ABSTRACT
Bilberry has been reported to have anti-oxidant and anti-inflammatory properties. We studied the effect of bilberry (Vaccinium myrtillus L.) fruits extracts (BEs) on the pathogenesis caused by lipid accumulation in fatty liver and non-alcoholic steatohepatitis (NASH). 5 μg/ml of BEs was enough to suppress lipid accumulation in the fatty liver model of the mouse hepatic AML12 cells. BEs increased cell viability and anti-oxidant capacity, presumably by activating (phosphorylating) Akt/STAT3 and inducing MnSOD/catalase. BEs also significantly reduced Rubicon and induced p62/SQSTM1, possibly contributing to reduce cellular lipids (lipophagy). When the mice were fed supplemented with BEs (5% or 10%, w/w), hepatic steatosis, injury, and hypercholesterolemia/hyperglycemia were significantly improved. Furthermore, histological and cytokine studies indicated that BEs possibly suppress hepatic inflammation (hepatitis) and fibrosis. Therefore, BEs improved liver steatosis and injury, and potentially suppress fibrosis by suppressing inflammatory response, which therefore may prevent the progression of fatty liver to NASH.
Graphical abstract
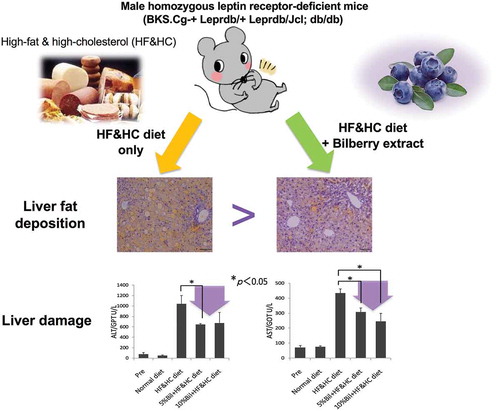
Bilberry fruits extracts suppressed hepatocyte injury caused by early fat accumulation. Therefore, bilberry is expected to be a liver-friendly food.
The perennial dwarf shrub bilberry (Vaccinium myrtillus L.) is also known as the European blueberry and is widely grown in the northern hemisphere across Europe and Central Asia [Citation1]. Although bilberry leaves are a promising source of bioactive natural products [Citation2], bilberry fruits are better known for the health benefits of its phytochemicals. Bilberry fruits are rich in anthocyanidins and anthocyanins [Citation3] and other potent natural antioxidants like flavanols and phenolic compounds. Several studies have shown that V. myrtillus berries contain higher anthocyanin and antioxidant levels than cultivated blueberries (V. corymbosum) [Citation4].
Wild bilberry fruits are well established in pharmacognosy and have been used as a food source for centuries. Bilberry is a rich source of anthocyanins [Citation3] and its extracts are extensively used in dietary supplements and pharmaceutical products. Bilberry fruits extracts are antioxidant [Citation5–Citation8], can decrease capillary permeability and fragility [Citation9], inhibit platelet aggregation [Citation10], and strengthen collagen matrix cross-linkages [Citation11,Citation12]. Bilberry crude extracts are used in the treatment of ophthalmological diseases and blood vessel disorders [Citation13]. Recently, they have been administered to treat dysentery, diarrhea, and mouth and throat inflammations [Citation14]. Certain studies have shown that the bilberry anthocyanins may prevent metabolic syndrome [Citation15–Citation22]. However, the mechanisms by which bilberry influences diabetes and lifestyle-related diseases have not yet been identified. Since bilberry seems to prevent diabetes mellitus, it may also prevent non-alcoholic steatohepatitis (NASH), which is a progressive and severe form of non-alcoholic fatty liver disease (NAFLD). Fat accumulation in liver is involved in the onset and progression of these conditions [Citation23,Citation24].
In the present study, we investigated the effects of dried bilberry (Vaccinium myrtillus) fruits extracts (BEs) especially on mouse hepatic steatosis and injury in cellular and animal models. We elucidated the possible mechanisms of BEs on the prevention of fatty liver and NASH, with regards to hepatic steatosis and injury. Also, we proposed the possible molecular mechanisms of BEs effects on the prevention of fatty liver and NASH, regarding autophagy, fat metabolism, oxidative stress, and mitogenesis.
