ABSTRACT
Dementia and cognitive decline have become public health issues worldwide and life-style-related diseases and obesity have recently been reported as key risk factors. We have recently demonstrated that short-term administration of iso-α-acids (IAAs), hop-derived bitter components of beer, improves spatial and object recognition memory. However, the short-term effects of IAAs on obesity-induced cognitive impairment remain to be investigated. Furthermore, although matured hop bitter acids (MHBAs) are also derived from hops, their effect on obesity-induced cognitive decline remains unknown. In the present study, the short-term administration of IAAs improved memory deficits in high-fat diet (HFD)-fed mice, as assessed in the novel object recognition test (NORT). Dietary MHBAs supplementation administered to HFD-fed mice prevented obesity and improved memory deficits in the NORT. Moreover, the short-term administration of MHBAs improved episodic and spatial reference memory in obese mice. These hop-derived bitter acids may contribute toward improving obesity-induced cognitive impairments.
Abbreviations: IAAs: iso-α-acids; MHBAs: matured hop bitter acids; HFD: high fat diet; NORT: novel object recognition test; NOLT: novel object location test
Graphical abstract
Hop-derived bitter acids (IAAs and MHBAs) improve obesity-induced cognitive impairment by both long-term and short-term treatment.
The rapid aging of the population together with lifestyle changes led to the occurrence of various health problems in our societies, including dementia, cognitive decline, and lifestyle-related diseases. Increasing evidence has shown a strong association between lifestyle-related diseases, such as obesity and type II diabetes, and the risk of dementia [Citation1–Citation5]. Epidemiological studies have reported that body mass index (BMI) is negatively correlated with cognitive function both in healthy adults and in Alzheimer’s disease patients [Citation6]. Preclinical studies have also shown that high fat diet (HFD) feeding induces cognitive decline assessed in various behavioral tests [Citation7,Citation8]. Both obesity and lifestyle-related diseases induce neuroinflammation in various brain regions, including the hypothalamus and hippocampus. This, in turn, may lead to the onset of cognitive decline or dementia [Citation9–Citation11]. Since dementia is difficult to treat post-onset, preventing obesity and lifestyle-related diseases through everyday lifestyle, including dietary habits or exercise, might be beneficial for its prevention. Although some food-derived components independently prevent cognitive decline or obesity, few studies have reported the simultaneous effects of food-derived components in preventing obesity- or lifestyle-related cognitive decline.
Hops (Humulus lupulus L.) have been used as beer’s main ingredient to add bitterness and flavor. Hops contain both α- and β-acids, and during the brewery process α-acids are converted into iso-α-acids (IAAs) – beer’s main bitter components. Various physiological functions of IAAs have been investigated. We have previously reported that IAAs prevent lipid accumulation and insulin resistance in diet-induced obese rodents, and improve glucose metabolism and body fat accumulation in humans [Citation12–Citation15]. In terms of cognitive function, dietary supplementation with IAAs was shown to prevent cognitive impairment in mouse models of Alzheimer’s disease [Citation16] and obesity [Citation17]. More recently, both single and short-term IAAs administration was reported to improve spatial working memory and object recognition memory via vagus nerve activation [Citation18]. This finding suggests that IAAs may not only prevent the occurrence of obesity-induced cognitive impairments, but also improve it after its onset. However, the short-term therapeutic effects of IAAs on obesity-induced cognitive impairment remain to be investigated.
