still [Citation26]. After an initial resting period to collect baseline data, exercise commenced at 20rpm running speed. The onset of exhaustion was noted when the mice could not keep up at the treadmill pace [Citation27]. The Exercise and NAT mice were given intense exercise for 3 h from 2 p.m. to 5 p.m., continuous exercise for 2 days and then intermittent 5 days. In this way, the same intensive exercise program was carried out for 16 days.
Histological analysis
The liver and jejunum samples were routinely processed with the hematoxylin and eosin (HE) stain [Citation28]. After standard fixation and dehydration processes, the liver and jejunum tissue was sectioned at a 5um thickness and stained with hematoxylin (#: 517-28-2) and eosin (#: 17372-87-1). Histological parameters, such as morphological structure, edema, and hemorrhage, were observed by light microscopy (Olympus BX-41: Olympus Optical Co. Ltd, Tokyo, Japan). The ratio of the villus length to the crypt depth was calculated as an index indicator of the comprehensive function of the small intestine [Citation28].
The detection of liver parameters
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined spectrophotometrically using standard testing kits [Citation20]. All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Liver endotoxin (LPS) concentration was measured using ELISA (R&D Systems, Minneapolis, MN, USA).
Bile acid quantification
Profiling of total bile acids and secondary bile acids in feces and serum was determined spectrophotometrically using Mouse total bile acid (TBA) and secondary bile acid (SBA) ELISA Kit. All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Sequencing using RNA-seq and data analysis
As previously described, total RNA from colon samples was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) [Citation28]. In brief, after the mice were sacrificed, a 2 cm colon was taken and the colon contents were extruded. Each sample was dissolved in 1 mL Trizol reagent by homogenization in a homogenizer based on the manufacturer’s instructions.
The RIN score of capillary electrophoresis on the Agilent Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA) was used as an indicator for verifying RNA integrity. High-integrity RNA (RIN > 9.0) was processed using an Illumina TruSeq RNA sample prep kit following the manufacturer’s instruction (Illumina, San Diego, CA, USA). And then sequenced at 50bp/sequence read using an Illumina HiSeq 2000sequencer as described previously [Citation29].
Group comparison and Differential gene expression analysis were based on DESeq2 v3.2 [Citation29].
Microbial analysis of the feces
Fecal samples for metagenomic analysis were collected from each mouse at necropsy. Total DNA was extracted from the fecal samples with a magnetic bead DNA extraction kit (Sangon, Shanghai, China), according to the manufacturer’s instruction [Citation24]. DNA integrity was verified using a BioAnalyzer 2000 (Agilent, Palo Alto, CA). The DNA concentration was then quantified using a QuantiFluor fluorometer (Promega, Madison, WI) [Citation30]. 16S rRNA gene was sequenced using an Illumina MiSeq Reagent Kit on an Illumina MiSeq sequencer. QIIME pipeline (v1.9.1) was used to analyze the 16S rRNA gene sequences. A “closed reference” protocol in the pipeline was used for Operational Taxonomic Unit (OTU) OTU picking [Citation30]. The latest version of GreenGene database (v13.8) was used for taxonomy assignment (greengenes.lbl.gov). PyNAST (v1.2.2) was used for sequence alignment [Citation30].
Molecular ecological network analyses
We constructed ecological association networks named molecular ecological networks (MENs) through Random Matrix Theory (RMT)-based methods [Citation31–Citation33]. We determined the network structure of microbial communities based on pyrosequencing data of 16S rRNA genes. The microbiota module profiles showed by Eigengene analysis. And network interactions between several major liver traits and microbial communities were examined. All these methods and statistical tools have been integrated into a comprehensive Molecular Ecological Network Analysis Pipeline (MENAP), which is open-accessible now (http://ieg2.ou.edu/MENA) [Citation34].
Statistical analysis
All of the values in the tables and figures are expressed as the mean ± S.E.M. Statistical comparisons of the results were performed using Tukey’s post-hoc test (ANOVA) analysis of variance by SPSS 18.0. p < 0.05 was considered statistically significant.
