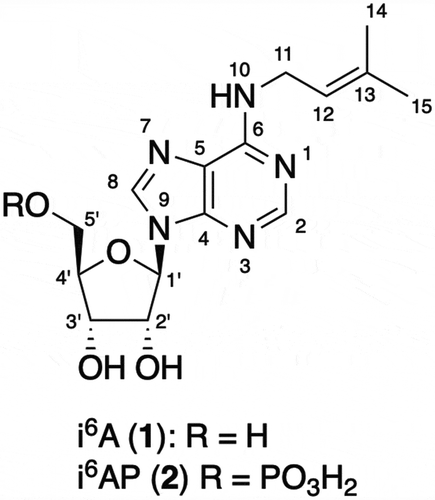
ABSTRACT
N6-Isopentenyladenosine (i6A) was isolated from a marine sponge Oceanapia sp. as the major cytotoxic constituent along with N6-isopentenyladenosine 5ʹ-monophosphate (i6AP) which was inactive. The structures of i6A and i6AP were assigned by a combination of the analysis of NMR spectroscopy and mass spectrometry. This is the first isolation of i6A and i6AP from a marine sponge.
Sponges tend to show the highest incidence of activity in the screening of marine organisms for cytotoxicity against cultured mammalian cells [Citation1]. As a result, a wide variety of novel cytotoxic compounds have been isolated from marine sponges. Some of them exhibit a unique mode of action and have been used as reagents for cell biology or a scaffold for cancer chemotherapy, e. g. okadaic acid and calyculins inhibit protein phosphatases 1 and 2A [Citation2,Citation3]; halichondrins and discodermolide affect functions of microtubules [Citation4,Citation5]; mycalolides and misakinolides depolymerize F-actin [Citation6,Citation7]; and polytheonamides form a pore in the cell membrane [Citation8], just to mention a few. Although discovery studies on conspicuous sponge species inhabiting in shallow waters have been conducted intensively, this effort frequently results in the rediscovery of known metabolites. On the other hand, sponges inhabiting in deep-sea, which cannot be collected by scuba diving, are relatively unexplored. Therefore, we have been collecting deep-sea sponges by dredging and studied their potential in drug discovery. Our cytotoxicity screening showed that a sponge Oceanapia sp. collected at Oshimashinsone at a depth of 150 m was active. In this paper we describe the isolation and identification of the active constituent and its congener from the sponge.
The sponge Oceanapia sp. (50 g) was extracted with EtOH to afford an aqueous suspension of the extract, which was extracted with CHCl3 and n-BuOH. The n-BuOH fraction, which showed cytotoxic activity, was concentrated and fractionated by ODS flash chromatography (aq. MeCN containing 0.5% AcOH, stepwise gradient elution with 20%, 40%, 60%, 80% and 100% of MeCN in H2O). The 40% MeCN fraction was purified followed by ODS-HPLC (20% to 70% MeCN containing 0.5% AcOH) to afford 1 (1.3 mg) as the predominant cytotoxic constituent as well as 2 (0.4 mg).
Compound 1, obtained as a colorless amorphous solid, had the molecular formula of C15H21N5O4 as determined by HRESIMS (calcd. for C15H21N5O4Na [M + Na]+ m/z 358.1491, found 358.1468). The UV spectrum in MeOH (λmax (log ε) 268 (4.1), 215 (4.0) nm) suggested the presence of a chromophore similar to purine bases [Citation9]. Interpretation of the 1H NMR spectrum in conjunction with the HSQC spectrum showed the presence of two aromatic singlets, one olefinic proton, one anomeric proton, three oxygenated methines, one oxygenated methylene, one nitrogen-bearing methylene, and two vinylic methyls (). Interpretation of the COSY spectrum revealed the presence of a pentofuranose unit and an isopentenyl unit substituted by a nitrogen atom. The furanose was assigned as ribose by comparing the 1H-1H coupling constants and 13C chemical shifts with those in the literature [Citation10]. 13C NMR chemical shifts of the aromatic carbons together with the HMBC correlations observed for the aromatic protons permitted the assignment of an adenine unit. Because of signal broadening, HMBC correlation was not observed from the methylene protons in the isopentenyl moiety. However, coincidence of the chemical shifts of aromatic carbons with those of adenine suggested that the isopentenyl group was connected to the nitrogen attached to C-6 of the adenine system (). HMBC correlations from the anomeric proton to C-4 and C-8 demonstrated that N-9 was substituted by a ribose. Therefore, 1 was assigned as N6-isopentenyladenosine (i6A). The 1H NMR spectrum of the commercial i6A in CD3OD was identical with that of 1, confirming our assignment (Figure S8).
