 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, a sulfated polysaccharide (BFP) was isolated from the edible red alga Bangia fusco-purpurea. Gel-filtration and thin layer chromatographically analyses suggested that BFP was a homogenous polysaccharide. The chemical structural analysis revealed that BFP mainly consisted of galactose together with a small amount of uronic acid, mannose, and glucose. Its molecular mass was estimated to be 133.18 kDa by high-performance liquid chromatography (HPLC) analysis. BFP inhibited α-amylase and α-glucosidase in a concentration-dependent manner. The IC50 values of BFP against α-amylase and α-glucosidase were estimated to be 1.26 ± 0.11 mg/mL and 1.34 ± 0.07 mg/mL, respectively. Kinetic analyses suggested that BFP showed competitive and non-competitive inhibition against α-amylase and α-glucosidase, respectively. Circular dichroism spectral and fluorescence spectral analyses suggested that BFP affects the conformational structures of these enzymes, which may lead to the inhibition of the enzymatic activities.
Abbreviations: Ara: D-arabinose; AnGal: anhydro-L-galactose residues; CD spectroscopy: Circular Dichroism spectroscopy; DNS: dinitrosalicylic acid; FT-IR: fourier transform infrared spectra; Fuc: L-fucose; Gal: D-galactose; Glc: D-glucose; GlcA: D-Glucuronic acid; HPLC: high performance liquid chromatography; Man: D-mannose; pNPG: p-nitrophenyl-α-D-glucoside; TFA: trifluoroacetic acid; TLC: thin-layer chromatography; PMP: 1-phenyl-3-methyl-5-pyrazolone; Xyl: D-xylose
Graphical abstract

Inhibitory effects of a sulfated polysaccharide isolated from edible red alga Bangia fusco-purpurea on α-amylase and α-glucosidase
Edible seaweeds contain abundant non-starch polysaccharides, which can function as dietary fibers due to their indigestibility by human digestive enzymes [Citation1,Citation2]. It has been reported that some seaweed polysaccharides can delay the digestion and absorption of nutrients including sugar and fatty acid, and then decrease the levels of glucose and cholesterol in blood [Citation2]. Moreover, the consumption of seaweed polysaccharides results in the reduction of the risks of some chronic diseases such as diabetes, obesity, and hypertension [Citation3]. In addition to the functions as soluble fibers, seaweed polysaccharides have been proved to possess numerous biological activities, and thus drawn a great attention in different research fields as the promising sources of new drugs and health foods or supplements [Citation4,Citation5]. Recently, special interests are focusing on sulfated polysaccharides isolated from several seaweeds such as Fucus vesiculosus, Ascophyllum nodosum, and Sargassum wightii. Some of them show inhibitory effects on carbohydrate digestive enzymes such as α-amylase or α-glucosidase with different extents depending on the saccharides [Citation3,Citation6–Citation9]. Since it has been known that the absorption of glucose into blood can be decreased significantly via inhibition of α-amylase and α-glucosidase [Citation3,Citation6,Citation10], the use of effective inhibitors of the enzymes is one of the feasible therapeutic approaches to ameliorate Type 2 diabetes through alleviating the postprandial hyperglycemia [Citation7,Citation11]. At present, anti-diabetic drugs such as acarbose and miglitol, which are potent inhibitors of α-amylase and α-glucosidase, have been clinically used for the treatment of Type 2 diabetes. However, the persistent use of these inhibitors is limited due to their undesirable side effects [Citation12,Citation13]. Therefore, novel and safe inhibitors against α-amylase and α-glucosidase from edible natural sources are urgently required.
Bangia fusco-purpurea, belonging to Bangia species, is a commercially important edible red alga, and abundantly cultivated in Fujian province in China. This red alga is superior to Porphyra sp. in terms of nutritional value and taste [Citation14]. In addition, it has been reported that B. fusco-purpurea can reduce the risks of the cardiovascular diseases and chronic metabolic diseases [Citation15,Citation16], although there is no available information on the effective ingredients responsible for the health benefits. Therefore, in this study, we prepared the polysaccharide fraction (BFP) from B. fusco-purpurea, and investigated its effects on α-amylase and α-glucosidase. Since there are no detail studies for the inhibitory mechanisms of seaweed polysaccharides on the enzymes from the viewpoint of the interaction between polysaccharides and enzymes, we also investigated the effects of BFP on the conformational structures of the enzymes as well as the kinetic analysis of its inhibitory effects.