Both α- and β-acids are converted into oxidized components called matured hop bitter acids (MHBAs), which contain a β-tricarbonyl formula and a chemical structure similar to IAAs [Citation19]. We have previously demonstrated that dietary supplementation with MHBAs reduces body weight gain and epididymal fat accumulation in rodents fed a high-fat diet, and that oral MHBAs administration promotes thermogenesis in brown adipose tissues (BAT) by elevating sympathetic nerve activity [Citation20]. BAT stimulation is blocked by vagotomy, suggesting that the effect of MHBAs is mediated by vagus nerve activation. Furthermore, continuous ingestion of MHBAs reduced body fat in a clinical trial [Citation21]. These observations suggest that the long-term intake of MHBAs prevents obesity-induced cognitive impairment via obesity suppression. In addition, our latest research indicates that a single administration of MHBAs improves spatial working memory in a scopolamine-induced model of amnesia and episodic memory function in normal mice via vagus nerve stimulation [Citation22]. However, the effects of short- and long-term MHBAs administration on obesity-induced cognitive impairment have not been investigated.
In the present study, we found that short-term IAAs administration improves episodic memory impairment in obese mice, assessed by the novel object recognition test (NORT). Moreover, long-term MHBAs administration prevents HFD-induced obesity and episodic memory impairment, whereas its short-term administration improves episodic and spatial reference memory, as assessed in the NORT and the novel object location test (NOLT), respectively.
Materials and methods
Materials
MHBAs were prepared from hop pellets as previously described [Citation19,Citation23]. Isomerized hop extract (IHE) was purchased from Hopsteiner (Mainburg, Germany). IHE comprises an aqueous solution of IAAs as a potassium salt and thus was used as a source of IAAs. The content of IHE was previously analyzed and described [Citation16]. Briefly, IHE contains 30.5% (w/v) IAAs, including trans-isocohumulone (1.74% w/v), cis-isocohumulone (7.61% w/v), trans-isohumulone (3.05% w/v), cis-isohumulone (14.0% w/v), trans-isoadhumulone (0.737% w/v), and cis-isoadhumulone (3.37% w/v).
Animals
For the long-term dietary MHBAs supplementation experiment, male, 5-week-old C57BL/6J mice were purchased from Charles River Japan Inc. (Tokyo, Japan) and maintained at room temperature (23 ± 1°C) under constant 12-h light/dark cycles (light period from 8:00 am to 8:00 pm). All mice were acclimatized for 1 week, during which they were fed a standard rodent diet, CE-2 (Clea Japan, Tokyo, Japan). After acclimatization, the mice were divided into three experimental groups: normal diet-fed group (ND group), HFD-fed group (HFD group), and HFD plus 0.05% (w/w) MHBAs group (HFD + MHBA group). The prvious study has shown that MHBA supplementation do not affect food intake [Citation20]. For the ND group, a 10 kcal% fat containing diet (D12450J, Research Diets) was used. The formulation and composition of both HFD and ND are presented in . Weekly body weight measurements were performed.
Table 1. High-fat diet and normal diet formulations.
For the short-term intragastric administration experiment, male, 11-week old C57BL/6J mice that had been fed either ND or HFD for 7 weeks were purchased from Charles River Japan Inc and maintained at room temperature (23 ± 1°C) under constant 12-h light/dark cycles (light period from 8:00 am to 8:00 pm). All mice were acclimatized for 1 week prior to the behavioral pharmacological tests. All animal care and experimental procedures were in accordance with the guidelines of the Animal Experiment Committee of the Kirin Company Ltd., and all efforts were made to minimize suffering. All studies were conducted in 2017, after approval by the Animal Experiment Committee of the Kirin Company Ltd (approval IDs: AN10379-Z01 and AN10415-Z01).
Analysis of metabolic and cognitive parameters
To evaluate the effects of MHBAs on the metabolic state and cognitive function of HFD-fed mice, euthanasia was performed at the eighth week of feeding (13 weeks of age) by isoflurane. Blood samples were collected from the heart into heparin-coated tubes and centrifuged at 3,000 × g for 5 min, and supernatants were collected as plasma samples. The glucose and insulin levels were analyzed using the Glucose Assay Kit II (BioVision, Mountain View, USA) and the Lebis Insulin Elisa kit (Shibayagi, Gunma, Japan), respectively. The left hippocampus was collected and homogenized using a multi-beads shocker (Yasui Kikai, Osaka, Japan) in TBS buffer containing a protease inhibitor cocktail (BioVision). The homogenates were centrifuged (50,000 × g, 30 min) and the supernatants were used to determine total protein content using the BCA protein assay kit (Thermo Scientific, Rockford, USA). Inflammatory cytokines and chemokines were evaluated using the Bio-Plex assay system (Bio-Rad, Richmond, USA).