Results
Effects of orally administered NAT on organ weight with intense exercise
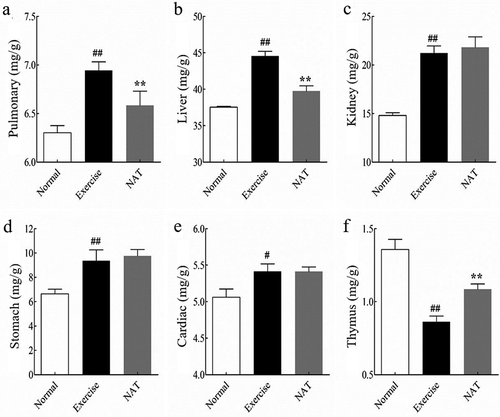
To evaluate the effect of intense exercise and orally administered NAT on mice, organ weight analysis was performed. As shown in , the high pulmonary weight and liver weight induced by intense exercise were significantly reduced by the oral administration of NAT (p < 0.01). Also, thymus weight was largely reduced by intense exercise but increased by NAT significantly (p < 0.01). These data suggest the possibility that orally administered NAT is an effective way to relieve organ injury in mice induced by intense exercise.
Figure 1. Effects of orally administered NAT on organ weight changes.
(a) Pulmonary; (b) Liver; (c) Kidney; (d) Stomach; (e) Cardiac; (f) Thymus. Error bars indicate standard deviations. Normal: normal control group. Exercise: intense exercise group. NAT: intense exercise + NAT administration. P values were performed using Tukey’s post-hoc test ANOVA using SPSS 18.0. #p < 0.05, ##p < 0.01 (Exercise versus Normal), *p < 0.05, **p < 0.01 (NAT versus Exercise).
Oral administration of NAT is effective to suppress liver injury caused by intense exercise
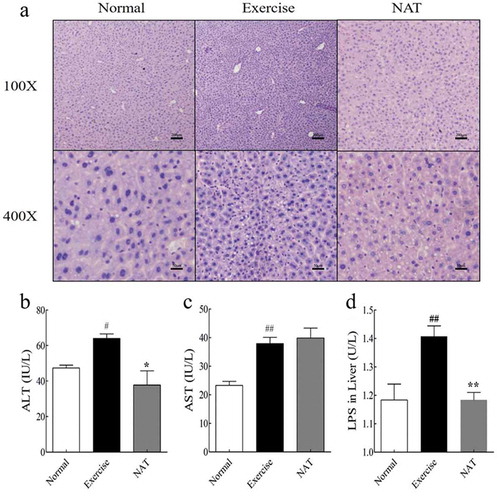
Light microscopic examination indicated the morphological structure of the liver. The liver tissues in the normal group had typical hepatic lobule structure, and the liver cells arranged orderly. Nuclei are large and rich in chromatin. While, in the exercise group, the liver cells arranged disorderly; the cell shrinkage was obvious; the cytoplasm was sparse. After 16 days of NAT treatment, the shrinkage and thinning of liver cells were gradually alleviated ()). To assess liver injury induced by intense exercise, Liver ALT and AST activities were determined. Liver ALT and AST activities were significantly increased by intense exercise (p < 0.05). NAT markedly decreased ALT level ()). But there was no significant difference in AST level between Exercise group and NAT group ()). Taken together, our results suggest that 16-day NAT feeding sensitizes liver to intense exercise-induced liver injury. We also indicated that the concentration of liver endotoxin (mean ± SEM = 1.41 ± 0.04 U/L for Exercise and 1.18 ± 0.03 U/L for NAT) was substantially decreased by NAT (p < 0.01). Our results showed that intense exercise largely increased the level of liver endotoxin, and this effect was reversed by NAT supplementation (p < 0.01) ()).
Figure 2. Effect of NAT on liver injury caused by intense exercise.
(a) Histological photomicrograph of HE stained sections; (b) ALT activity in liver; (c) AST activity in liver; (d) LPS in liver. Data are expressed as mean ± SEM (n = 8). #p < 0.05, ##p < 0.01 (Exercise versus Normal), *p < 0.05, **p < 0.01 (NAT versus Exercise). Abbreviation: ALT, alanine transaminase; AST, aspartate transaminase.