Materials and methods
Cell culture, reagents and bilberry fruits extracts (BEs)
Alpha mouse liver 12 cells (AML12; ATCC® CRL-254™, Manassas, VA, USA) were maintained at 37°C and 5.0% CO2 in DMEM/Nutrient Mixture F-12 (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS) and 1× ITS-A (Insulin-Transferrin-Selenium-Sodium Pyruvate Supplement, Thermo Fisher Scientific, Waltham, MA, USA). Fatty acids (FA; oleic and linoleic) and T0901317 (T090; LXRα agonist) were purchased from TCI Chemicals (Tokyo, Japan) and Sigma-Aldrich (St. Louis, MO, USA), respectively. To induce steatosis of hepatocytes, T090 (1 μM) or FA (100 μM for each oleic and linoleic acids) were added to the culture media daily for 3 or 5 days [Citation25–Citation27].
A 90% ethanolic extracts of bilberry (Vaccinium myrtillus L.) fruits was purified with an adsorbent resin to obtain bilberry fruits extracts containing ≥36% anthocyanin glycosides. The purified product was lyophilized, homogenized, and used as bilberry fruits extracts (BEs). BEs were added daily to the culture media at the designated concentration. We used BEs in the concentrations from 1 μg/ml to 10 μg/ml, which was proved to show no cytotoxicity to AML12 hepatocytes by lactose dehydrogenase (LDH) assay using LDH Cytotoxicity Detection Kit (Takara Bio Inc., Shiga, Japan) (Figure S1).
Hepatic lipid accumulation analysis
AML12 cells were rinsed with PBS, fixed with 4% paraformaldehyde/PBS for 5 min, and stained with Nile red (AdipoRed Assay Reagent, Lonza, Basel, Switzerland). After incubation at room temperature for 10–15 min, cells were quantified and observed for fluorescence with excitation at 485 nm and emission at 572 nm (expressed as relative fluorescence units, RFU) in a multimode plate reader (Infinite® 200 PRO, TECAN, Zurich, Switzerland) and under a fluorescence microscope (Biozero; Keyence, Osaka, Japan). Hoechst33342 (Sigma-Aldrich) was used for nuclear counterstaining. Relative lipid accumulation (RLA) was determined as the ratio of AdipoRed to Hoechs33342 intensity values to calculate well-level normalization. For all doses, the average RLAs of the technical replicates were calculated and normalized to vehicle control wells to determine the mean fold-change values across the test plates. The mean dose-response and standard error were derived from eight independent experiments.
For animal experiments, liver triacylglycerol (TG) contents were measured with an adipogenesis colorimetric/fluorometric assay kit (BioVision, Milpitas, CA, USA) according to the manufacturer’s instructions [Citation28]. The TG concentration was calculated from a standard curve and normalized to mass per μg protein.
Monitoring and evaluation of hepatocyte growth (survival and proliferation)
The effects of BEs on the growth (survival/proliferation) of non-steatotic or steatotic AML12 cells were determined with the xCELLigence System (Roche, Basel, Switzerland). The survival/proliferation analyses of non-steatotic and steatotic hepatocytes were performed in the following way. Briefly, for the non-steatotic hepatocytes, untreated AML12 cells were plated at a density of 1.5 × 104 cells/well in an E-Plate 16 PET (ACEA Biosciences, San Diego, CA, USA), and for the steatotic hepatocytes, pre-steatotic AML12 cells were plated at a density of 1.2 × 103 cells/well in the plate. The plates were then placed in the xCELLigence System and BEs were administered daily at the designated concentrations. The above-mentioned experiments were monitored by real-time quantitation.
Evaluation of cellular redox states in vitro
Cellular redox states were imaged/evaluated with fluorescence microscopy using Redox-sensitive roGFP as previously described [Citation29].
RNA extraction and qRT-PCR
Total hepatocyte RNA was isolated with an Aurum Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cDNA was synthesized with an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. The quantitative real-time PCR (qRT-PCR) was performed on the Bio-Rad CFX Connect System with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The used primers were listed in . The qRT-PCR data were analyzed by the comparative Ct Method (2−ΔΔCt) [Citation30]. The mRNA expressions were normalized to 36B4 (acidic ribosomal phosphoprotein P0 (RPLP0)) mRNA, and the relative mRNA expression levels were calculated using the control mRNA.
Table 1. Primer list for the quantitative real-time PCR (qRT-PCR).
Animal care, experimental design, and sample preparation
Male homozygous leptin receptor-deficient (BKS.Cg-+ Leprdb/+ Leprdb/Jcl; db/db) mice (10 weeks) were obtained from CLEA Japan (Tokyo, Japan). The mice were divided into four groups. Group 1 received a normal control diet. Group 2 received a high-fat and high-cholesterol (HF&HC) diet. Groups 3 and 4 received HF&HC diet supplemented with 5% and 10% BEs, respectively. The HF&HC diet (No. D09100301) was purchased from Research Diet Inc. (New Brunswick, NJ, USA), mixed with BEs, and freshly prepared every 2 days.