NORT
The NORT is a behavioral test that based on the rodent curiosity for novel object rather than familiar object, assessing object reference memory or episodic memory. The NORT was performed as previously described [Citation24]. The experimental apparatus was a square open field (40 cm × 40 cm × 40 cm) made of gray polyvinyl chloride. The box was placed in a sound-isolated experimental room. Two pairs of wooden blocks were used as objects: one pair of triangle prisms and one pair of square pyramids. Each object was placed in a corner, on the same side as its matching shape. The test comprised two periods: an acquisition and a recall. Prior to the test, the mice were acclimated to the experimental room for at least 16 h. Each mouse was then placed into the experimental apparatus in the presence of two objects and allowed to explore freely for 10 min. After 24 h, the recall period was performed, by replacing one object of each pair with a novel object (wooden white sphere). Each mouse was placed once more into the apparatus for 5 min, and the time spent exploring the familiar and the novel object was measured. In the short-term experiment, either IAAs (1 mg/kg), MHBAs (10 mg/kg), or distilled water were intragastrically administered 60 min prior to both the acquisition and the recall periods. The discrimination index was calculated using the following formula: (novel object exploration time – familiar object exploration time)/(total exploration time).
NOLT
The NOLT is a behavioral test based on the rodent curiosity for novel location rather than familiar location, which assesses spatial reference memory. The NOLT was performed by placing visual cues (black, white, or stripe-pattern picture) on the wall of the square open field, thus allowing the mice to distinguish each direction. Brown glass vials were used as objects. This test comprised an acquisition and a recall period. The mice were acclimated to the experimental room for at least 16 h, after which habituation to the test apparatus was performed. During habituation, each mouse was placed in the experimental apparatus for 10 min without any objects and then returned to its home cage. After 24 h the acquisition was performed by placing each mouse in the apparatus for 5 min with two objects placed at the corners of the same side. After 4 h, the recall period was performed by reintroducing each mouse in the apparatus for 8 min, with two objects placed at the two diagonal corners. In the short-term experiment, either IAAs (1 − 10 mg/kg), MHBAs (10 mg/kg), or distilled water were intragastrically administered 60 min before the acquisition and recall periods. Similar to the NORT, the time spent exploring the familiar and novel locations was measured, and the discrimination index was calculated.
Statistical analysis
All values are expressed as means ± SEM. Body weight changes were analyzed by two-way ANOVA, comparing the effects of diet and time. Two-group comparisons were analyzed using a Student’s t test. All other experimental data were analyzed with one-way ANOVA, followed by Tukey-Kramer’s test. P < 0.05 was considered statistically significant.
Results
Short-term IAAs administration improves obesity-induced cognitive decline
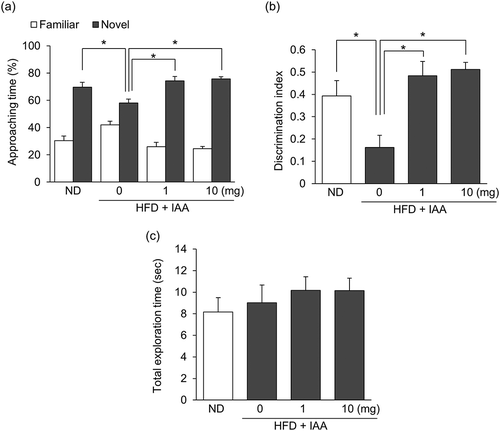
The effect of short-term IAAs administration on obesity-induced cognitive impairment was evaluated using NORT. Mice were fed with either ND or HFD for 8 weeks, after which the body weight of HFD-fed mice was significantly increased compared with that of ND-fed mice (data not shown). Consistent with previous reports, both the time spent exploring a novel object and the discrimination index were significantly decreased in HFD-fed control mice compared with those of ND-fed control mice. The oral administration of IAAs (1–10 mg/kg body weight) significantly increased these scores compared with HFD-fed control mice (, b)). The total exploration time was not affected by HFD feeding or IAAs treatment ()). These results indicate that the short-term administration of IAAs attenuates obesity-induced episodic memory impairment.