Materials and methods
Materials and reagents
Seaweed B. fusco-purpurea was harvested from the coast of Nan’ri island of Fujian province, China. Standard dextrans (5, 12, 50, 270, and 410 kDa), standard monosaccharides, L-fucose (Fuc), D-glucose (Glc), D-glucuronic acid (GlcA), D-mannose (Man), D-xylose (Xyl), D-galactose (Gal), D-arabinose (Ara), and D-galacturonic acid, starch, α-amylase (16 units/mg solid, porcine pancreas, Enzyme Commission number 3.2.1.1), and α-glucosidase (≥100 units/mg protein, Saccharomyces cerevisiae, Enzyme Commission number 3.2.1.20) were purchased from Sigma-Aldrich (St. Louis, USA). P-nitrophenyl-α-D-glucoside (pNPG) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Acarbose was obtained from Bayer China Ltd. (Shanghai, China). Other chemicals were of the highest grade commercially available.
Preparation and purification of polysaccharide
Fresh seaweeds were rinsed with distilled water extensively and air-dried at 50°C until the mass becomes constant. Crude polysaccharides were extracted from B. fusco-purpurea according to the method reported previously [Citation17]. The purification of polysaccharide was carried out by using gel permeation chromatography [Citation18]. Briefly, 3 mL of crude polysaccharide fraction (5 mg/mL) was loaded onto a Sephadex G75 column (1.6 cm I.D. × 100 cm) (GE Healthcare, Uppsala, Sweden), and eluted with 0.1 M NaCl solution (pH 7.0) at a flow rate of 0.5 mL/min at room temperature. Each elution fraction of 5 mL/tube was collected and the chromatographic separations were monitored by the phenol-sulfuric acid method [Citation19]. After combining each peak fraction in accordance with the elution profile, the sample was dialyzed against distilled water through a cellulose membrane (3500 Da cut-off) for 2 days at 4°C. After lyophilization, the dried powder was considered as purified B. fusco-purpurea polysaccharide (BFP).
Thin-layer chromatography (TLC) analysis
TLC analysis was used to analyze the purity of polysaccharide fractions [Citation20]. BFP solution (5 µL, 1 mg/mL) was loaded onto a cellulose acetate membrane (5 cm × 10 cm) and developed with a solvent system consisting of n-butanol/glacial acetic acid/water (2: 2: 1, v/v/v). After development, the TLC membrane was dried and stained by dipping in a sulfuric acid/ethanol reagent (1: 9, v/v) at room temperature for 3 s and then heated at 90°C for 5 min.
Molecular mass and composition analysis
The apparent molecular mass of BFP was estimated by a Shimazu® prominence high performance liquid chromatography (HPLC) system (including Shimadzu® SIL-20A prominence auto sampler, CBM-20A communications bus module, LC-20AT reciprocating pumps connected to a DGU 20A3R degassing unit, CTO-20A column oven, and ELSD-LT II low temperature evaporative light scattering detector) (Shimazu Corporation, Kyoto, Japan) equipped with a TSK-gel G4000 PWXL column (7.8 mm I.D. × 300 mm) (Tosoh Bioscience, Tokyo, Japan), as reported previously [Citation21]. Standard dextrans (5, 12, 50, 270, and 410 kDa) were applied to the same column under the same chromatographic conditions for the calibration curve. Data were analyzed using an LC solution software (Shimadzu Corporation, Kyoto, Japan).