The mice were sacrificed before BEs administration and after 8 weeks to collect liver and blood specimens. The liver/body weight ratios were calculated to estimate relative changes in liver mass. Liver specimens were prepared for histological and western blot analyses. Blood specimens were used in biochemical analyses (alanine transaminase/glutamate pyruvate transaminase (ALT); aspartate transaminase/glutamate oxaloacetate transaminase (AST); glucose (GLU); and total cholesterol (T-CHO)). The animal care and experimental procedures were approved by the Animal Research Committee of Hokkaido University (Permission No. 16–0120) and performed in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of Hokkaido University. All efforts were made to minimize animal suffering.
For histological analysis, liver specimens were fixed in 10% buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) or Azan. To visualize hepatic lipid accumulation, frozen sections of formalin-fixed liver tissue were stained with Sudan III. Briefly, frozen liver sections (8 μm thick) were mounted on slide glasses, air-dried, and rinsed with 50% v/v ethanol. Next, the specimens were stained in Sudan III stain for 10 min at room temperature and rinsed with 50% (v/v) ethanol to remove excess stain. Hematoxylin nuclear counterstain was applied for 3 min. The stained liver sections were then washed several times in water, mounted with a coverslip, and viewed under a microscope.
Immunoassay
For expression analysis of proteins associated with autophagy (lipophagy) and fatty acid (FA) synthesis, antibodies against the following proteins were used; Rubicon, p62/SQSTM1 (sequestosome-1, Cell Signaling Technology, Danvers, MA, USA), FASN (fatty acid synthase, BD Transduction Laboratories, Franklin Lakes, NJ, USA) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase, Cell Signaling Technology, Danvers, MA, USA) using standard western blot analysis protocol.
For expression analysis of proteins associated with autophagy, survival/proliferation, and antioxidant properties, protein separation, and detection were performed using an automated capillary electrophoresis system, Wes™ (ProteinSimple, San Jose, CA, USA) and antibodies against the following proteins were used; STAT3 (signal transducer and activator of transcription 3, Santa Cruz Biotechnology, Dallas, TX, USA), p62/SQSTM1, phospho-Akt, Akt, phospho-STAT3 (Tyr705) (Cell Signaling Technology, Danvers, MA, USA), MnSOD (manganese-dependent superoxide dismutase, BD Transduction Laboratories, Franklin Lakes, NJ, USA), catalase (EMD Biosciences, Darmstadt, Germany), and GAPDH. Signals were detected with an HRP (horseradish peroxidase)-conjugated secondary anti-rabbit or anti-mouse antibody, and were visualized using Compass for ProteinSimple software.
Statistical analysis
All results were expressed as means ± standard error of the mean (SEM). ANOVA followed by Tukey–Kramer test were performed to compare the means. Statistical difference at p < 0.05 were considered as significant.
Results
Effects of BEs on hepatic cells
To determine the effects of BEs on hepatocyte steatosis, we created a steatotic murine hepatocytes in two ways by adding fatty acids (FA; oleic and linoleic) or a nuclear receptor LXRα agonist (T090) to AML12 cells. ) shows lipid accumulation induced by FA or T090, which are standardized by untreated AML12 cells (left panel). Fluorescence photomicrographs of cells showing lipids (green color) and nuclei (red color) are shown in ) (right panel). Both FA and T090-induced lipid accumulation in AML12 cells, though FA was more efficient. Cell growth (survival/proliferation) of the FA- and T090-induced steatotic AML12 cells was significantly lower than that of the untreated controls ()). The addition of FA and T090 both inhibited the proliferation of AML12 cells, but the inhibitory effect of FA was observed at an early stage of proliferation, whereas that of T090 was observed at a later stage of proliferation. However, steatosis was significantly improved by adding BEs into the culture media. This effect of BEs was most apparent at 5 μg/mL BEs, indicating an optimum concentration for this effect ()). BEs seem to suppress cellular lipid accumulation by both types of hepatic steatosis (FA and T090) dose-dependently ()).
Figure 1. Bilberry fruits extracts (BEs) improved steatotic hepatocyte growth (survival/proliferation) (AML12 cells). (a) Administration of free fatty acids (FA) consisting of 100 μM oleic and linoleic acids or 1 μM T0901317 (T090, an agonist for LXRα) induced substantial lipid deposition in murine AML12 hepatocytes (Day 5). Right panel: lipids in cytosol and nuclei stained with AdipoRed (green) and Hoechst33342 (red, pseudo-color), respectively. Scale bar: 50 μm. Different letters indicate statistically significant differences among groups (p < 0.05); n = 4; mean ± SEM. (b) Cell growth deteriorated similarly in both FA- and T090-induced steatotic hepatocytes. The same letter or no letter indicates that the difference is not significant within each time-point group (p < 0.05); n = 3–4; mean ± SEM). (c) BEs promoted cell growth of steatotic hepatocytes induced by FA- and T090-administration (left and right panels, respectively) with a peak concentration at 5 μg/mL. Since no significant difference in cell growth was observed between 12 h and 48 h, only the data after 60 h are shown. The same letter or no letter indicates that the difference is not significant within each time-point group (p < 0.05); n = 3–4; mean ± SEM.
