ABSTRACT
A novel labdane type diterpenoid, 15-nor-8-labden-13-ol, named kujigamberol C, was isolated from Kuji amber using a modified isolation method to increase the yield of biologically active compounds. The structure was determined using HREIMS, 1D and 2D NMR. Kujigamberol C showed growth-restoring activity against mutant yeast via Ca2+-signal transduction.
Graphical abstract
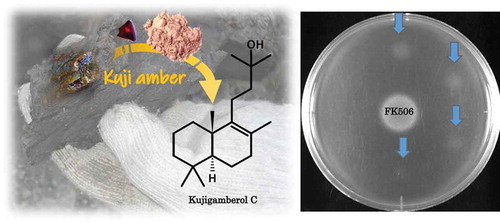
A novel Ca2+-signal transduction inhibitor, kujigamberol C, was isolated from Kuji amber. It showed the growth-restoring activity against the mutant yeast.
Kuji amber is a fossilized tree resin found around the Kuji region in Japan, dated at around 90–86 million years (Late Cretaceous) (Prof. Hisao Ando, Ibaraki University, personal communication). We focused on the biological activity of the MeOH extract of Kuji amber (MEKA) and the biologically active compounds in it for the first time [Citation1]. Three novel compounds, kujigamberol [15,20-dinor-5,7,9-labdatrien-18-ol (1)], kujiol A [13-methyl-8,11,13-podocarpatrien-19-ol (2)], and kujigamberol B [15,20-dinor-5,7,9-labdatrien-13-ol (3)] (Figure S1, Supporting information) were isolated from Kuji amber using the hypersensitive mutant Saccharomyces cerevisiae (zds1Δ erg3Δ pdr1Δ pdr3Δ: YNS17 strain) with respect to Ca2+-signal transduction [Citation1–Citation6]. In addition, amberene [15,19,20-trinor-5,7,9-labdatriene (or 1,6-dimethyl-5-isopentyltetralin)] and 1-methylamberene [15,20-dinor-5,7,9-labdatriene (or 1,1,6-trimethyl-5-isopentyltetralin)], which seem to be biomarkers for Cretaceous ambers, were also isolated from Kuji amber [Citation7,Citation8]. MEKA and 1 inhibited degranulation and leukotriene C4 (LTC4) production in rat basophilic leukemia-2H3 (RBL-2H3) cells. Nasal administration of MEKA and 1 inhibited nasal blockade more strongly compared to the clinical steroid mometasone, in an ovalbumin-induced rhinitis model in guinea pigs [Citation9,Citation10]. The potent anti-allergy activity of MEKA and the kujigamberol content in MEKA indicated the presence of additional potent compounds and/or compounds having different mechanisms [Citation10]. We tried to improve the isolation method for the isolation of additional biological compounds from MEKA. We explored the effect of EtOAc extraction but since this cannot extract more polar compounds, the quantity of 1, 2 and 3 were decreased. Thus, omitting the EtOAc extraction and after the silica gel column chromatography, medium pressure liquid chromatography (MPLC) (ODS) was used for the isolation of non-detected biologically active compounds. In this paper, we report the isolation by biological activity-guided fractionation, structure elucidation and biological activity of a new compound, named kujigamberol C.
Materials and methods
General
Kuji amber was excavated from mines located in the upper part of the Tamagawa Formation of Kuji Group in Kuji city, Iwate Prefecture, northeastern Japan by Kuji Kohaku Co. Ltd. The yeast strain used was YNS17 strain (MATa zds1::TRP1 erg3::HIS3 pdr1::hisG URA3 hisG pdr3::hisG), a derivative of strain W303-1A [Citation2–Citation4]. Difco® YPD broth and YPD agar were obtained from Becton Dickinson (Franklin Lakes, NJ, USA). FK506 was kindly provided by the Fujisawa Pharmaceutical Co., Ltd. (now Astellas Pharma Inc., Tokyo, Japan). The HPLC system (Shimadzu Corp., Kyoto, Japan) was equipped with a pump (LC-20AD) and a photodiode array (PDA) detector (SPD-M20A). Unless otherwise stated, chemicals used were of the best commercially available grade.
