ABSTRACT
Sterol regulatory element-binding proteins (SREBPs) are transcription factors that regulate the expression of genes involved in fatty acid and cholesterol biosynthetic pathways. The present study showed that the flavonoid chrysin impairs the fatty acid synthase promoter. Chrysin reduces the expression of SREBP target genes, such as fatty acid synthase, in human hepatoma Huh-7 cells and impairs de novo synthesis of fatty acids and cholesterol. Moreover, it reduces the endogenous mature, transcriptionally active forms of SREBPs, which are generated by the proteolytic processing of precursor forms. In addition, chrysin reduces the enforced expressing mature forms of SREBPs and their transcriptional activity. The ubiquitin–proteasome system is not involved in the chrysin-mediated reduction of SREBPs mature forms. These results suggest that chrysin suppresses SREBP activity, at least partially, via the degradation of SREBPs mature forms.
Abbreviations: ACC1: acetyl-CoA carboxylase 1; DMEM: Dulbecco’s modified Eagle’s medium; FAS: fatty acid synthase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; 25-HC: 25-hydroxycholesterol; HMGCS: HMG-CoA synthase; LDH: lactate dehydrogenase; LPDS: lipoprotein-deficient serum; PI3K: phosphatidylinositol 3-kinase; SCD1: stearoyl-CoA desaturase; SREBPs: sterol regulatory element-binding proteins.
Graphical abstract
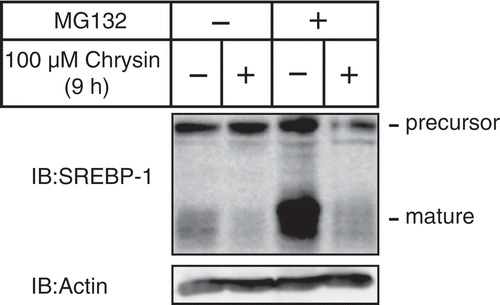
Chrysin decreases the protein level of SREBPs mature forms regardless of the presence of MG132, a proteasome inhibitor.
KEYWORDS:
In recent years, an increasing number of people suffer from metabolic diseases, such as type-II diabetes and cardiovascular diseases conditions [Citation1]. The onset of metabolic diseases is mainly caused by lipid homeostasis disruption [Citation2]. Sterol regulatory element-binding proteins (SREBPs), a family of transcription factors including SREBP-1a, SREBP-1c, and SREBP-2, regulate the expression of the genes involved in fatty acid and cholesterol biosynthetic pathways [Citation3]. SREBP-1c dysregulation is considered an important factor involved in metabolic diseases, and its overexpression in transgenic mouse liver leads to the development of fatty liver, hypertriglyceridemia, and insulin resistance [Citation4]. Moreover, obese mice exhibit increased mRNA and proteolytic processing levels of SREBP-1c in the liver [Citation5,Citation6]. Therefore, repression of SREBP-1c activity may constitute a promising drug target for metabolic diseases.
Chrysin is abundantly present in many plant extracts, including propolis, blue passion flower, and honey, and exerts several pharmacologic activities against cancer, diabetes, cardiovascular diseases, and rheumatoid arthritis [Citation7]. We have previously reported that several small compounds of food ingredients suppress SREBPs, using a luciferase reporter gene assay with the FAS gene promoter region [Citation8–Citation11]. In the present study, we used this cell line to identify the flavonoid chrysin as a suppressor of SREBP activity.
Materials and methods
Materials
Cholesterol, 25-hydroxycholesterol (25-HC), fluvastatin, and lipoprotein-deficient serum (LPDS), were purchased from Sigma. Dulbecco’s modified Eagle’s medium (DMEM) was from Wako. Blasticidin S was from Invitrogen. Chrysin was from LKT laboratories.
Antibodies
Monoclonal anti-SREBP-1 (2A4) antibody was purchased from Santa Cruz. Monoclonal anti-β-actin (AC-15) antibody was purchased from Sigma. Monoclonal anti-SREBP-2 (1D2) antibody was purchased from MBL. Polyclonal anti-SREBP-2 (RS004) antibody has been previously described [Citation12]. Peroxidase-conjugated affinity-purified donkey anti-mouse IgG and peroxidase-conjugated affinity-purified donkey anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratories.