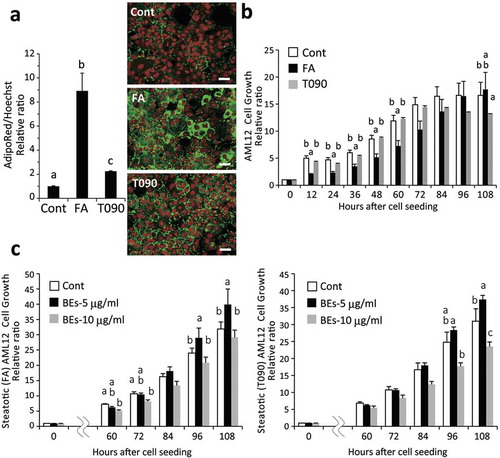
Figure 2. Bilberry fruits extracts (BEs) reduced lipid deposition in steatotic hepatocytes, promoted cell growth, and conferred resistance to oxidative stress. (a) BEs significantly suppressed lipid accumulation in hepatocytes. Right panel: lipids in cytosol and nuclei in green (AdipoRed) and red (Hoechst33342, pseudo-color), respectively. Scale bar: 50 μm. Different letters indicate statistically significant differences among groups (p < 0.05); n = 4–6; mean ± SEM. (b) BEs promoted cell growth at peak concentrations of 1–5 μg/mL in non-steatotic hepatocytes. The same letter indicates that the difference is not significant (p ≥ 0.05); n = 3–6). (c) BEs conferred resistance to a 24-h H2O2 challenge (500 μM) in hepatocytes. Lower panel: more live cells were observed in BEs-treated hepatocytes (1 μg/mL) than untreated liver cells. Scale: 50 μm. The same letter indicates that the difference is not significant (p ≥ 0.05); n = 3–6). (d) BEs did not directly react with and/or scavenge cellular ROS (left panel; n = 8).
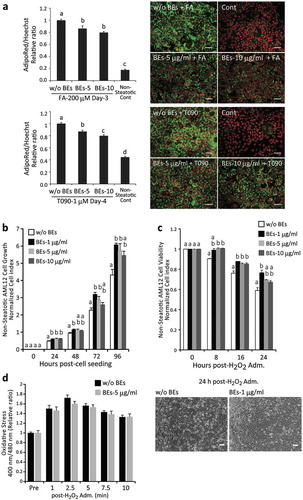
We examined the pro-survival effects of BEs in non-steatotic AML12 cells. BEs significantly promoted cell survival/proliferation showing a peak at 1 μg/mL ()). Oxidative stress (H2O2, 500 μM) reduced cell survival in non-steatotic AML12 cells, which was improved significantly by the treatment with BEs ()). These results indicate that BEs confer a pro-survival property and a resistance against oxidative stress to hepatocytes. Therefore, we monitored changes of reactive oxygen species (ROS) levels over time in AML12 cells treated with H2O2. Interestingly, there were no significant differences between cells added with and without BEs in terms of their cellular ROS levels ()). These data may indicate that BEs do not directly eliminate intracellular ROS, but BEs provide the resistance against oxidative stress. Although NF-E2-related factor 2 (Nrf2), activated by oxidative stress and toxic substances in the environment, is well known to protect cells from environmental stresses including ROS, significant activation of an antioxidative Nrf2-DNA binding was not observed (data not shown).
Effect of BEs on mouse with fatty liver
Next, we challenged BEs against mouse fatty liver model to confirm the BEs’ in vivo effects. HF & HC diet markedly increased liver/body weight ratios, hepatic steatosis/TG contents, and injuries, which were suppressed by BEs in a dose-dependent manner ()). There was no significant difference in dietary intake between the groups receiving BEs and those not receiving them (). Histological study and hepatic TG measurement revealed that BEs administration undoubtedly suppressed fat accumulation/TG contents in mouse liver ()). The HF & HC diet caused mild fibrosis around portal area in mouse livers (), arrowhead), whereas those being administered BEs presented much less fibrosis. Blood biochemistry of the mice fed with the normal and HF & HC diet are shown in ). Plasma ALT and AST levels in the 5% BEs group were significantly lower than those in the group receiving HF & HC diet alone (p < 0.05). Plasma GLU and T-CHO levels for the 10% BEs group were also significantly lower than those for the HF & HC diet group. Furthermore, compared with those given the normal diet, a series of pro-inflammatory cytokine levels (e.g. TNF-α, IL-9, IL-1β, and IFN-γ) were higher in mice receiving the HF & HC diet, which were likely to be reduced by BEs administration though no significant difference was found among them (Figure S2).