Table 1. 1H and 13C NMR data of i6A (1) and i6AP (2) in CD3ODa.
Compound 2, obtained as a colorless amorphous solid, had the molecular formula of C15H22N5O7P as determined by HRESIMS (calcd. for C15H21N5O7P [M – H]− m/z 414.1179, found 414.1204), indicating that 2 was a phosphorylated form of 1. A comparison of the NMR data showed that all the 1H and 13C signals found in 1 were also present in 2, with chemical shift perturbations observed for H2-5ʹ (). The site of phosphorylation was determined on the basis of the 1H-31P HMBC data which gave intense correlations between H2-5ʹ and the phosphorus signal, confirming the site of phosphorylation to be the C-5ʹ oxygen atom. Therefore, 2 was assigned as N6-isopentenyladenosine 5ʹ-monophosphate (i6AP).
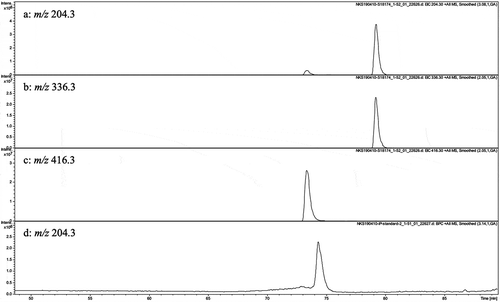
The presence of i6A and i6AP in the sponge prompted us to search for the related metabolites by LC-MS (). Although N6-isopentenyl adenine (iP) was not detected, there was a peak eluting earlier than i6A and 16 mass units larger than that of i6A, indicating the presence of zeatin riboside albeit with ca. 1/100 of the amount of i6A. Due to its small amount, it was not possible to isolate and characterize this putative zeatin riboside. However, the co-incidence of its retention time in the LC-MS with that of the commercial zeatin riboside indicated the presence of zeatin riboside in the sponge (Figure S18).
Figure 2. (a)-(c) LC/MS chromatograms of the crude extract of Oceanapia sp. and (d) that of iP standard. (a) Mass chromatogram observed at m/z 204.3. (b) mass chromatogram observed at m/z 336.3. (c) mass chromatogram observed at m/z 416.3. (d) mass chromatogram of iP standard observed at m/z 204.3.
i6A (1) shows cytotoxic activity against HeLa cells with an IC50 value of 2.1 µM, which is comparable to the previously reported values [Citation11]. On the other hand, i6AP (2) was inactive at a concentration of 50 µM.
i6A was first isolated and characterized during the course of the search for a minor component of soluble ribonucleic acid fraction of yeast [Citation12]. i6A has been shown to be a component of tRNA in most organisms and is distributed at position 37 of tRNA, whose isopentenyl group being installed by the action of tRNA-isopentenyl transferase (tRNA-IPT), which is encoded by homologous genes in all domains of life [Citation13,Citation14]. Marine sponges sometimes hold a large amount of associated microorganisms within their body [Citation15]. The origin of i6A in the sponge Oceanapia sp. can be traced to either sponge cells or symbiotic microorganisms. With our current analytical data, it is not possible to speculate their biosynthetic pathway nor the producing organism(s).
Immediately after the discovery of i6A, its cytotoxicity against tumor cells was reported [Citation16]. Deribosylated bases, iP and trans-zeatin, do not show cytotoxicity [Citation16]. Our result showed that i6AP was also inactive, although i6AP was considered as the metabolized active form within the cells in inducing apoptotic cell death [Citation17], in which involvement of Akt/NF-κB pathway was reported [Citation18]. We have examined the effect of i6A against Fucci2-HeLa cells with time-lapse imaging. Our observations demonstrated that the cell-cycle was arrested at the G1 phase (data not shown), which is in agreement with targeting the Akt/NF-κB pathway.