The neutral monosaccharide composition of BFP was analyzed by an Agilent® HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with an InertSustain® C18 column (4.6 mm I.D. × 250 mm) (GL Sciences Inc., Tokyo, Japan) as reported previously [Citation22]. In brief, 0.5 mg of BFP was hydrolyzed with 100 µL of trifluoroacetic acid (TFA) (2 M) at 100°C for 4 h. The hydrolysates were reacted with the 1-phenyl-3-methyl-5-pyrazolone (PMP) reagent solution and applied to the HPLC system. The PMP-conjugates of hydrolysates were eluted with 0.1 M phosphate buffer (pH 6.7) containing 17% acetonitrile at a flow rate of 1.0 mL/min at 30°C for 70 min. A known concentration of PMP-monosaccharide (PMP-Gal, -Man, -Glc, -Xyl, -Ara, and -Fuc) were used as standards, and PMP-monosaccharides were detected at the wavelength of 254 nm by an Agilent® UV-Detector (Agilent Technologies, Santa Clara, CA, USA), and analyzed by the LC-1260 software. Uronic acids level in BFP was determined by a modified carbazole-sulfuric acid method [Citation23]. The content of 3, 6-anhydro-L-galactose (3,6-AnGal) was detected by the resorcinol method [Citation24]. The sulfate level of BFP was analyzed with barium sulfate turbidimetric method [Citation25]. The protein content of BFP was determined by Bradford protein assay kit (Beyotime Inst. Biotech., Jiangsu, China) according to the manufacturer’s instruction. The phenolic compound in the BFP was determined by using the Folin–Denis method [Citation26].
Infrared spectroscopy
BFP (20 mg) was kept dry and ground with 400 mg of potassium bromide (KBr) powder followed by pressing into pellet for Fourier transform infrared spectra (FT-IR) analysis. FT-IR spectrum of BFP was recorded in the infrared region between 4000 and 400 cm−1 with a Nicolet iS50 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) at room temperature.
Enzyme inhibition assay
The effect of BFP on α-amylase was examined according to the method described previously with slight modification [Citation12]. In brief, 125 µL of BFP solutions at varying final concentrations (0, 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) in 20 mM phosphate buffer (pH 6.9) containing 6 mM sodium chloride were mixed with 125 µL of α-amylase (16 units/mL) in the same buffer. After pre-incubation at 25°C for 30 min, 500 µL of starch (1%, w/v) in the buffer was added into each tube and the mixture was further incubated at 37°C for 30 min, followed by adding 1000 µL of dinitrosalicylic acid (DNS) reagent solution prepared as described previously [Citation27]. The reaction was terminated by heating at 100°C for 5 min and then cooled down to room temperature. The reaction mixture was diluted with 10 mL of distilled water and the absorbance was measured at 540 nm with a Unico® 7200 spectrophotometer (Unico Instrument Corporation, Dayton, NJ, USA). The effect of BFP on α-glucosidase was measured by the method described previously [Citation12]. Briefly, 125 µL of α-glucosidase (≥2 units/mL) in 100 mM phosphate buffer (pH 6.9) was mixed with 125 µL of BFP solutions (at different final concentrations of 0, 0.2, 0.4, 0.6, 0.8, or 1.0 mg/mL) in the buffer, and incubated for 10 min at 37°C, and then 250 µL of pNPG (5 mM) was added. After a further 10 min incubation at 37°C, the reaction was terminated by heating at 100°C for 5 min, followed by cooling down to room temperature. The reaction mixture was diluted with 10 mL of distilled water and the absorbance was measured at 405 nm with a Unico® 7200 spectrophotometer. A reaction mixture without α-amylase or α-glucosidase was referred to the negative control. The IC50 value, which is defined as the concentration of BFP to inhibit 50% of enzyme activity, was estimated by linear fitting. As a positive control, acarbose, a known inhibitor of α-amylase and α-glucosidase, was examined under the same conditions. Acarbose was examined at 0.01 mg/mL and 0.50 mg/mL, which were nearly IC50 values for α-amylase or α-glucosidase, respectively.
The % inhibition was calculated as follows:
where Asample is the absorbance in the presence of sample (BFP), and Acontrol is the absorbance in the absence of sample.
Kinetics analysis
The inhibitory kinetics of BFP on α-amylase or α-glucosidase was evaluated with the Lineweaver–Burk double reciprocal equation. In brief, α-amylase or α-glucosidase at the final concentrations of 0–0.10 mg/mL was pre-incubated with or without BFP (final 500 µg/mL), followed by adding same volume of starch solution (1%, w/v) or pNPG solution (5 mM) as the substrates for α-amylase or α-glucosidase. The reaction rate was determined as described above. For Lineweaver–Burk diagram, the activities of α-amylase (16 units/mL) or α-glucosidase (≥2 units/mL) at different concentrations of starch (final 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 mg/mL) or pNPG (final concentration of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mmol/L) were measured as described above, respectively, in the presence or absence of BFP (final 500 µg/mL). The inhibition constant (Ki) of BFP on α-amylase or α-glucosidase was simultaneously evaluated with Lineweaver–Burk double reciprocal equations [Citation28].