Table 2. Body weight and food intake in mice fed a high-fat and high-cholesterol (HF&HC) diet with/without bilberry fruits extracts (BEs) for 8 weeks. Data are means ± SEM; n = 4–8 per group. *p < 0.05 vs. HF&HC group. There were no significant differences in initial body weight or food intake between groups.
Figure 3. Bilberry fruits extracts (BEs) improved liver steatosis, liver damage, and hyperglycemia in mice fed HF&HC diets. (a) HF&HC diet (8 weeks) induced liver mass enlargement with no increase in body weight. The same letter indicates that the difference is not significant (p ≥ 0.05); n = 4. (b) BEs improved hepatic steatosis. Scale bar: 100 μm. Hepatic TG levels were reduced in BEs-treated livers (right panel). The same letter indicates that the difference is not significant (p ≥ 0.05); n = 4. (c) Fibrosis stained blue with Azan and was observed in the periportal area (arrowhead) of HF&HC-treated livers. (d) Blood biochemistry revealed that BEs improved steatosis-induced liver damage (ALT and AST) and hypercholesterolemia (total cholesterol, T-CHO). The same letter indicates that the difference is not significant within each group (p ≥ 0.05); n = 4. Mean ± SEM.
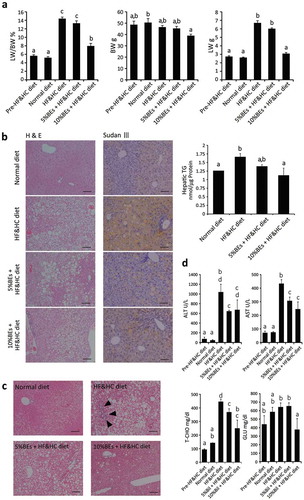
These data indicate that BEs improve hyperglycemia/hypercholesterolemia, liver injury and inflammation, and presumably liver fibrosis.
Effects of BEs on the expression levels of the genes and proteins regulating lipid metabolism, survival/proliferation, antioxidant, and autophagy in hepatocytes
The mouse fatty liver model clearly showed that BEs improve fat accumulation, injury, inflammatory reaction, and fibrosis of liver. To elucidate the underlying molecular mechanisms, we examined the expressions of the genes and proteins related to these events using mouse AML12 cells.
) shows the protein expressions of autophagy- and fat metabolism-associated proteins including Rubicon (run domain beclin-1-interacting and cysteine-rich domain-containing protein, a cellular protein that suppresses late stage of autophagy), p62/SQSTM1 (an autophagy marker), and FASN (fatty acid synthase) [Citation31–Citation34] in AML12 cells. Fat accumulation in hepatocytes was stimulated by the addition of FA and T090 to the culture media ()). When AML12 cells were subjected to FA, Rubicon expression was dramatically elevated. The addition of BEs downregulated Rubicon significantly and concurrently upregulated p62/SQSTM1 expression significantly (), upper panel). Therefore, it is suggested that BEs enhanced autophagy (lipophagy) by suppressing Rubicon expression, and eventually reduced lipid accumulation. T090 clearly upregulated FASN, which was certainly suppressed by the addition of BEs. On the contrary, FASN was neither induced in FA-treated AML12 cells nor affected by BEs administration at all (), upper panel). Taken together, BEs may improve fat accumulation by enhancing autophagy (lipophagy) or suppressing FA synthesis in the steatotic hepatocytes, depending on the causes of steatosis.
Figure 4. Molecular analyses of the effects of bilberry fruits extracts (BEs) on murine AML12 hepatocytes. (a) Effects of BEs on autophagy (lipophagy) (Rubicon, p62/SQSTM1) and fatty acid (FA) synthesis (fatty acid synthase, FASN) were examined in FA-/T0901317 (T090; LXRα agonist)-treated AML12 cells by western blot. Each blot represents ≥3 independent experiments. The duplicates of immunoblots are taken from the specimens of experiments performed at different times. ImageJ (NIH, Bethesda, MD, USA) was used for the quantitative analysis of western blot. The same letter indicates that the difference is not significant among groups (p ≥ 0.05). (b) Effects of BEs on FA synthesis were examined by qRT-PCR. The same letter indicates that the difference is not significant among groups (p ≥ 0.05). (c) Levels of proteins associated with autophagy, survival/proliferation, and antioxidant properties were slightly increased by the addition of BEs in non-steatotic AML12 cells (Day 3 and/or Day 7). Each blot represents ≥3 independent experiments. Each experiment was performed 3× and the data are expressed as means ± SEM (a, b, and c). The same letter or no letter indicates that the difference is not significant within each time-point group (p ≥ 0.05).