Effects of orally administered NAT on liver function with intense exercise
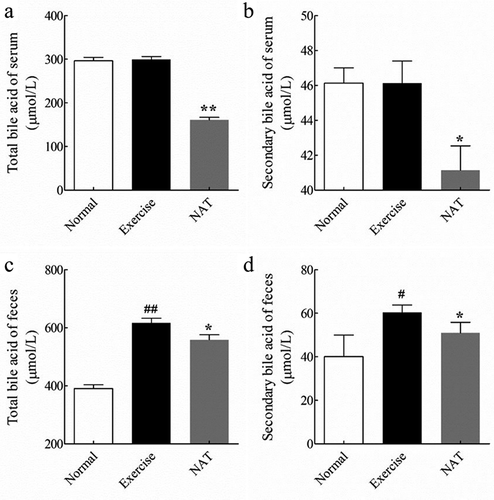
Intense exercise mouse model has also provided evidence that intense exercise could be linked to altered fecal and serum bile acid profiles and the potential for liver function. Our results have shown that intense exercise leads to increased total bile acid (TBA) (p < 0.01) ()) and secondary bile acid (SBA) ()) of feces (p < 0.05), which were significantly decreased by NAT treatment. Although intense exercise has no significant impact on serum total bile acid and secondary bile acid (, )), they were significantly decreased by NAT (p < 0.05).
Figure 3. Bile acids concentration changes in the fecal and serum.
(a) Total bile acid of serum; (b) Secondary bile acid of serum; (c) Total bile acid of feces; (d) Secondary bile acid of feces. Data are expressed as mean ± SEM (n = 8). #p < 0.05, ##p < 0.01 (Exercise versus Normal), *p < 0.05, **p < 0.01 (NAT versus Exercise).
Effects of orally administered NAT on gut epithelial integrity in mice under intense exercise
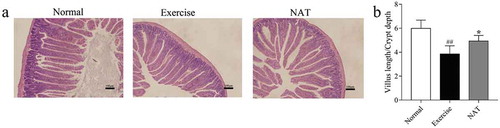
The morphological structure of the jejunum changed ()). In the intense exercise mice, the villi were significantly shortened and thickened, while the NAT group showed a certain degree of remission. Specifically, the length of villi was increased, and the swelling was alleviated. The V/C ratio of jejunum was reduced by intense exercise (p < 0.01), but increased by NAT significantly (p < 0.05) ()). These data showed that NAT significantly improves the intestinal barrier damage caused by intense exercise and protect the intestinal integrity effectively.
Figure 4. Effects of NAT on gut epithelial integrity in mice under intense exercise.
(a) Histological photomicrograph of HE stained sections; (b) Villus length/Crypt depth ratio. Data are expressed as mean ± SEM (n = 8). #p < 0.05, ##p < 0.01 (Exercise versus Normal), *p < 0.05, **p < 0.01 (NAT versus Exercise).
Colonic transcriptome analysis displayed genes significantly impacted by intense exercise and NAT treatment
Each colon sample was detected and at least 21,290 genes were identified. The screening conditions were fold change greater than 2.0 for significant up-regulation and less than 0.7 for significant down-regulation. Exhaustive exercise led to a significant up-regulation () or down-regulation () of 1053 genes. The results showed that intense exercise significantly up-regulated the solute transporter family members, apoptosis-related factors, interferon, and apolipoprotein overexpression. Simultaneously, exhaustive exercise significantly reduced the expression levels of proliferating proteins, transferrin and macrophage-associated factors. The results indicated that intense exercise could promote the apoptosis of intestinal epithelial cells, inhibit the expression of proliferating proteins, and reduce the body’s own immunity and induce inflammation.
Table 1. Genes significantly increased by intense exercise in the colon samples detected by RNA-seq technology.
Table 2. Genes significantly decreased by intense exercise in the colon samples detected by RNA-seq technology.