Circular dichroism (CD) spectroscopy analysis
To examine the effects of BFP on the secondary structure of α-amylase or α-glucosidase, CD spectroscopy analysis was employed according to the method reported previously with some modifications [Citation29,Citation30]. Briefly, α-amylase or α-glucosidase (final 5.0 mg/mL) was incubated with BFP (final 0 or 1000 μg/mL) in the buffer used for enzyme activity assay at 25°C for 15 min. After that, each α-amylase-BFP mixture or α-glucosidase-BFP mixture was injected into a 1 mm-path length quartz cuvette and the CD spectrum of each mixture was recorded with a Jasco-810 spectrophotometer (JASCO, Tokyo, Japan). The detection conditions were as follows: the scanning wavelength was from 260 to 200 nm, the scanning rate was 100 nm/min, a resolution was 0.5 nm, the respond time was 1 s, and the bandwidth was 2 nm. Baseline was corrected using a pure buffer. The data were analyzed by the CDNN program, and the errors of prediction on the range of 190–260 nm and 200–260 nm were 5% as described previously [Citation31,Citation32].
Fluorescence spectroscopy analysis
To investigate the influence of BFP on the tertiary structure of α-amylase or α-glucosidase, the intrinsic fluorescence emission spectrum of the α-amylase or α-glucosidase was measured as previously reported [Citation29]. Briefly, α-amylase or α-glucosidase (final 1.0 mg/mL) was incubated with BFP (final 0 or 1000 μg/mL) in the buffer used for enzyme activity assay at 25°C for 15 min. The fluorescence emission spectrum of each reaction mixture was recorded at 25°C with a 1 mm-path length quartz cuvette in a Varian Cary Eclipse fluorescence spectrometer (Varian Inc., California, USA). The emission spectra were recorded from 300 to 500 nm at the excitation wavelength of 295 nm.
Statistical analysis
All the experiments were repeated at least three times. The results were expressed as a mean ± standard deviation (SD), and the data were analyzed by using one-way ANOVA followed by a t-test to determine any significant differences. p < 0.05 was considered as statistically significance.
Results and discussion
Isolation and purification of BFP
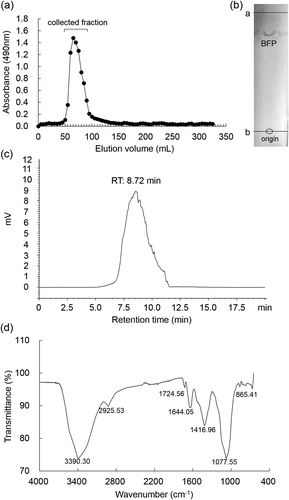
A polysaccharide fraction was obtained from B. fusco-purpurea by hot water extraction and ethanol precipitation procedures. After ethanol precipitation, the amount of polysaccharide fraction obtained from 100 g of the dried seaweed was 6.89 ± 0.44 g. The fraction was further purified by a Sephadex G75 column chromatography, and a single peak of polysaccharide was detected (). The elution fractions from 50 to 100 mL were collected and dialyzed against distilled water. After lyophilization, the yield of the fraction was 5.67 ± 0.36 g. TLC analysis showed that the fraction had a single band (). Furthermore, BFP was detected as a single peak in HPLC analysis (). These results suggest that BFP may be a homogeneous polysaccharide. Thus, this faction was used as purified BFP thereafter.
Figure 1. Purification and characterization of BFP.
(a) Elution profile of polysaccharide fraction extracted from B. fusco-purpurea on a Sephadex G75 column. (b) TLC pattern of BFP developed with n-butanol/glacial acetic acid/water, 2: 2: 1, v/v/v. The first line (a line) is the solvent front, and the second line (b line) is the origin. (c) Profile of BFP in HPLC on a TSK-gel G4000 PWXL column (7.8 mm I.D. × 300 mm) eluted with ultra-pure water (pH = 7.0) at a flow rate of 1.0 mL/min. (d) FT-IR spectrum of BFP.