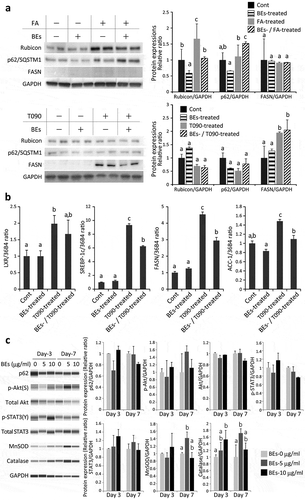
We then studied the expression of genes related to FA synthesis including LXR (a member of the family of nuclear receptor transcription factors involved in lipid synthesis), SREBP-1c (transcription factor suppressed in FA, TG, and cholesterol synthesis), FASN, and ACC-1 (acetyl-CoA carboxylase, a key enzyme for FA synthesis). As shown in ), these four genes were upregulated by LXR stimulus (T090), which were significantly suppressed by BEs administration except LXR. Therefore, BEs suppress cellular FA synthetic pathway stimulated by LXR agonist. In case where the cells were stimulated by FA, BEs did not affect the expression levels of LXR, SREBP-1c, FASN, or ACC-1 (data not shown).
To elucidate the molecular mechanisms of BEs for autophagy, survival/proliferation, and antioxidant properties, we additionally examined the following protein expressions in non-steatotic AML12 cells ()). p62/SQSTM1 protein was increased very mildly and transiently after the BEs treatment even in non-steatotic cells (day 3). This suggests that BEs did not directly affect p62/SQSTM1 expression in non-steatotic cells not expressing Rubicon ()), and the increase of p62/SQSTM1 ()) was secondary to reduction of Rubicon. Akt and STAT3 were both phosphorylated after the BEs treatment with slight increase of these protein expressions. Regarding the antioxidant proteins, the increase of protein expression was observed in MnSOD and catalase ()).
Discussion
In the present study, we demonstrated that BEs improve fatty liver and the following liver injury using mouse liver cells and fatty liver model. We proposed the unique mechanisms of BEs’ these effects on autophagy (Rubicon and p62/SQSTM1), lipid metabolism (FASN, STAT3), survival/proliferation (Akt/STAT3), and antioxidant (MnSOD/catalase).
We showed that BEs reduced the oxidative injury induced by hydrogen peroxide ()). BEs did not eliminate ROS directly ()), but BEs induced the antioxidant proteins, MnSOD and catalase significantly ()). Although Nrf2 is known to eventually confer resistance against oxidative stress to cells, significant activation of Nrf2 was not observed (data not shown). Though anthocyanin rich in BEs is known to suppress ROS production and prevent resulting toxic injury [Citation35], it is not clear whether anthocyanin plays a central role for BEs’ these effects.
We also showed that BEs may affect hepatocyte survival and/or proliferation. BEs improved cell viability in both non-steatotic and steatotic cells (), , c)). As shown in ), Akt and STAT3 seem to be both phosphorylated after the BEs treatment with slight increase of these protein expressions. The activation of these proteins by BEs should contribute to cell survival and proliferation in steatotic and non-steatotic cells. These data indicate that BEs may have pro-survival and regenerative potentials of tissues/organ in various conditions, though further investigation is required.
As we have already shown that STAT3 activation suppressed steatosis by inhibiting lipogenic genes such as SREBP-1c [Citation36], a STAT3/SREBP pathway would definitely play an important role for the suppression of hepatic steatosis. Therefore, an activation of STAT3 may contribute at least partly to reduction of lipid accumulation by BEs.
We carefully examined the influence of BEs on fatty liver using both cultured liver cells and mouse liver. BEs suppressed lipid accumulation and its progression in both models () and )). BEs suppressed lipid accumulation in two different models (FA addition and TG synthesis) (,b)) and enhanced lipolysis by autophagy (FA addition model) ()). Most notable was Rubicon, which suppresses the late stage of autophagy and accelerates lipid accumulation [Citation34]. It suppresses autophagosome fusion with lysosomes, which is the final step of autophagy. The rate of autophagy decreases with increasing intracellular Rubicon levels. Therefore, fatty liver may have progressed with increased hepatic Rubicon levels suppressing lipophagy. In the present study, we first report that BEs markedly downregulate Rubicon and promote lipophagy (shown by an increase of p62/SQSTM1) in FA-induced steatotic hepatocytes [Citation32,Citation33]. BEs also reduce FASN expression, which is directly related to fat accumulation [Citation31]. Therefore, BEs are predicted to suppress lipid accumulation in the liver by promoting lipolysis (decrease of Rubicon) and inhibiting TG synthesis (decrease of FASN). Because p62/SQSTM1 is also known to possess a cytoprotective effect [Citation28], the increase of p62/SQSTM1 by BEs may also contribute to prevention against liver injury.