NAT could significantly increase the expression of the heat shock protein family (). Studies have shown that heat shock proteins can increase the stress ability of cells, promote the gluconeogenesis and glycogen production in cells, increase the amount of glycogen stored in cells, and thus improve stress ability. In addition, NAT significantly increased the expression of ribosomal protein. Ribosome proteins are the major components of ribosomes and play an important role in intracellular protein biosynthesis. NAT could significantly reduce transmembrane protein, nuclear transcription factor NF-κB, phosphatase, metal peptidase, CD46 antigen, cytochrome P450, chemokine receptor 6, phospholipase Relative expression level (). NF-κB is a transcription factor with multi-directional regulation function, which is widely involved in the transcriptional regulation of various genes, and is often present in the cytoplasm in the form of P50/P65 heterodimer. When stimulated by inflammatory cytokines, IKB is ubiquitinated, NF-κB is activated and enters the nucleus, which activates transcriptional activation of target genes such as COX-2 and Bcl-2, and participates in tumor angiogenesis, proliferation, anti-apoptosis and Transfer and other processes. Studies have shown that chemokine receptor 6 is directly involved in the development of atherosclerosis. In inflammatory bowel disease, chemokine receptor 6 participates in intestinal inflammatory responses to tissue damage and trauma through regulation of macrophages. In addition, the study also found that knockdown of chemokine receptor 6 is resistant to sepsis caused by intestinal flora and endotoxin translocation. All the above results indicated that NAT can significantly regulate the expression of colon genes.
Table 3. Genes significantly increased by NAT in the colon samples detected by RNA-seq technology.
Table 4. Genes significantly decreased by NAT in the colon samples detected by RNA-seq technology.
Dietary NAT altered the microbiota composition of mice under intense exercise
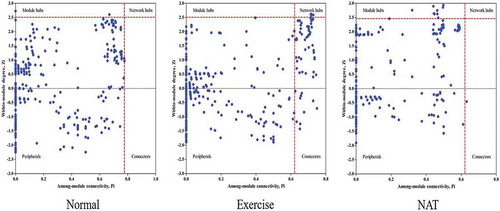
The topological roles of gut microbiota in mice were illustrated in ZP-plot (). According to the values of Zi and Pi, the gut microbiota was classified into four categories: peripherals, connectors, module hubs, and network hubs (). In brief, Peripherals were only responsible for the implementation of its own functions, did not interact with other OTU; Connectors were only responsible for the connection between Module; Module hubs were mainly responsible for the internal connection of its own module; Network hubs were responsible for the internal contact of its own module. It also played an important role in the connection between modules. The ZP-plot results showed a distinct difference in the microbial composition between the normal, exercise, and NAT treatment mice. For example, the number of connectors was significantly increased in intense excise mice when compared with normal group. And NAT tended to alter the microbial composition of mice under intense exercise. This result demonstrated that NAT significantly regulates the potential network relationship of gut microbiota in exhausted mice.
Table 5. The roles of microbiota were classified into four categories according Zi and Pi.
Figure 5. ZP-plot showing distribution of OTUs based on their module-based topological roles.
Each dot represents an OTU in the dataset of normal, exercise or NAT group. The topological role of each OTU was determined according to the scatter plot of within-module connectivity (Zi) and among-module connectivity (Pi).
The correlations and heat-map to show the network interactions between liver traits and microbial communities
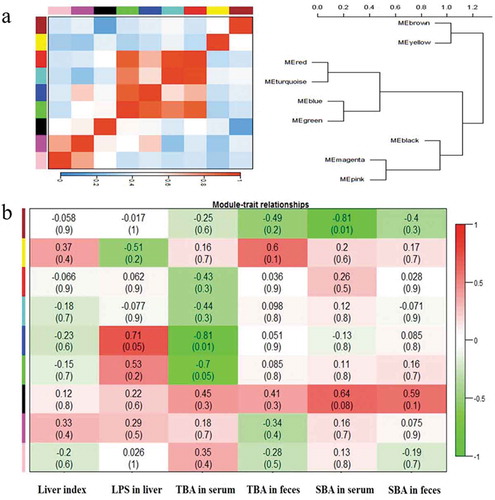
In NAT mice, 9 largest modules were visualized as a heat-map and hierarchical clustering diagram (). The modules showed significant correlations and clustered together as super-groups, such as module 1 (brown) and module 2 (yellow), module 7 (black), module 8 (magenta), and module 9 (pink) ()). In NAT-treatment mice, the similarity coefficients and P values were shown in a heat-map ()). Module 5 (blue) was positively correlated with the LPS in liver significantly (p = 0.05) but negatively with TBA in serum (p = 0.01), indicating that LPS concentration in liver and TBA in serum might be stimulated by the microbiota members in module 5. Also, module 1 (brown) was negatively correlated with SBA in serum (p = 0.01) and module 6 (green) was negatively correlated with TBA in serum (p = 0.05). All above results demonstrated that different modules in NAT mice responded to the liver traits changes differently, and the gut microbiota could have significant impacts on the changes of TBA in serum.