Estimation of molecular mass and composition analysis of BFP
The molecular mass of BFP was estimated by HPLC analysis with a calibration (y = −0.3187x + 7.9035; y = lg molecular mass, x = retention time) using standard dextrans (Figure S1). Based on the retention time of BFP in HPLC and the calibration, the apparent molecular mass of BFP was estimated to be 133.18 kDa (, ).
Table 1. Chemical compositions and molecular mass of polysaccharide isolated from B. fusco-purpurea.
The monosaccharide composition analysis revealed that BFP consisted of galactose (66.55%; w/w) as the major monosaccharide together with a small amount of uronic acid (7.27%; w/w), mannose (6.04%; w/w), glucose (5.92%; w/w), 3,6-AnGal (2.81%; w/w), fucose (1.82%; w/w), xylose (1.71%; w/w), and arabinose (1.27%; w/w) as shown in and Figure S2. The content of sulfate group in BFP was estimated to be 10.34 ± 0.01% (w/w) (). The contents of proteins and phenolic compounds in BFP were estimated to be 0.31 ± 0.01% (w/w) and trace level, respectively (). The sum of the percentages of neutral monosaccharides, 3,6-AnGal, UA, and SO42- ion is 103.73% (). It is presumed that the overlapping determination of galactose, 3,6-AnGal, and uronic acids might occur during the composition analysis by PMP derivation method. During the analysis, the internal ether bonds of the 3,6-AnGal were destroyed by acid hydrolysis and further reduced to galactose form. The carboxyl groups of uronic acids in BFP might be reduced to hydroxyl groups. These structural changes may lead to the overlapping determination. Even so, our results suggest that BFP is a highly purified sulfated galactan. Based on the amount of purified BFP obtained, the yield of BFP was estimated to be 5.14 ± 0.32% of the dried seaweed.
FT-IR spectrometric characterization of BFP
As shown in ), an intense and broad absorption peak at 3390 cm−1 for O-H stretching vibrations was observed. A small absorption peak at 2925 cm−1 for C-H stretching and bending vibration was also observed. The characteristic absorption peak at 1077 cm−1 suggests the structure of galactans [Citation33], and a small absorption peak at 865 cm−1 may be attributed to a characteristic signal for the vibrations of the anomeric C-H groups, suggesting the presence of galactopyranose and α-type glycosidic linkages in BFP [Citation34]. Furthermore, the absorption peaks at 1644 cm−1 and 1416 cm−1 are assigned to asymmetrical and symmetrical COO− stretching vibrations in uronic acids of BFP [Citation35], which is in good agreement with the results of composition analysis (). An absorption peak at 1724 cm−1 suggests the presence of O-acetyl groups in BFP [Citation36].
It has been reported that the typical polysaccharides in red algae are galactan, classified as agaran or carrageenan [Citation37]. Actually, an agar-type galactan extracted from B. fusco-purpurea has been found to consist of DL-galactose, 3,6-anhydro-L-galactose and 6-O-methyl-D-galactose [Citation38]. Usov et al. have revealed that the hot water-soluble galactan from Bangia atropurpurea, a freshwater homotypic synonym with B. fusco-purpurea belonging to Bangia sp., was similar to agarose or porphyran. Porphyran, a sulfated polysaccharide, existing in the intercellular matrix of red algal tissues, is one of the main constituents of red alga Porphyra yezoensis. This polysaccharide is a linear sulfated polysaccharide consisting of repeating disaccharide units with alternating 3-linked β-D-galactose and 4-linked α-L-galactose-6-sulfate or 3,6-anhydro-α-L-galactose units (AnGal) [Citation39,Citation40]. Furthermore, it has been reported that the porphyran-like polysaccharides isolated from B. atropurpurea contains mannose, galactose, glucose, xylose, and 3,6-anhydrogalactose residues [Citation41]. Composition similarities of BFP to the previously reported porphyran-like polysaccharides suggest that BFP is classified as a porphyran-like polysaccharide. This notion is also supported by the FT-IR spectrometric characterization of BFP as described above.