It has been reported that bilberry is antioxidant [Citation5–Citation8] and anti-inflammatory [Citation37–Citation39]. Also, in the present study, BEs showed antioxidant and anti-inflammatory properties other than defatting and protective property. This may suppress the steatosis-induced hepatic inflammation (hepatitis). As shown in Figure S2, BEs restrained biological effects against inflammatory cytokines such as TNF-α, IL-9, IL-1β, and IFN-γ. This means that BEs have inhibitory effects against inflammation as well as defatting and protection, suggesting that BEs potentially prevent the progression from fatty liver to NASH. It is interesting to know that BEs may control these chemokine activities directly or indirectly, which may be important clinical targets of the future study of BEs.
Morrison et al. [Citation40] comprehensively investigated the effects of Mirtoselect, an anthocyanin-rich bilberry extracts, on NASH and associated fibrosis. They examined the effects of anthocyanins-rich bilberry extracts on late pathology of NASH by animal experiment only. They concluded that Mirtoselect reduced development of NASH, attenuating both steatosis and inflammation as well as the development of hepatic fibrosis. On the other hand, we focused on the effect of BEs on earlier pathological events of fatty liver developing into NASH. We also studied its mechanism of action in detail by cell experiments, focusing on cell survival/proliferation, fat accumulation (incorporation, degradation), antioxidant, and inflammation. The results obtained from cell experiments were verified by animal experiments. Our study gives new mechanistic/therapeutic insights on the effect of bilberry for the liver pathology developing into NASH.
Conclusion
Overall, we identified the factors that improve lipid accumulation, cytotoxicity, oxidative stress, and inflammation by BEs. Because hepatocyte fat accumulation, insulin resistance, oxidative stress, injury and sugar/fat metabolism are of particular importance in NASH progression, BEs were proved to target some of these crucial factors. We also showed that BEs potentially suppresses inflammation and fibrosis induced by fat accumulation and injury of liver. Based on the results detailed above, bilberry is expected to be a liver-friendly food supplement.
Author Contribution
M. O. designed and supervised the study. N. M., S. H. and M. O. wrote and revised the manuscript. S. H., Y, S. J. and H. Y. performed cell experiments. S. H. and M. O. performed animal experiments. S. H., T. S., N. M. and M. O. analyzed and discussed the results. All authors gave final approval and consented to be accountable on all matters.
Supplemental_data_ID_BBB-190270_R1.docx
Download MS Word (36.6 KB)Fig_S2_Manuscript_ID_BBB-190270_R1.tiff
Download TIFF Image (985.1 KB)Fig_S1_Manuscript_ID_BBB-190270_R1.tiff
Download TIFF Image (463.3 KB)Acknowledgments
We are particularly grateful for generous donation from Mr. & Mrs. Fujikawa (to M.O.).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Chu W-K, Cheung SCM, Lau RAW, et al. Bilberry (Vaccinium myrtillus L.). In: Benzie IFE, Wachtel-Galor S, editors Herbal Medicine: biomolecular and Clinical Aspects. Florida (FL): CRC Press; 2011. p. 55–71.
- Ferlemi AV, Lamari FN. Berry leaves: an alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants. 2016;5:17.
- Akerström A, Jaakola L, Bång U, et al. Effects of latitude-related factors and geographical origin on anthocyanidin concentrations in fruits of Vaccinium myrtillus L. (bilberries). J Agric Food Chem. 2010;58:11939–11945.
- Burdulis D, Sarkinas A, Jasutiené I, et al. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Polym Pharm. 2009;66:399–408.
- Erlund I, Marniemi J, Hakala P, et al. Consumption of black currants, lingonberries and bilberries increases serum quercetin concentrations. Eur J Clin Nutr. 2003;57:37–42.
- Matsunaga N, Tsuruma K, Shimazawa M, et al. Inhibitory actions of bilberry anthocyanidins on angiogenesis. Phytother Res. 2010;24:S42–S47.
- Viljanen K, Kylli P, Kivikari R, et al. Inhibition of protein and lipid oxidation in liposomes by berry phenolics. J Agric Food Chem. 2004;52:7419–7424.