Figure 6. The correlations and heat-map to show the network interactions between liver traits and microbial communities.
(a) The hierarchical clustering based on the Pearson correlations among module. One color represents one microbiota module; (b) Heat-map shows the coefficient values between liver traits and gut microbiota. Red color means higher correlation whereas green color signified lower correlation. The above values represent the similarity coefficients. The values in parentheses represent P value.
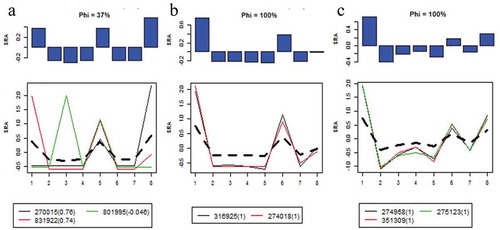
Analysis of the key microbiota members and relative abundance of each module
Three OTUs belonged to module 1 ()) that was negatively related to SBA in serum. OTU270015 and OUT 831922 could be assigned to Clostridiales, while OTU801995 belonged to Staphylococcus. Two OTUs belonged to module 5 ()) that was related to LPS in liver and TBA in serum. Three OTUs belonged to module 6 ()) that was negatively related to TBA in serum. Interestingly, all three modules (module1, module5, and module6) were mostly derived from Clostridiales, which indicated that the liver injury induced by intense exercise may be related to Clostridiales.
Figure 7. Microbiota profiles and relative abundance of each module.
(a) Module 1; (b) Module 5; (c) Module 6. Notes: (1) SRA: Standardized relative abundance. (2) Y-axis is SRAs and the X-axis is individual samples. (3) Black dotted lines and blue bar graphs represent averages of SRAs. (4) The values in parentheses are module memberships.
Discussion
Fatigue has emerged as an important health issue in humans, and intense exercise can lead to the production and accumulation of reactive free radicals and reduce gastrointestinal tract perfusion, which results in oxidative injury and energy source depletion as well as excess metabolite accumulation [Citation35]. In our study, a forced exercise wheel-track treadmill was used to establish intense exercise animal model. And we found that intense exercise can lead to acute liver injury. For example, firstly, we calculated organ weight of each mouse involved in intense exercise. Our data showed that NAT could relieve pulmonary injury and increase thymus weight which reflect the host immune function on the whole. But NAT played the most significant protective effect on liver.
Next, we detected the morphological structure of the liver. After 16 days of NAT treatment, the shrinkage of liver cells was gradually alleviated. To assess how the dietary NAT protect liver of intense exercise mice, we measured ALT and AST activity in the liver. ALT and AST levels are predictive of liver disease and liver-related mortality. In clinical practice, blood levels of ALT and AST are used to index liver injury. AST and ALT blood levels increase when the liver cell membrane is damaged and thus mark hepatocellular injury [Citation36]. Our results suggested that NAT markedly decreased ALT level. But there was no significant difference in AST level. Furthermore, we explored the mechanism of liver damage induced by intense exercise. Our results showed that intense exercise largely increased the level of liver endotoxin, and this effect was reversed by NAT supplementation.
Moreover, we found that intense exercise could be linked to altered bile acid profiles and the potential for liver damage. Total bile acids and secondary bile acid concentration changes in the fecal and serum were determined. Bile acids are produced in hepatocytes, stored in the gall bladder, and released into the duodenum upon ingestion of a meal to facilitate absorption of triglycerides, cholesterol, and lipid-soluble vitamins [Citation37]. Bile acids are synthesized from cholesterol in the liver and further metabolized by the gut microbiota into secondary bile acids [Citation38]. Secondary bile acid is the product of microbial metabolism and promotes liver cancer [Citation39,Citation40]. Microbial modifications of bile acids influence host metabolism via the bile acid receptors FXR and TGR5 [Citation41,Citation42]. Our results have shown that total bile acid (TBA) and secondary bile acid (SBA) in serum and feces were significantly decreased by NAT treatment. It indicates that NAT could alter bile acid profiles and has the potential to improve liver function, which may be related to gut microbiota [Citation43].