Screening of Ca2+-signal transduction inhibitors
Screening was carried out using the YNS17 strain and 5 μL of each sample was added on a plate as described previously [Citation4]. The inhibitory activity on Ca2+-signal transduction was determined by examining the yeast growth zone. An immunosuppressive drug, FK506 [tacrolimus; 2.5 ng/spot, calcineurin (protein phosphatase 2B) specific inhibitor] was used as a positive control.
Isolation
Kuji amber (Kuji Kohaku Co. Ltd., Iwate, Japan) was ground to a powder (480.73 g) and extracted with MeOH for three days at room temperature. Extracted samples of 10 mg/mL in MeOH were prepared for subsequent assays. The extract (20.76 g) was directly subjected to silica gel column chromatography [75–150 μm, 75 × 155 mm (300 g), Wako Pure Chemical Industries Ltd., Osaka, Japan] with hexane:EtOAc = from 20:1 to 1:3 as a solvent without the extraction by EtOAc [Citation1,Citation6]. Seventeen fractions were obtained and active fractions were collected and concentrated under reduced pressure to obtain the crude material. The active fractions [hexane:EtOAc = 3:1 (Frs. 7 and 8), 2:1 (Frs. 9 and 10) and 1:1 (Fr. 11), 4.63 g] were purified using MPLC [Ultra Pack ODS (37 mm i.d. × 300 mm), (Yamazen Co., Osaka, Japan)] by isocratic elution with 100% MeOH at a flow rate of 3 mL/min. Finally, purification using HPLC [Capcell Pak C18 column, 20 mm i.d. × 250 mm (Shiseido Co. Ltd., Tokyo, Japan)] was performed by isocratic elution with 80% MeOH for 1, or 80% MeOH followed by 70% MeOH for 2 and 3 at a flow rate of 5 mL/min. The pure compounds were yielded as 1 (59.3 mg), 2 (9.9 mg) and 3 (4.0 mg), respectively.
In the comparison of HPLC charts for the 17 fractions eluted by silica gel column chromatography with and without EtOAc extraction, we focused on increased peaks of fractions without EtOAc extraction. An increased peak was detected in the silica gel column fraction (hexane:EtOAc = 3:1 (Fr.7), 1.21 g) followed by MPLC [Ultra Pack ODS (37 mm i.d. × 300 mm)] using isocratic elution with 100% MeOH at a flow rate of 3 mL/min. It was finally purified by HPLC (Capcell Pak C18 column, 20 mm i.d. × 250 mm) using isocratic elution with 70% MeOH at a flow rate of 5 mL/min. Compound 4 was yielded at 21.4 mg as a colorless oil.
Structure elucidation
Structure elucidation of 4 was performed using HREIMS (JMS700, JEOL Ltd. Tokyo, Japan) and NMR (ECA-600, JEOL Ltd.), including 1H NMR, 13C NMR, DQF-COSY, HSQC, HMBC, and NOESY. UV spectrum was measured by UV mini 22 (Shimadzu Corp., Kyoto, Japan).
Kujigamberol C (4)
Colorless oil; [α]D27 −17.0 (c 0.1, MeOH); UV λmax nm (MeOH) End absorption (Figure S2, Supporting Information); 1H NMR and 13C NMR, see ; EIMS m/z 278 [M]+ (Figure S3, Supporting Information), HREIMS m/z 278.2613 [M]+ (calcd for C19H34O, 278.2610).
Table 1. NMR spectroscopic data for kujigamberol C (4).