- Yao Y, Vieira A. Protective activities of Vaccinium antioxidants with potential relevance to mitochondrial dysfunction and neurotoxicity. Neurotoxicology. 2007;28:93–100.
- Mian E, Curri SB, Lietti A, et al. Anthocyanosides and the walls of the microvessels: further aspects of the mechanism of action of their protective effect in syndromes due to abnormal capillary fragility. Minerva Med. 1977;68:3565–3581.
- Bottecchia D, Bettini V, Martino R, et al. Preliminary report on the inhibitory effect of Vaccinium myrtillus anthocyanosides on platelet aggregation and clot retraction. Fitoterapia. 1987;58:3–8.
- Harvsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–1148.
- Gabor M. Pharmacologic effects of flavonoids on blood vessels. Angiologica. 1972;9:355–374.
- Tumbasa V, Čanadanović-Brunet J, Gille L, et al. Superoxide anion radical scavenging activity of bilberry (Vaccinium myrtillus L.). J Berry Res. 2010;1:13–23.
- Valentová K, Ulrichová J, Cvak L, et al. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2007;101:912–917.
- Lankinen M, Schwab U, Kolehmainen M, et al. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the sysdimet study. PLoS One. 2011;6:e22646.
- Lehtonen HM, Suomela JP, Tahvonen R, et al. Different berries and berry fractions have various but slightly positive effects on the associated variables of metabolic diseases on overweight and obese women. Eur J Clin Nutr. 2011;65:394–401.
- Mauray A, Felgines C, Morand C, et al. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 2010;5:343–353.
- Mykkänen OT, Huotari A, Herzig K-H, et al. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS One. 2014;9:e114790.
- Persson IA, Persson K, Andersson RG. Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J Agric Food Chem. 2009;57:4626–4629.
- Suzuki R, Tanaka M, Takanashi M, et al. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr Metab. 2011;8:14.
- Takikawa M, Inoue S, Horio F, et al. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140:527–533.
- Ziberna L, Lunder M, Tramer F, et al. The endothelial plasma membrane transporter bilitranslocase mediates rat aortic vasodilation induced by anthocyanins. Nutr Metab Cardiovasc Dis. 2013;23:68–74.
- Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295.
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273.
- Cha J-Y, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751.
- Nagrath D, Xu H, Tanimura Y, et al. Metabolic preconditioning of donor organs: defatting fatty livers by normothermic perfusion ex vivo. Metab Eng. 2009;11:274–283.
- Nativ NI, Yarmush G, So A, et al. Elevated sensitivity of macrosteatotic hepatocytes to hypoxia/reoxygenation stress is reversed by a novel defatting protocol. Liver Transplant. 2014;20:1000–1011.
- Haga S, Min Y, Ozaki M. Relevance of FXR-p62/SQSTM1 pathway for survival and protection of mouse hepatocytes and liver, especially with steatosis. BMC Gastroenterol. 2017;17:9.
- Haga S, Remington SJ, Morita N, et al. Hepatic ischemia induced immediate oxidative stress after reperfusion and determined the severity of the reperfusion-induced damage. Antioxid Redox Signal. 2009;11:2563–2572.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2DDCT method. Methods. 2001;25:402–408.
- Dorn C, Riener MO, Kirovski G, et al. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int J Clin Exp Pathol. 2010;3:505–514.
- Haga S, Ozawa T, Yamada Y, et al. p62/SQSTM1 plays a protective role in oxidative injury of steatotic liver in a mouse hepatectomy model. Antioxid Redox Signal. 2014;21:2515–2530.
- Park JS, Oh SY, Lee DH, et al. p62/SQSTM1 is required for the protection against endoplasmic reticulum stress-induced apoptotic cell death. Free Radical Res. 2016;50:1408–1421.
- Tanaka S, Hikita H, Tatsumi T, et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64:1994–2014.
- Pandey KB, Rizv SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278.
- Inoue H, Ogawa W, Ozaki M, et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med. 2004;10:168–174.
- De Mello VD, Schwab U, Kolehmainen M, et al. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the sysdimet study. Diabetologia. 2011;54:2755–2767.
- Domitrović R, Jakovac H. Effects of standardized bilberry fruit extract (Mirtoselect®) on resolution of CCl4-induced liver fibrosis in mice. Food Chem Toxicol. 2011;49:848–854.
- Miyake S, Takahashi N, Sasaki M, et al. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: cellular and molecular mechanism. Lab Investig. 2012;92:102–109.
- Morrison MC, Liang W, Mulder P, et al. Mirtoselect, an anthocyanin-rich bilberry extract, attenuates non-alcoholic steatohepatitis and associated fibrosis in ApoE∗ 3Leiden mice. J Hepatol. 2015;62:1180–1186.