Media and buffers
Medium A contained DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% (v/v) fetal bovine serum. Medium B contained DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 10% fetal bovine serum, 50 μM sodium mevalonate, and 12.5 μM fluvastatin. Furthermore, medium C contained DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 5% (v/v) LPDS, 50 μM sodium mevalonate, and 12.5 μM fluvastatin. Medium D contained DMEM supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 5% LPDS, 10 μg/mL cholesterol, and 1 μg/mL 25-HC.
Cell culture
Huh-7 cells (a human hepatoma line) were maintained in medium A. Huh-7/FAS-luc (a stable cell line of Huh-7 that expresses a luciferase reporter driven by an SRE-containing fatty acid synthase (FAS) promoter) [Citation8] cells were maintained in medium A containing 2 μg/mL blasticidin S. The cells were incubated at 37°C under a 5% CO2 atmosphere.
Luciferase assays in Huh-7/FAS-Luc cells
Huh-7/FAS-luc cells were plated in 12-well plates at a density of 1.0 × 10 [Citation5] cells/well, cultured with medium A for 24 h, and the cells were then switched to medium B for 16 h. After incubation for another 24 h in the absence or presence of 100 µM chrysin, the luciferase activity and protein contents of the cell extracts were measured as described previously [Citation13]. The normalized luciferase values were determined by dividing the luciferase activity by the protein content in the cell extracts quantified using the BCA protein assay (Pierce).
Luciferase assays
Huh-7 cells were plated in 12-well plates at a density of 2.0 × 10 [Citation5] cells/well, cultured with medium A for 24 h, and transfected plasmid as indicated in the figure legends. Twenty-four hours later, the cells were then cultured with vehicle or 100 µM chrysin. After incubation for another 24 h, luciferase and β-galactosidase activities were determined as described previously [Citation14]. Normalized luciferase values were determined by dividing the luciferase activity by the β-galactosidase activity.
Real-time quantitative PCR
Total RNA was extracted from Huh-7 cells using ISOGEN (Nippon Gene) according to the manufacturer’s instructions. RNA was reverse transcribed using a high capacity cDNA reverse transcription kit (Applied Biosystems). Real-time quantitative PCR (TaqMan probe and SYBR green) analysis was performed on StepOnePlus Real-Time PCR Systems. Expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The TaqMan ID numbers for genes analyzed are as follows: human (h) FAS, Hs00188012_m1; h stearoyl-CoA desaturase (SCD1), Hs00748952_s1; hGAPDH, 4,352,934. The sequences of the primer set used were as follows: h acetyl-CoA carboxylase 1 (ACC1), 5′-TGGGCCTCAAGAGGATTTGT-3′ and 5′-TCCACTGTTGGCTGATACATAGATG-3′; h HMG-CoA synthase (HMGCS), 5′-GACTTGTGCATTCAAACATAGCAA-3′ and 5′-GCTGTAGCAGGGAGTCTTGGTACT-3′.
Immunoblotting
Cells and mouse liver tissue were lysed in RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% (v/v) Triton X-100, 0.5% (w/v) deoxycholate, and 0.1% (w/v) SDS] supplemented with a protease inhibitor cocktail. The lysates were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore), and probed with the antibodies indicated in the figure legends. Immunoreactive proteins were visualized using ECL (GE Healthcare) or Immobilon (Millipore) immunoblotting detection reagents. The signals on the membrane were detected using an ImageQuant LAS 4000 mini biomolecular imager (GE Healthcare).
Plasmid constructs
The luciferase reporter plasmid pFAS-Luc has been described previously [Citation8]. Expression plasmids for mature form of human SREBP-1c and SREBP-2 [pME-SREBP1c(1–463) and pME-SREBP-2(1–481)] have been described previously [Citation12].
Measurement of de novo fatty acid and cholesterol synthesis
Huh-7 cells were plated in 12-well plates at a density of 2 × 10 [Citation5] cells/well and cultured with medium A for 24 h. The cells were then switched to medium C for 16 h. After incubation for another 18 h in the absence or presence of 50 or 100 μM chrysin, the cells were placed in fresh medium supplemented with 1.6 μCi/mL [Citation14C] acetate and incubated for 6 h. Further, the cells were washed with phosphate buffered saline (PBS), scraped, and collected by centrifugation. The cell pellets were mixed with 1 ml of 8 N potassium hydrate and 1 ml of ethanol. The mixture was heated at 100°C for 2 h and extracted two times with 2 ml of petroleum ether (cholesterol extract). Moreover, 2 ml of the lower aqueous layer was mixed with 1 mL of 12 N hydrochloric acid and then extracted two times with 3 mL of petroleum ether (FA extract). The lipid extracts were then purified by thin-layer chromatography on Silica gel 60 (Merck, Darmstadt, Germany) and quantified with a BAS2000 image analysis system (Fujifilm, Tokyo, Japan). Normalized fatty acid and cholesterol synthesis rates were determined by dividing the signals of [Citation14C]-fatty acid and [Citation14C]-cholesterol by the amounts of total cellular protein quantified using the BCA protein assay.