Figure 1. Short-term IAAs administration improves object recognition memory in HFD-fed obese mice. (a) The time spent exploring a novel or familiar object over 5 min in the NORT was measured. (b) The discrimination index (DI; (time spent investigating novel object minus time spent investigating familiar object)/(total exploration time)) was calculated. (c) The total time spent exploring both objects was calculated. All values are expressed as means ± SEM (n = 8–10 mice per group). *P < 0.05 versus each group.
Long-term dietary intake of MHBAs improves obesity-induced cognitive impairment
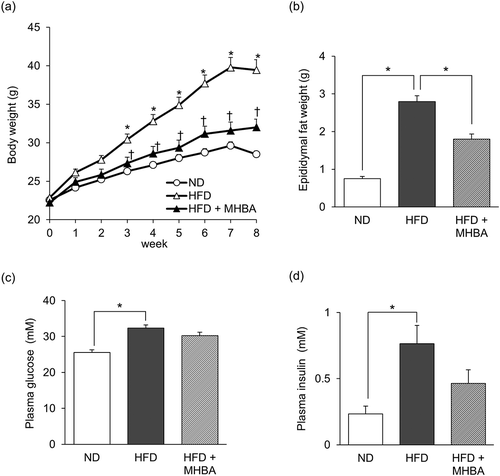
To investigate the preventive effects of MHBAs on obesity-induced cognitive impairment, mice were fed ND, HFD, or HFD containing 0.05% w/w MHBAs (HFD + MHBA) for 8 weeks. After 3 weeks of feeding, the body weight of HFD-fed mice was significantly increased compared with that of ND-fed mice, whereas the body weight of the HFD + MHBA group was significantly decreased compared with that of the HFD group ()). At the eighth week of feeding, the mice were sacrificed and obesity-related parameters were measured. Visceral fat weight was significantly increased in the HFD group compared with that of the ND group, and this increase was significantly suppressed in the HFD + MHBA group ()). The plasma glucose and insulin levels were significantly increased in HFD-fed compared to ND-fed mice, whereas no significant difference was observed between the HFD- and the HFD + MHBA-fed groups (, d)). Consistent with our previous report, MHBAs exhibited anti-obesity effect.
Figure 2. Long-term dietary MHBAs intake prevents HFD-induced excessive body weight and adipose tissue gain. Male C57BL/6J mice were either fed a normal diet, a high-fat diet (HFD), or HFD with 0.05% (w/w) MHBAs supplementation for 8 weeks. (a) Body weight was measured every week. (b-d) Epididymal fat weight (b), plasma glucose level (c), and plasma insulin level (d) at the eighth week of feeding, when mice were sacrificed. All values are expressed as means ± SEM (n = 10 mice per group). (a) *P < 0.05 versus ND-fed group, †P < 0.05 versus HFD-fed group. (b-d) *P < 0.05 versus each group.