In addition, we examined the morphological structure of the jejunum, the results showed that NAT may improve intestinal barrier Integrity in mice undergoing intense exercise, and maintain the normal structure and physiological function of the intestine. NAT was utilized by the colonic microbiota to function. Colonic transcriptome analysis showed that intense exercise significantly altered the expression of colon genes. NAT could significantly regulate the stress ability of intestinal cells and increase the glucose metabolism process in the intestine. In addition, NAT inhibited the expression of nuclear transcription factors and the expression of intestinal chemokine receptors and reduced intestinal inflammation. At this point, we speculate that NAT protects the liver by regulating intestinal microbiota and intestinal integrity [Citation19,Citation44].
Our results proposed that intense exercise could significantly change the gut microbiota structure. The ZP-plot results showed a distinct difference in the microbial composition between the normal, exercise, and NAT treatment mice. And NAT tended to alter the microbial composition of mice under intense exercise by decreasing the gut microbiota classified into connectors. Then, we develop a novel random matrix theory (RMT)-based bioinformatic approach to define and characterize ecological networks in microbial communities based on high-throughput metagenomic sequencing data and distribute the gut microbiota into clearly delimited modules. Moreover, we analyze the correlations between liver traits and microbial communities using Molecular Ecological Network Analysis Pipeline (MENAP) [Citation33]. Our results showed that LPS in liver was correlated with Module 5; TBA in serum was correlated with Module 5 and Module 6; SBA in serum was correlated with Module 1. Furthermore, we analyzed the key microbiota members and relative abundance of each module (). We came to the conclusion that Staphylococcus and Clostridiales were closely related to liver traits. Staphylococcus can cause a wide variety of diseases in humans and animals through either toxin production or penetration [Citation45]. The Clostridiales are a highly polyphyletic class of Firmicutes. For example, Clostridium difficile become established in the human colon.
Table 6. Annotations of OTUs of each module.
In this study, we demonstrated that NAT has the potential to alleviate intense exercise-induced liver damage by modulating the gut microbiome in mice. This would indicate the possibility that NAT could be a safe supplemental food supplement. Further investigations are required to establish the efficacy of NAT for the prevention of liver damage as a supplement for humans.
Author contributions
Conceived and designed the experiment: XC, XZM, QJT. Performed the experiment: XC, XZM, RW.L, YMW, CHX. Analyzed the data: XC, JHY, RW.L. Wrote the manuscript: XC, XZM, JHY. All authors reviewed and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pauly M, Assense A, Rondon A, et al. High intensity aerobic exercise training improves chronic intermittent hypoxia-induced insulin resistance without basal autophagy modulation. Sci Rep. 2017;7:43663.
- Rode B, Shi J, Endesh N, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8(1):350.
- Hoene M, Li J, Li Y, et al. Muscle and liver-specific alterations in lipid and acylcarnitine metabolism after a single bout of exercise in mice. Sci Rep. 2016;6(1):22218.
- Morland C, Andersson KA, Haugen ØP, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun. 2017;8:15557.
- Bermon S, Petriz B, Kajėnienė A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21(21):70–79.
- Shukla SK, Cook D, Meyer J, et al. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Plos One. 2015;10(12):e0145453.
- Liu F, Zhang N, Li Z, et al. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci Rep. 2017;7(1):6783.
- Lu J, Ge GL, Zhang HL, et al. Effect of moxibustion on hepatic glycogen and ultrastructure of exercise-induced fatigue rats. Acupuncture Res. 2011;36(1):32–35.
- Rasmussen BA, Breen DM, Lam TK. Lipid sensing in the gut, brain and liver. Trends Endocrinol Metab. 2012;23(2):49–55.
- Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18(4):337–346.
- Balmer ML, Slack E, de Gottardi A, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6(237):237ra66.
- Chen J, Wei Y, He J, et al. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota. Sci Rep. 2014;4:7259.
- Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–516.
- Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25(5):1054–1062.
- Bajaj JS, Hylemon P, Younossi Z. The intestinal microbiota and liver disease. Am J Gastroenterol. 2012;1(1):9–14.