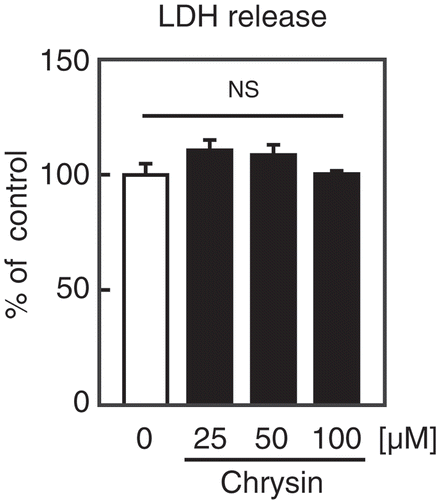
Determination of plasma membrane damage
The plasma membrane damage of Huh-7 cells was determined by LDH assay (Dojindo). Huh-7 cells were plated in 96-well plates at a density of 0.8 × 10 [Citation4] cells/well and were cultured with medium A for 24 h. After incubation for another 9 h in the absence or presence of 25 μM, 50 μM, or 100 μM chrysin, LDH assay was performed according to the manufacturer’s instructions.
Statistical analysis
All data are presented as means ± standard error of the mean (SE). Statistical analyses were performed using Ekuseru-Toukei Ver.2.0 software (Social Survey Research Information). Comparisons between two treatments were made using Student’s t-test, whereas more than two treatments were compared using one-way analysis of variance (ANOVA) followed by the Bonferroni procedure. Differences were considered statistically significant when p < 0.05.
Results
Chrysin suppresses the FAS gene promoter activity and the expression levels of the SREBP target gene as well as the de novo synthesis of fatty acids and cholesterol in Huh-7 cells
To determine whether chrysin ()) suppresses the activity of SREBP, reporter assays were performed using a human hepatoma Huh-7 cell line that stably expresses a luciferase reporter gene under the control of an SRE-containing FAS promoter (Huh-7/FAS-luc) [Citation8]. The activity of the FAS gene promoter was attenuated by a 24 h treatment with 50 or 100 μM chrysin ()). Quantitative real-time PCR analysis in Huh-7 cells revealed that a 24 h treatment with chrysin reduced the mRNA levels of two SREBP-1 target genes involved in fatty acid synthesis – FAS and ACC1, but not SCD1 ()). Similarly, the SREBP-2 target gene HMGCS, which is involved in cholesterol synthesis, was down-regulated by chrysin ()). Consistent with such gene expression changes, chrysin decreased the de novo synthesis of both fatty acids and cholesterol ()). These results imply that chrysin suppresses SREBP activity, thereby reducing the expression of its target genes and the synthesis of lipids.
Figure 1. Chrysin reduces the activity of the SRE-containing FAS promoter.
(a) Chrysin structure (b) Huh-7/Fas-luc cells were depleted of sterols through a 16 h incubation in medium B. The cells were then switched to medium B in the presence of vehicle, 50 μM chrysin, or 100 μM chrysin. After a 16 h incubation, a luciferase assay was performed and the relative activity of luciferase was obtained by normalization to the activity in the presence of vehicle. All data are presented as means ± SE (n = 3). Different superscript letters indicate statistical significance (p < 0.05).
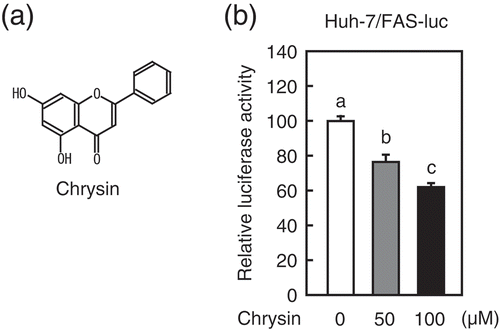
Figure 2. Chrysin reduces SREBP target gene expression and de novo synthesis of fatty acids and cholesterol.
Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. (a) The cells were then switched to medium C in the presence of either vehicle or the indicated chrysin concentrations. After a 24 h incubation, total RNA was isolated from the cells. Quantitative real-time PCR was performed, and the relative mRNA levels were obtained by normalization to GAPDH. (b) The cells were then switched to medium C in the presence of vehicle, 50 μM chrysin, or 100 μM chrysin. After an 18 h incubation, the cells were treated with 1.6 μCi/mL [Citation14C]acetate and cultured for an additional 6 h. Fatty acids and cholesterol were extracted, and the rates of synthesis were determined. All data are presented as means ± SE (n = 3). Different superscript letters indicate statistical significance (p < 0.05).
![Figure 2. Chrysin reduces SREBP target gene expression and de novo synthesis of fatty acids and cholesterol.Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. (a) The cells were then switched to medium C in the presence of either vehicle or the indicated chrysin concentrations. After a 24 h incubation, total RNA was isolated from the cells. Quantitative real-time PCR was performed, and the relative mRNA levels were obtained by normalization to GAPDH. (b) The cells were then switched to medium C in the presence of vehicle, 50 μM chrysin, or 100 μM chrysin. After an 18 h incubation, the cells were treated with 1.6 μCi/mL [Citation14C]acetate and cultured for an additional 6 h. Fatty acids and cholesterol were extracted, and the rates of synthesis were determined. All data are presented as means ± SE (n = 3). Different superscript letters indicate statistical significance (p < 0.05).](/cms/asset/302f8f00-f392-4591-8ad9-a081fe15da26/tbbb_a_1608806_f0002_b.gif)
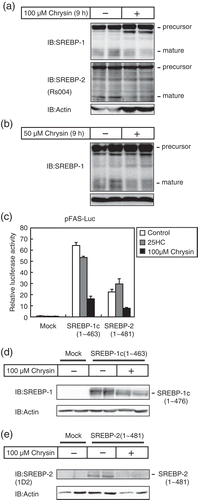
Chrysin reduces the protein level and activity of SREBPs mature forms
SREBPs are synthesized as inactive precursors. Once cellular cholesterol is low, the inactive precursors are proteolytically processed into mature forms that function as active transcription factors. To investigate the effects of chrysin on the protein level of SREBPs mature forms, Huh-7 cells were treated with chrysin for 9 h under low cholesterol conditions, and cell lysates were subjected to immunoblotting. In these experiments, the cells were pre-cultured under low cholesterol conditions for 16 h to increase the mature forms of SREBP protein levels. As shown in ), a 9 h treatment with 50 or 100 μM chrysin decreased the levels of SREBP-1 and SREBP-2 mature forms. Next, we investigated the effect of chrysin on the transcriptional activity and protein level of SREBPs mature forms. Enforced expression of SREBP-1c(1–463) and SREBP-2(1–481) mature forms stimulated the activity of the FAS gene promoter, which was not suppressed by treatment with sterols ()) as the enforced expression SREBPs mature forms are neither mediated by the precursor forms nor affected by the treatment with sterols, which suppresses SREBP processing. On the other hand, chrysin suppressed the activity of enforced expressing SREBPs mature forms ()) and lowered the protein levels of the enforced expressing mature SREBP-1c(1–463) and SREBP-2(1–481) () and (d)). These results imply that chrysin lowers the protein levels of SREBP mature forms, thereby suppressing their transcriptional activity.
Figure 3. Chrysin decreases the protein levels and activity of SREBPs mature forms.
Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. (a and b) The cells were then switched to medium C in the presence of vehicle, 50 μM chrysin (a), or 100 μM chrysin (b). After a 9 h incubation, whole-cell extracts underwent immunoblotting (IB) with anti-SREBP-1 (2A4), anti-SREBP-2 (Rs004) (b), or anti-β-actin antibodies. Similar results were obtained in three separate experiments. (c) Huh-7 cells were transfected with 400 ng of a reporter plasmid, pFAS-luc, together with 200 ng of pCMV-β-gal in the presence or absence of 400 ng of the indicated expression plasmids for mature forms of SREBP-1c or SREBP-2. Twenty-four hours after transfection, the cells were cultured with 1 μg/mL 25-hydroxycholesterol (25HC) or 100 μM chrysin for 24 h. Luciferase assays were performed as described under “materials and methods.” The promoter activities without 25HC and chrysin in the absence of SREBP-1c and −2 are represented as 1. All data are presented as means ± SE (n = 3). (d and e) Huh-7 cells were transfected with pME-18S (Mock), pME-SREBP-1c(1–463), or pME-SREBP-2(1–481) and cultured for 24 h in medium A. The cells were trypsinized, seeded in six well plates, and cultured further for 24 h under the same conditions, after which they were cultured for 24 h with either vehicle or 100 μM chrysin. Whole-cell extracts underwent immunoblotting (IB) with anti-β-actin and anti-SREBP-1 (2A4) (c) or anti-SREBP-2 (1D2) (d) antibodies. Similar results were obtained in three separate experiments.