In general, seaweed-derived polysaccharides have complex and high molecular mass, and their compositions and entire structures can vary depending on algal spices, the extraction process, the harvest seasons, and even the local climatic conditions [Citation42,Citation43]. Therefore, the BFP obtained in this study may have specific structural features different from previous porphyran-like polysaccharides.
Inhibitory effects of BFP on α-amylase and α-glucosidase
As shown in , BFP inhibited both α-amylase and α-glucosidase in a concentration-dependent manner. The IC50 values of BFP against α-amylase and α-glucosidase were estimated to be 1.26 ± 0.11 mg/mL and 1.34 ± 0.07 mg/mL, respectively. Since no significant levels of phenolic compounds and proteins were detected in the purified BFP, the inhibitory activities of BFP are attributed to the polysaccharide. The enzymes used in this study are commercially available and are often used to screen the clinically applicable inhibitors [Citation9,Citation12]. Actually, we have found that a seaweed-derived polysaccharide, which had identified as a potent inhibitor of these enzymes in in vitro study, showed the blood glucose lowering effect after oral administration in a clinical trial [Citation9]. Since it has been reported that carboxylic groups in soluble low molecular weight glycosides are involved in the inhibition of α-glucosidase [Citation44], the carboxylic groups of uronic acids portion in BFP may play a role in the inhibition of α-glucosidase.
Figure 2. Inhibitory effects of BFP on α-amylase and α-glucosidase.
(a) Inhibitory effects of BFP (0–1.0 mg/mL) or acarbose (0.01 mg/mL) on α-amylase. (b) Inhibitory effects of BFP (0–1.0 mg/mL) or acarbose (0.50 mg/mL) on α-glucosidase. All experiments were performed in triplicate, and values are the means ± SD. Asterisks indicate significant differences between with and without polysaccharides (or acarbose). *p <0.05.
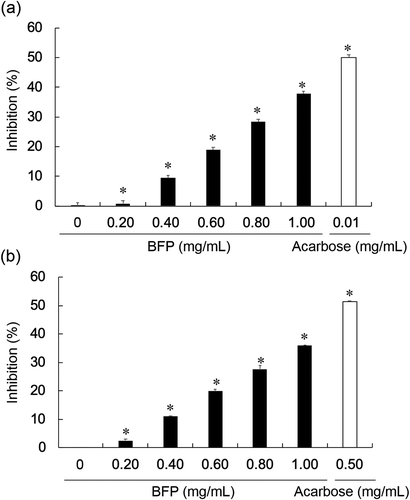
Acarbose, an inhibitor of α-amylase and α-glucosidase, which has been developed as a drug, is clinically used in the treatment of Type-2 diabetes. However, its usage is often limited because of its undesirable side effects such as diarrhea, flatulence, and abdominal distention. It is considered that these side effects are caused by the prolonged potent inhibition of α-amylase [Citation13]. In fact, our results showed that the IC50 value of acarbose against α-amylase was nearly 50 times lower than that against α-glucosidase (). Although the inhibition of α-amylase contributes to the decrease in maltose and glucose release from starch, the excessive inhibition can lead to the accumulation of undigested starch that could serve as the substrate for the fermentation of intestinal flora, and consequently cause intestinal disorders [Citation7]. Therefore, partial or mild inhibition of α-amylase may be preferable. Since BFP is derived from the edible seaweed, and showed inhibitory effects on α-amylase and α-glucosidase almost equally, BFP is a promising candidate as a safe food additive allowing long-term consumption for the postprandial hypoglycemic control, although further in vivo studies are necessary to verify these points.
Kinetics of inhibitory effects of BFP on α-amylase and α-glucosidase
To understand the inhibitory types of BFP on α-amylase or α-glucosidase, linear regression curve was plotted with the reaction rates versus different concentrations of α-amylase or α-glucosidase in the presence or absence of BFP. In the both the enzymes, linear relationships between the concentrations of the enzymes and the reaction rates were observed, and the slope of the line obtained in the presence of BFP declined as compared to the one obtained in the absence of BFP (). These results suggest that BFP exhibited reversible inhibition on α-amylase and α-glucosidase [Citation12,Citation34]. Probably, BFP can act on the enzyme molecules through the reversible non-covalent interaction.
Figure 3. The inhibitory kinetics of BFP on α-amylase and α-glucosidase.