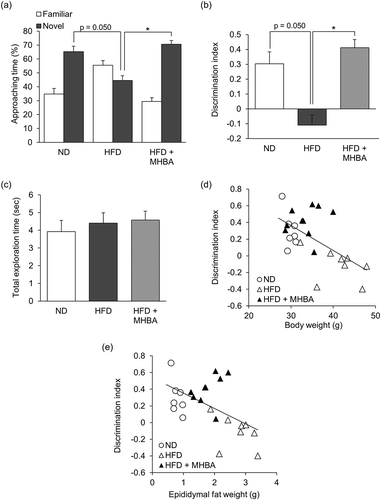
Next, we performed the NORT to investigate the effect of long-term MHBAs administration on the episodic memory impairment of HFD-fed obese mice. Both the time spent exploring a novel object and the discrimination index were decreased in HFD-fed mice compared with those of ND-fed mice (p = 0.050). This decrease was significantly improved by MHBAs treatment (, b)). The total exploration time was not affected by either HFD feeding or MHBAs treatment ()). The correlation among body weight, epididymal fat weight, and memory performance was also analyzed, revealing a negative correlation between the discrimination index in the NORT and both the body weight (r = 0.601, p < 0.01) and the epididymal fat weight (r = 0.546, p < 0.01). These results indicate that dietary MHBAs intake prevents obesity-induced episodic memory impairment via suppressing excessive body weight gain and lipid accumulation.
Figure 3. Long-term dietary intake of MHBAs improves obesity-induced object recognition memory impairment. (a) Time spent exploring a novel or a familiar object over 5 min in the NORT was measured. (b) The discrimination index (DI; (time spent investigating novel object – time spent investigating familiar object)/(total exploration time)) was calculated. (c) The total time spent exploring both objects was calculated. All values are expressed as means ± SEM (n = 8–10 mice per group). *P < 0.05 versus each group. (d,e) The discrimination index was negatively correlated with body weight (r = −0.601, p = 0.0002) and epididymal fat weight (r = −0.546, p = 0.0021).
Short-term MHBAs administration improves obesity-induced cognitive impairment
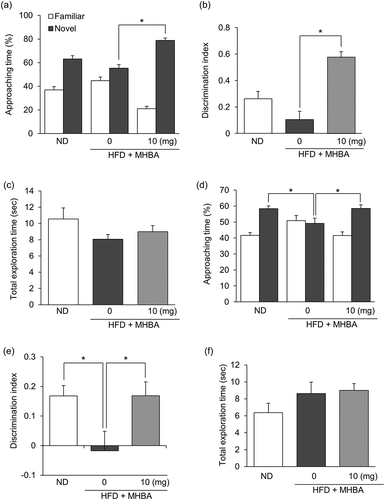
Mice were fed with either ND or HFD for 8 weeks, after which HFD-fed mice had a significantly increased body weight, compared with ND-fed mice (data not shown). Oral MHBAs administration (1 mg/kg) significantly increased the time spent exploring a novel object and the discrimination index in the NORT compared with HFD feeding (, b)). The total exploration time was not affected by either HFD feeding or MHBAs treatment ()). These results indicate that short-term MHBAs administration attenuates episodic memory in obese mice. We also performed NOLT, which is designed to test spatial reference memory function. Both the time spent exploring a novel location and the discrimination index were significantly decreased in HFD-fed mice compared with those of ND-fed mice. This decrease was significantly improved by short-term MHBAs compared with HFD feeding (, e)). No difference was found between groups regarding the total exploration time ()). These results indicate that short-term MHBAs administration attenuates obesity-induced spatial reference memory impairment.
Figure 4. Short-term administration of MHBAs improves object recognition and spatial reference memory in HFD-fed mice. (a) The time spent exploring a novel or a familiar object over 5 min in the NORT was measured. (b) The discrimination index (DI; (time spent investigating novel object minus time spent investigating familiar object)/(total exploration time)) in the NORT was calculated. (c) The total time spent exploring both objects was calculated. (d) The time spent exploring a novel or a familiar location over 8 min in the novel location recognition test was measured. (e) The discrimination index in the novel location recognition test was calculated. (f) The total time spent exploring both locations was calculated. Either MHBAs (10 mg/kg) or distilled water were orally administered 60 min prior to both the acquisition and recall periods in each test. All values are expressed as means ± SEM (n = 8–10 mice per group). *P < 0.05 versus each group.