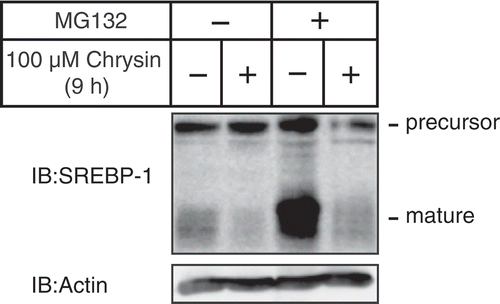
Next, we investigated whether the ubiquitin–proteasome system was involved in the chrysin-mediated decrease of SREBP-1 mature forms since SREBPs mature forms have reportedly been degraded by the ubiquitin–proteasome pathway [Citation15]. Consistent with previous reports, the amount of SREBP-1 mature forms increased in cells treated with MG132, a proteasome inhibitor (, lines 1 and 3). Importantly, a 9 h treatment with chrysin decreased the levels of SREBP-1 mature forms, regardless of the presence of MG132 (), which suggested that the ubiquitin–proteasome system does not contribute to the chrysin-mediated decrease of SREBP-1 mature forms.
Figure 4. Chrysin-mediated decrease of mature SREBP is not abolished by treatment with the proteasome inhibitor MG132.
Huh-7 cells were depleted of sterols through a 16 h incubation in medium C. After pre-treatment with 10 μM MG132 for 0.5 h, the cells were incubated for 9 h with medium C supplemented with 10 μM MG132 in the presence of either vehicle or 100 μM chrysin. Whole-cell extracts underwent immunoblotting (IB) with anti-SREBP-1 (2A4) or anti-β-actin antibodies.
Finally, We investigated the cytotoxic effect of chrysin on Huh-7 cells. LDH analysis revealed that treatment with chrysin for 9 h did not affect the release of LDH from Huh-7 cells (). These results indicate that chrysin exhibits low cytotoxicity to the cells.
Discussion
Our results demonstrate that the flavonoid chrysin suppresses SREBP activity through a decrease in SREBPs mature forms. It has been reported that SREBPs mature forms are rapidly degraded by the ubiquitin–proteasome system, and that these degradations are accelerated by inactivation of the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway [Citation16]. Chrysin reportedly suppresses the phosphorylation of Akt at Thr308 and Ser473 in human melanoma A375.S2 cells [Citation17], thus, it has been considered to stimulate the proteasome-mediated degradation of SREBPs mature forms through PI3K-Akt inactivation in Huh-7 cells. However, this assumption was refuted by the observation that chrysin reduces SREBPs mature forms, even in the presence of the proteasome inhibitor, MG132. Further studies are required to determine which degradation pathway is involved in the chrysin-mediated degradation of SREBPs mature forms.
It has been known that the proteolytic processing of SREBPs is regulated by the levels of intracellular cholesterol and several kinases, including AMP-activated protein kinase and PI3K-Akt signaling pathway. In addition, the extracellular signal-regulated kinase 1/2-mediated phosphorylation of SREBPs mature forms stimulates their transcriptional activity [Citation18–Citation20]. In the present study, we demonstrated that chrysin suppresses SREBPs activity, at least partially, by stimulating the mature forms of SREBPs degradation. As mentioned above, chrysin inhibits the PI3K-Akt signaling pathway, possibly suppressing the proteolytic processing of SREBPs. Further studies are required to determine whether chrysin suppresses the processing and activity of SREBPs mature forms by changing certain signaling pathways.
Does chrysin administration to animals suppress the activity of hepatic SREBPs? In adult rats, oral administration of chrysin (100 mg/kg/day) for 18 weeks has been shown to decrease the level of fructose-induced serum triacylglycerol [Citation21]. Although this improvement is thought to be mediated by chrysin’s antioxidant effects, its suppressive effect of SREBPs activity may also contribute to this amelioration. However, the oral bioavailability of chrysin was very low with estimated range of 0.003–0.02%[Citation22]. After a single oral administration (30 mg/kg), chrysin was undetectable in plasma. One of its major metabolites, chrysin glucuronide, was detected at a 0.8 μM concentration [Citation23]. Although in the present study, 100 μM chrysin reduced SREBPs mature forms, the concentration of chrysin in circulation is unlikely to reach that value and further studies are required to determine whether lower concentrations of chrysin and chrysin glucuronide exhibit a similar effect.