In the field of marine natural products chemistry, sponge-derived nucleosides are important molecules, because the discovery of spongothymidine (Ara T) and spongouridine (Ara U) led to the development of anticancer or antiviral agents, Ara-A and Ara-C [Citation19]. Their discovery stimulated the field of marine natural products chemistry. Despite the intensive studies toward finding cytotoxic compounds from marine organisms and the isolation of a variety of modified nucleosides from marine sponges [Citation19], this is the first isolation of i6A from the sponge. Our discovery demonstrates that the deep-sea sponges are untapped promising resources for natural product chemistry.
Author contribution
SN isolated and identified the compounds and wrote the draft of the manuscript. YI identified the sponge. SOh conducted the collection of the sponge. SOk participated to design the study. SM designed the study and prepared the manuscript. All authors reviewed and approved the final manuscript.
Supp_Mat_.docx
Download MS Word (6.6 MB)Acknowledgments
We thank the captain and crew of the T/S Toyoshio-maru for sample collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Munro HGM, Blunt JW, Dumdei EJ, et al. The discovery and development of marine compounds with pharmaceutical potential. J Biotechnol. 1999;70:15–25.
- Takai A, Bialojan C, Troschka M, et al. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1984;217:81–84.
- Suganuma M, Fujiki H, Furuya-Suguri H, et al. Calyculin A, an inhibitor of protein phosphatases, a potent tumor promoter on CD-1 mouse skin. Cancer Res. 1990;50:3521–3525.
- Bai R, Nguyen TL, Burnett JC, et al. Interactions of halichondrin B and eribulin with tubulin. J Chem Inf Model. 2011;51:1393–1404.
- Ter HE, Kowalski RJ, Hamel E, et al. Discodermolide, a cytotoxic marine agent that stabilizes microtubules more potently than taxol. Biochemistry. 1996;35:243–250.
- Saito S, Watabe S, Ozaki H, et al. Mycalolide B, a novel actin depolymerizing agent. J Biol Chem. 1994;269:29710–29714.
- Terry DR, Spector I, Higa T, et al. Misakinolide A is a marine macrolide that caps but does not sever filamentous actin. J Biol Chem. 1994;272:7841–7845.
- Hamada T, Matsunaga S, Fujiwara M, et al. Solution structure of polytheonamide B, a highly cytotoxic nonribosomal polypeptide from marine sponge. J Am Chem Soc. 2010;132:12941–12945.
- Leigh BC, Ignacio T Jr. Correlations in the ultraviolet spectra of the purine and pyrimidine bases. J Am Chem Soc. 1965;87:11–15.
- Doi Y, Ishibashi M, Kobayashi J. Isolation and structure of shimofuridins B-G from the Okinawan marine tunicate Aplidium multiplicatum. Tetrahedron. 1994;50:8651–8656.
- Spinola M, Colombo F, Falvella FS, et al. N6‐isopentenyladenosine: A potential therapeutic agent for a variety of epithelial cancers. Int J Cancer. 2007;120:2744–2748.
- Ross HH, Morris JR, Lubomyr S, et al. Isolation of N6-(γ,γ-dimethylallyl)adenosine from soluble ribonucleic acid. J Am Chem Soc. 1966;88:2614–2615.
- Persson BC, Esberg B, Ólafsson Ó, et al. Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie. 1994;76:1152–1160.
- Ulrich S, Simon B, Noelia FV. The modified base isopentenyladenosine and its derivatives in tRNA. RNA Biol. 2017;14:1197–1208.
- Taylor MW, Radax R, Steger D, et al. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev. 2007;71:295–347.
- Fleysher MH, Hakala MT, Bloch HA, et al. Synthesis and biological activity of some N6-alkyladenosines. J Med Chem. 1968;11:717–720.
- Ranieri R, Ciaglia E, Amodio G, et al. N6-isopentenyladenosine dual targeting of AMPK and Rab7 prenylation inhibits melanoma growth through the impairment of autophagic flux. Cell Death Differ. 2018;25:353–367.
- Laezza C, Malfitano AM, Di Matola T, et al. Involvement of Akt/NF-κB pathway in N6-isopentenyladenosine-induced apoptosis in human breast cancer cells. Mol Carcinog. 2010;49:892–901.
- Huang R-M, Chen Y-N, Zeng Z, et al. Marine nucleosides: structure, bioactivity, synthesis and biosynthesis. Mar Drugs. 2014;12:5817–5838, and references cited therein.