Cell culture and cytotoxicity
RBL-2H3 cells (ATCC CRL-2256, Manassas, VA, USA) were maintained in DMEM supplemented with 10% heat-inactivated FBS (Biowest SAS, Nuaillé, France) and antibiotics [penicillin (50 units/mL)-streptomycin (50 μg/mL), Gibco, Thermo Fisher Scientific Inc., Waltham, MA, USA] [Citation11,Citation12]. Cell viability was determined using the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (Dojindo Lab., Kumamoto, Japan). RBL-2H3 cells were plated in triplicate at a concentration of 3 × 104 cells/well in a 96-well plate and incubated overnight prior to treatment with various concentrations of 4 for 48 h. Cytotoxicity was measured as described previously [Citation9,Citation10].
Measurement of the degranulation (β-HEX assay)
RBL-2H3 cells were grown overnight in 96-well plates (3 × 104 cells/well) and the degranulation activity of 4 against them stimulated with antigen [IgE + DNP-BSA (Ag)], thapsigargin (Tg), and A23187 was determined as described previously [Citation9,Citation10].
Results and discussion
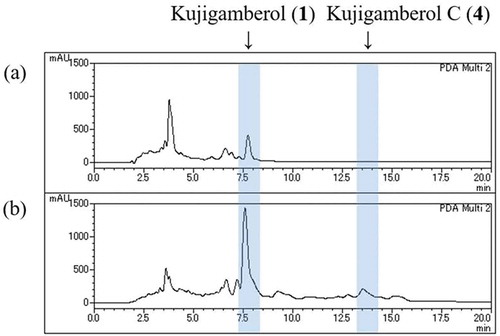
The quantity of the previously isolated new compounds such as kujigamberol, kujiol A, and kujigamberol B was calculated to be 8.3, 9.9 and 7.5 times higher than the previous isolation method by the peak area of HPLC charts, respectively. The peak of 4 could not be confirmed on the HPLC chart of any silica gel column chromatography fractions by the previous isolation method ().
Figure 1. HPLC profiles of silica gel column chromatography Fr. 7 with (a) or without (b) EtOAc extraction. Column: Capcell Pak C18 (4.6 × 150 mm, 5 µm, UG120A), Solvent: 85% MeOH, Flow rate: 1 mL/min, Detector: PDA (205 nm), Injection: 50 µg.
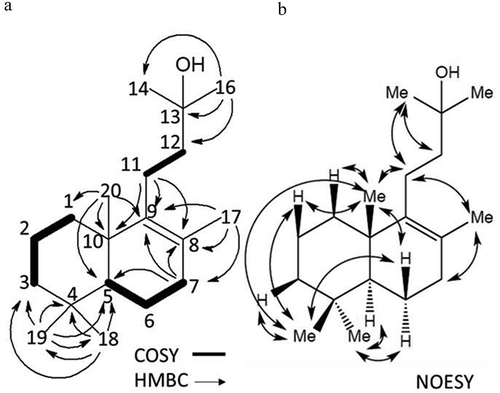
The molecular formula of 4 was determined to be C19H34O by HREIMS, with m/z 278.2613 as the molecular ion (calcd; 278.2610). The UV spectrum of 4 (end absorption) (Figure S2, Supporting Information) suggested absence of an aromatic ring. In the 13C NMR spectrum (Figure S4, Supporting Information), two non-protonated olefinic sp2 carbons were observed at 125.7 and 140.0 ppm. Olefinic proton signals were not observed in the 1H NMR spectrum of 4 (Figure S5, Supporting Information), which indicated that carbons of the double bond were tetra substituted. Six methyl groups were observed in the 1H NMR spectrum of 4 and they were 0.83, 0.88, 0.95, 1.24 (6H) and 1.57 ppm, respectively. The latter methyl (1.57 ppm) could be assigned to an olefinic methyl group from its chemical shift value (Figure S5, Supporting Information). From the DQF-COSY (Figure S6, Supporting Information) and the HSQC (Figure S7, Supporting information) spectra, other partial structures were elucidated; one is trimethylene consisting of three methylene signals of 37.1 (C-1), 19.1 (C-2), 41.8 (C-3) ppm, the second is dimethylene having two methylene signals of 22.5 (C-11) and 44.1 (C-12) ppm, and the third is a propane-1,1,3-triyl group consisting of two methylene signals 19.1 (C-6), 33.7 (C-7) ppm, and a methine signal of 51.9 ppm (C-5) (). One of the non-protonated sp3 carbons at 71.3 ppm indicated that oxygen is attached to this as a tertiary hydroxyl group (Figures S4 and S7, Supporting Information). Connectivity of each partial structure was established by analysis of long-range correlations in the HMBC spectrum () and Figure S8, Supporting Information). Long-range correlations from a