In summary, the present study demonstrated that 100 μM chrysin stimulates the degradation of SREBPs mature forms, thereby suppressing their activity. Our results suggest a new function for chrysin, improving lipid metabolism through suppression of SREBP activity.
Author contributions
JI and RS conceived the project. MI, KW, and JI designed the study. MI and KW performed the experiments. MI, KW, TS, YY, MS, and JI analyzed the data. JI wrote the manuscript. All authors read and approved the manuscript.
Acknowledgments
We would like to thank Enago (www.enago.jp) for the English language review.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200.
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361.
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048.
- Knebel B, Haas J, Hartwig S, et al. Liver-specific expression of transcriptionally active SREBP-1c is associated with fatty liver and increased visceral fat mass. PLoS One. 2012;7:e31812.
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032.
- Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215.
- Mani R, Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–196.
- Miyata S, Inoue J, Shimizu M, et al. 4‘-Hydroxyflavanone suppresses activation of sterol regulatory element-binding proteins and de novo lipid synthesis. FEBS Lett. 2012;586:1778–1782.
- Miyata S, Inoue J, Shimizu M, et al. Xanthohumol improves diet-induced obesity and fatty liver by Suppressing Sterol Regulatory Element-binding Protein (SREBP) Activation. J Biol Chem. 2015;290:20565–20579.
- Miyata S, Inoue J, Shimizu M, et al. Allyl isothiocyanate suppresses the proteolytic activation of sterol regulatory element-binding proteins and de novo fatty acid and cholesterol synthesis. Biosci Biotechnol Biochem. 2016;80:1006–1011.
- Inoue J, Miyata S, Shimizu M, et al. Isoxanthohumol stimulates ubiquitin-proteasome-dependent degradation of precursor forms of sterol regulatory element-binding proteins. Biosci Biotechnol Biochem. 2018;82:1591–1598.
- Sato R, Miyamoto W, Inoue J, et al. Sterol regulatory element-binding protein negatively regulates microsomal triglyceride transfer protein gene transcription. J Biol Chem. 1999;274:24714–24720.
- Sato R, Inoue J, Kawabe Y, et al. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J Biol Chem. 1996;271:26461–26464.
- Li J, Inoue J, Choi JM, et al. Identification of the flavonoid luteolin as a repressor of the transcription factor hepatocyte nuclear factor 4alpha. J Biol Chem. 2015;290:24021–24035.
- Hirano Y, Yoshida M, Shimizu M, et al. Direct demonstration of rapid degradation of nuclear sterol regulatory element-binding proteins by the ubiquitin-proteasome pathway. J Biol Chem. 2001;276:36431–36437.
- Sundqvist A, Bengoechea-Alonso MT, Ye X, et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 2005;1:379–391.
- Chen HY, Jiang YW, Kuo CL et al. Chrysin inhibit human melanoma A375.S2 cell migration and invasion via affecting MAPK signaling and NF-kappaB signaling pathway in vitro. Environ. Toxicol. 2018;34:434–442.
- Kotzka J, Muller-Wieland D, Roth G, et al. Sterol regulatory element binding proteins (SREBP)-1a and SREBP-2 are linked to the MAP-kinase cascade. J Lipid Res. 2000;41:99–108.
- Roth G, Kotzka J, Kremer L, et al. MAP kinases Erk1/2 phosphorylate sterol regulatory element-binding protein (SREBP)-1a at serine 117 in vitro. J Biol Chem. 2000;275:33302–33307.
- Kotzka J, Lehr S, Roth G, et al. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding Protein-2 at serine residues 432 and 455 in vivo. J Biol Chem. 2004;279:22404–22411.
- Andrade N, Andrade S, Silva C et al. Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats. Eur. J. Nutr. 2019. doi: 10.1007/s00394-019-01895-9. [Epub ahead of print].
- Walle T, Otake Y, Brubaker JA, et al. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51:143–146.
- Noh K, Oh Do G, Nepal MR, et al. Pharmacokinetic interaction of Chrysin with Caffeine in rats. Biomol Ther (Seoul). 2016;24:446–452.