Discussion
Our recent study showed that short-term IAAs administration enhances hippocampus-dependent spatial and object memory, both in normal and scopolamine-induced amnesic mice. In the gastrointestinal tract, IAAs, agonist bitter taste receptors, activate the vagus nerve and increase the hippocampal dopamine level, leading to memory improvement. The present study demonstrates that short-term IAAs administration improves object recognition memory in obese mice, showing obesity-induced memory impairment without changing the body weight. HFD administration decreases dopamine turnover and long-term potentiation in the ventral hippocampus. Although the distinct involvement of hippocampal dopamine in this study is unclear, short-term administration of IAAs might reverse HFD-induced dopamine signaling deficits. Our previous report showed that long-term IAAs intake improves the memory impairment associated with HFD-induced obesity mice. Taken together, the results show that IAAs intake may be beneficial both to prevent and treat obesity-induced cognitive impairment.
While IAAs are converted from α-acids during the brewery process, MHBAs are generated from both α- and β-acids through oxidation. Due to the structural similarity between these compounds, MHBAs is expected to exert preventive effects both on obesity and obesity-associated cognitive impairment. In fact, the present results show that dietary MHBAs supplementation prevents HFD-induced excessive body weight and fat gain. These results are consistent with our previous study, which reports that MHBAs reduce body weight gain and epididymal fat accumulation through vagus nerve activation [Citation20]. In addition, we demonstrated that MHBAs improve obesity-induced episodic memory deficits in the NORT. Since the discrimination index in the NORT was negatively correlated with body and epididymal fat weight, the anti-obesity effect of MHBAs may contribute to the improvement of obesity-induced cognitive impairments. Recent studies show that obesity induces neuroinflammation in several brain regions, thereby leading to cognitive dysfunctions [Citation9–Citation11]. Chunchai et al revealed that electrical stimulation of the vagus nerve prevents HFD-induced lipid accumulation and hippocampal inflammation, leading to cognitive improvement [Citation25]. Taken together, these results indicate that MHBAs might improve obesity-induced cognitive impairments through vagus nerve activation, obesity prevention, and neuroinflammation suppression.
In addition to its long-term effect, short-term MHBAs administration improved object recognition and spatial reference memory in HFD-fed obese mice. Our previous findings revealed that both single and short-term MHBAs administration improve spatial working and episodic memory via vagus nerve activation, both in normal and in scopolamine-induced amnesic mice [Citation22]. Although in the present study the involvement of the vagus nerve was not investigated, the cognitive improving effect exerted by MHBAs in obese mice may also be mediated by vagus nerve activation. Further studies using obese and vagotomized mice are needed to clarify this matter.
Both IAAs and MHBAs are bitter components of beer derived from hops. While the effects of IAAs and MHBAs on human cognitive functions have not been investigated, clinical trials have been performed on the anti-obesity effects of these bitter acids. The intake of 32–48 mg/day of IAAs for 12 weeks decreased BMI, fasting blood glucose, and HbA1c [Citation13]. Similarly, the intake of 35 mg/day of MHBAs for 12 weeks reduced visceral and total fat areas in a clinical trial [Citation21]. The effective dose for short-term MHBAs administration (10 mg/kg) is equivalent to 48 mg/day in humans (60 kg body weight), calculated using the human equivalent dose modulus (0.08) [Citation26]. Taken together, these results show that the intake of 35 mg/day or 48 mg/day MHBAs for several weeks or a few days, respectively, may improve obesity-induced cognitive decline in humans. While popular types of beer contain 20–40 mg/L of MHBAs, some bitter beers (e.g. India Pale Ale) or those made of aged hops (e.g., Lambic) contain 150–210 mg/L and 100–150 mg/L of MHBAs, respectively [Citation23]. Thus, 0.24–0.48 L of these high-MHBA containing beers might constitute an effective dose. Since both IAAs and MHBAs have been consumed for a long time, the daily intake of these hop-derived bitter acids may constitute a safe and effective approach to prevent obesity-induced cognitive decline, which may lead to dementia.