singlet methyl signal at 1.24 ppm (H-14 or H-16) to the oxygenated tertiary carbon (C-13) at 71.0 ppm and to the methylene carbon (C-12) at 44.1 ppm, suggested that both were attached at C-13. Another methylene proton at 2.11 ppm of the dimethylene unit showed correlations to the olefinic carbons of C-8 and C-9 and a quaternary carbon of C-10. The other dimethyl groups at 0.83 and 0.88 ppm showed correlations to a methine carbon (C-5) at 51.9 ppm, and to an sp3 quaternary carbon (C-4) at 33.3 ppm, and also to one terminal methylene carbon (C-3) of the trimethylene group; this suggested that the singlet dimethyl groups were attached at C-4. The other singlet methyl protons at 0.95 ppm (H-20) showed correlations to the olefinic C-9, to one terminal methylene carbon (C-1) of the trimethylene group, and to a methine carbon (C-5) together with C-10. A correlation of the methylene protons H-7 (1.93 ppm) to the non-protonated olefinic carbons C-8 and C-9, respectively, was also observed. Correlations of the olefinic methyl at 1.57 ppm to C-7, C-8, and C-9 were also observed. Sequential assignments of HMBC correlations () and Figure S8, Supporting Information) and NOESY correlations () and Figure S9, Supporting Information) revealed the entire planar structure and the relative configuration of 4. Based on this collective evidence, the structure of 4 was determined to be 15-nor-8-labden-13-ol, and this new compound was named as kujigamberol C ( and S1, Supporting information). The 1H and 13C NMR spectral data of 4 are summarized in . As an HPLC peak of 4 was observed in MEKA, 4 is not an artefact compound.
Figure 3. Growth restoring activity of kujigamberol C (4) in the YNS17 strain. Growth restoring activity of kujigamberol C (4) against the YNS17 strain (zds1Δ erg3Δ pdr1Δ pdr3Δ) in the presence of 0.3 M CaCl2. 1: 0.5 μg/spot, 2: 0.25 μg/spot, 3: 0.13 μg/spot, 4. 0.063 μg/spot, 5: 0.031 μg/spot, 6: 0.016 μg/spot, 7: 2.5 ng/spot (FK506). Arrows indicate the growth restoring activity.
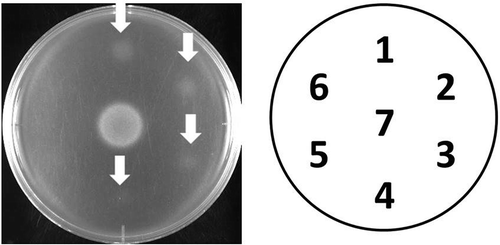
Compound 4 showed clear growth-restored zones of the YNS17 strain on the plate, in a dose-dependent manner (from 0.5 to 0.063 μg/spot), which is one-fourth lower activity than that of kujigamberol [Citation1] (). The cytotoxicity of 4 against RBL-2H3 cells was IC50 = 76.7 µM (MTT assay), but no inhibition was observed against degranulation of RBL-2H3 cells up to 50 μM stimulated by Ag, Tg, and A23187 (Figure S10, Supporting Information). The activity against the YNS17 strain parallels that observed in RBL-2H3 cells (degranulation), because kujigamberol [Citation10], kujiol A and kujigamberol B (data not shown), which have the same aromatic structure of the B ring, have potent biological activities against both YNS17 strain and RBL-2H3 cells, and more than those of kujigamberol C. Thus, the aromatic structure of B-ring may be crucial for these biological activities. Kujigamberol C could thus be suggested as an intermediate in the process of the diagenetic pathway involving the previously isolated biologically active compounds from Kuji amber [Citation1,Citation6,Citation11]. It has been reported that a compound having a B-ring with the same carbon skeleton as kujigamberol C was aromatized by heating with selenium at 320°C [Citation12]. Kujigamberol B that is formed by aromatization of the B-ring may be generated by diagenesis from kujigamberol C in nature.