- Liu N, Mao X, Du Z, et al. Cloning and characterisation of a novel neoagarotetraose-forming-β-agarase, AgWH50A from Agarivorans gilvus WH0801. Carbohydr Res. 2014;388:147–151.
- Hsu PH, Wei CH, Lu WJ, et al. Extracellular production of a novel endo-β-agarase agaa from pseudomonas vesicularis ma103 that cleaves agarose into neoagarotetraose and neoagarohexaose. Int J Mol Sci. 2015;16(3):5590–5603.
- Zhang N, Hou E, Song J, et al. Neoagarotetraose-modulated gut microbiota and alleviated gut inflammation in antibiotic treatment mice. Food Agric Immunol. 2017;28(6):1–16.
- Zhang N, Mao X, Li RW, et al. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol Nutr Food Res. 2017;61(8):1600585.
- Massey VL, Stocke KS, Schmidt RH, et al. Oligofructose protects against arsenic-induced liver injury in a model of environment/obesity interaction. Toxicol Appl Pharmacol. 2015;284(3):304–314.
- Wang X, Liu F, Wang Y, et al. The modulation effect of triglyceride type and phospholipids type ω-3 LCPUFA on mice gut microbiota. J Biosci Med. 2017;5:54–64.
- Zheng R, Li X, Cao B, et al. Dietary Apostichopus japonicus enhances the respiratory and intestinal mucosal immunity in immunosuppressive mice. Biosci Biotechnol Biochem. 2015;79(2):253–259.
- Shang Q, Shi J, Song G, et al. Structural modulation of gut microbiota by chondroitin sulfate and its oligosaccharide. Int J Biol Macromol. 2016;89:489–498.
- Tang Q, Zuo T, Lu S, et al. Dietary squid ink polysaccharides ameliorated the intestinal microflora dysfunction in mice undergoing chemotherapy. Food Funct. 2014;5(10):2529–2535.
- Zuo T, Cao L, Xue C, et al. Dietary squid ink polysaccharide induces goblet cells to protect small intestine from chemotherapy induced injury. Food Funct. 2015;6(3):981–986.
- Hao G, Zhang C, Cao W, et al. Effects of intragastric administration of five oyster components on endurance exercise performance in mice. Pharm Biol. 2014;52(6):723–728.
- Kastellorizios M, Burgess DJ. Continuous metabolic monitoring based on multi-analyte biomarkers to predict exhaustion. Sci Rep. 2015;5(1):10603.
- Zuo T, Li X, Chang Y, et al. Dietary fucoidan of acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 2015;6(2):415–422.
- Baldwin RL 6th, Wu S, Li W, et al. Quantification of transcriptome responses of the rumen epithelium to butyrate infusion using RNA-seq technology. Gene Regul Syst Bio. 2012;6:67–80.
- Li RW, Li W, Sun J, et al. The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Sci Rep. 2016;6:20606.
- Luo F, Yang Y, Zhong J, et al. Constructing gene co-expression networks and predicting functions of unknown genes by random matrix theory. BMC Bioinformatics. 2007;8(1):299.
- Zhou J, Deng Y, Luo F, et al. Functional molecular ecological networks. mBio. 2010;1(4):e00169–00110.
- Zhou J, Deng Y, Luo F, et al. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio. 2011;2(4):e00122–11.
- Deng Y, Jiang YH, Yang Y, et al. Molecular ecological network analyses. BMC Bioinformatics. 2012;13(1):113.
- Huang CC, Hsu MC, Huang WC, et al. Triterpenoid-rich extract from antrodia camphorata improves physical fatigue and exercise performance in mice. Evid Based Complement Alternat Med. 2012;2012(12):364741.
- van Beek JH, de Moor MH, de Geus EJ, et al. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav Genet. 2013;43(4):329–339.
- Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–235.
- Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14(7):676–684.
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563.
- Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101.
- Jones ML, Tomaro-Duchesneau C, Prakash S. The gut microbiome, probiotics, bile acids axis, and human health. Trends Microbiol. 2014;22(6):306–308.
- Wahlström A, Sayin SI, Marschall HU, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50.
- Nakajima M, Arimatsu K, Kato T, et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. Plos One. 2015;10(7):e0134234.
- Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096.
- Tong SY, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661.