Author Contribution
T.A. and Y.A. designed the most of experiments. T.A. and R.O. performed the experiments and analyzed the data. T.A. wrote the most of paper and Y.A. conceived and supervised the paper.
Acknowledgments
This research was supported by Kirin Company, Limited.
Disclosure statement
All authors are employed byKirin Company, Limited. The authors declare no other competinginterests with this manuscript.
Additional information
Funding
References
- Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst). 2017;8:165–178.
- Ohara T, Doi Y, Ninomiya T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–1134.
- Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67:843–847.
- Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064.
- Xu WL, Atti AR, Gatz M, et al. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574.
- Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond). 2006;30:201–207.
- Jeon BT, Jeong EA, Shin HJ, et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–1454.
- Wang D, Yan J, Chen J, et al. Naringin improves neuronal insulin signaling, brain mitochondrial function, and cognitive function in high-fat diet-induced obese mice. Cell Mol Neurobiol. 2015;35:1061–1071.
- Guillemot-Legris O, Muccioli GG. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017;40:237–253.
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4.
- Solas M, Milagro FI, Ramirez MJ, et al. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87–92.
- Miura Y, Hosono M, Oyamada C, et al. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARα activations in C57BL/6 mice. Br J Nutr. 2007;93:559–567.
- Obara K, Mizutani M, Hitomi Y, et al. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin Nutr. 2009;28:278–284.
- Yajima H, Ikeshima E, Shiraki M, et al. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor alpha and gamma and reduce insulin resistance. J Biol Chem. 2004;279:33456–33462.
- Yajima H, Noguchi T, Ikeshima E, et al. Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int J Obes (Lond). 2005;29:991–997.
- Ano Y, Dohata A, Taniguchi Y, et al. Iso-alpha-acids, bitter components of beer, prevent inflammation and cognitive decline induced in a mouse model of Alzheimer’s disease. J Biol Chem. 2017;292:3720–3728.
- Ayabe T, Ohya R, Kondo K, et al. Iso-alpha-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci Rep. 2018;8:4760.
- Ano Y, Hoshi A, Ayabe T, et al. Iso-alpha-acids, the bitter components of beer, improve hippocampus-dependent memory through vagus nerve activation. Faseb J. 2019 Apr;33:4987–4995.
- Taniguchi Y, Matsukura Y, Ozaki H, et al. Identification and quantification of the oxidation products derived from alpha-acids and beta-acids during storage of hops (Humulus lupulus L.). J Agric Food Chem. 2013;61:3121–3130.
- Morimoto-Kobayashi Y, Ohara K, Takahashi C, et al. Matured hop bittering components induce thermogenesis in brown adipose tissue via sympathetic nerve activity. PLoS One. 2015;10:e0131042.
- Morimoto-Kobayashi Y, Ohara K, Ashigai H, et al. Matured hop extract reduces body fat in healthy overweight humans: a randomized, double-blind, placebo-controlled parallel group study. Nutr J. 2016;15:25.
- Ayabe T, Ohya R, Taniguchi Y, et al. Matured hop-derived bitter components in beer improve hippocampus-dependent memory through activation of the vagus nerve. Sci Rep. 2018;8:15372.
- Taniguchi Y, Matsukura Y, Taniguchi H, et al. Development of preparative and analytical methods of the hop bitter acid oxide fraction and chemical properties of its components. Biosci Biotechnol Biochem. 2015;79:1684–1694.
- Ano Y, Ayabe T, Kutsukake T, et al. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol Aging. 2018;72:23–31.
- Chunchai T, Samniang B, Sripetchwandee J, et al. Vagus nerve stimulation exerts the neuroprotective effects in obese-insulin resistant rats, leading to the improvement of cognitive function. Sci Rep. 2016;6:26866.
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J. 2008;22:659–661.