Author contributions
K.K. designed the research and supervised manuscript preparation. H.T., H.K., M.M., and H.S. performed the experiments. All authors reviewed the manuscript.
Kujigamberol_C_Revised_SI_for_BBB__20190419.docx
Download MS Word (1.5 MB)Acknowledgments
We are grateful to Ms. Shizuko Nakajo from the Center for Regional Collaboration in Research and Education of Iwate University for HREIMS; to Prof. Hisao Ando, Ibaraki University for personal communications about Kuji amber; and to Emeritus Professor Tokichi Miyakawa of Hiroshima University for providing the YNS17 strain. We would like to thank Editage (www.editage.jp) and Emeritus Professor Don R Phillips, La Trobe University for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kimura K, Minamikawa Y, Ogasawara Y, et al. Kujigamberol, a new dinorlabdane diterpenoid isolated from 85 million years old Kuji amber using a biotechnological assay. Fitoterapia. 2012;83:907–912.
- Shitamukai A, Mizunuma M, Hirata D, et al. A positive screening for drugs that specifically inhibit the Ca2+-signaling activity on the basis of the growth promoting effect on a yeast mutant with a peculiar phenotype. Biosci Biotechnol Biochem. 2000;64:1942–1946.
- Chanklan R, Aihara E, Koga S, et al. Inhibition of Ca2+-signal-dependent growth regulation by radicicol in budding yeast. Biosci Biotechnol Biochem. 2008;72:132–138.
- Ogasawara Y, Yoshida J, Shiono Y, et al. New eremophilane sesquiterpenoid compounds, eremoxylarins A and B directly inhibit calcineurin in a manner independent of immunophilin. J Antibiot. 2008;61:496–502.
- Ye YQ, Koshino H, Hashizume D, et al. Synthesis and biological activity of both enantiomers of kujigamberol isolated from 85-million-years-old Kuji amber. Bioorg Med Chem Lett. 2012;22:4259–4262.
- Uchida T, Koshino H, Takahashi S, et al. Ca2+-signal transduction inhibitors, kujiol A and kujigamberol B, isolated from Kuji amber using a mutant yeast. J Nat Prod. 2018;81:1070–1074.
- Menor-Salván C, Simoneit BRT, Ruiz-Bermejo M, et al. The molecular composition of Cretaceous ambers: identification and chemosystematic relevance of 1,6-dimethyl-5-alkyltetralins and related bisnorlabdane biomarkers. Org Geochem. 2016;93:7–21.
- Kawamura T, Koshino H, Nakamura T, et al. Amberene and 1-methylamberene, isolated and identified from Kuji amber (Japan). Org Geochem. 2018;120:12–18.
- Abe T, Kobayashi M, Okawa Y, et al. Yeast Ca2+-signal transduction inhibitors isolated from Dominican amber prevent the degranulation of RBL-2H3 cells through the inhibition of Ca2+-influx. Fitoterapia. 2016;113:188–194.
- Maruyama M, Kobayashi M, Uchida T, et al. Anti-allergy activities of Kuji amber extract and kujigamberol. Fitoterapia. 2018;127:263–270.
- Menor-Salván C, Najarro M, Velasco F, et al. Terpenoids in extracts of lower Cretaceous ambers from the Basque-Cantabrian Basin (El Soplao, Cantabria, Spain): paleochemotaxonomic aspects. Org Geochem. 2010;41:1089–1103.
- Carman RM, Craig WJ, Diterpenoid XXX. The selenium dehydrogenation of agathic acid. Aust J Chem. 1971;24:2379–2388.