(a) Linear regression of reaction rates versus different concentrations of α-amylase in the presence (closed circles) and absence (open circles) of BFP. The final concentration of starch was 5.0 mg/mL, and the reaction time was 3 min. (b) Linear regression curves of reaction rates versus different concentrations of α-glucosidase in the presence (closed circles) and absence (open circles) of BFP. The final concentration of pNPG was 2.5 mM, and the reaction time was 3 min. (c) Lineweaver–Burk plot of the reactions of α-amylase in the presence (closed circles) and absence (open circles) of BFP. (d) Lineweaver–Burk plot of the reactions of α-amylase in the presence (closed circles) and absence (open circles) of BFP. All experiments were performed in triplicate, and values were the means ± SD.
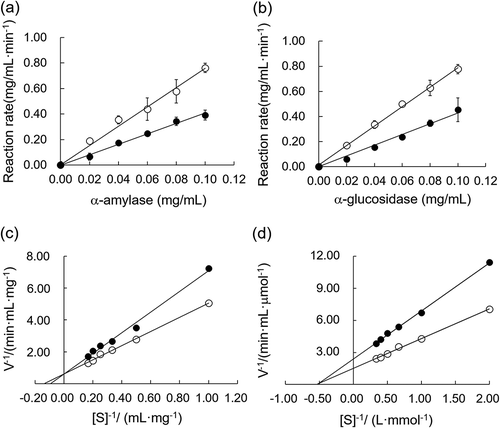
The kinetic parameters Km and Vmax of α-amylase or α-glucosidase in the presence or absence of BFP were further analyzed according to the Lineweaver–Burk double reciprocal plots. As shown in , the reciprocal plots of the reaction rates (V−1) versus the reciprocal of the α-amylase reaction system with or without BFP were characterized by straight lines intersected at the y-axis. In the case of α-amylase, the values of the Michaelis constant (Km) increased in the presence of BFP, while the maximum reaction rate of the enzymatic reaction (Vmax) remained constant, indicating that the inhibitory type of BFP against α-amylase was competitive inhibition [Citation34]. Namely, BFP can compete with starch to occupy the active site of α-amylase, thereby interfering the binding of the substrate to the enzyme. Contrast to the α-amylase, the graph of V−1 versus [S]−1 in the α-glucosidase reaction system gave a straight line, and the lines intersected at the negative x-axis. In addition, unchanged Km and decreased Vmax were observed in the presence of BFP in the α-glucosidase (). These results suggest that BFP exhibited non-competitive (or mixed) inhibition against α-glucosidase [Citation45]. BFP may be able to inhibit α-glucosidase through the interaction with other than substrate-recognition site on the enzyme molecule. Using the equations described previously [Citation28], the Ki of BFP against α-amylase and α-glucosidase were determined to be 1.64 mg/mL and 0.86 mg/mL, respectively.
Effects of BFP on the secondary and tertiary structures of α-amylase and α-glucosidase
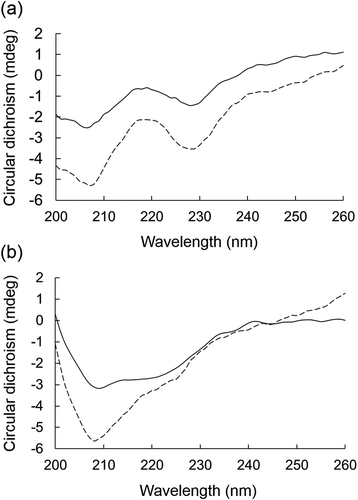
CD spectral analysis (260~200 nm) is usually employed to determine the secondary structure of a protein due to its accuracy and sensitivity. The characteristic CD bands reflect the content of secondary structure such as α-helix, β-sheet, β-turn, and random coil [Citation46]. The CD spectra of the α-amylase and α-glucosidase are shown in . Based on the spectra, the percentages of the secondary structures (α-helix, β-sheet, β-turn, and random coil) of the enzymes were estimated with 5% of prediction errors on the range of 200–260 nm by the CDNN program (). Similar to our results, it has been reported that the prediction errors of the percentages of secondary structures on the range of 190–260 nm are 5% in circular dichroism analysis [Citation32]. Therefore, the sums of percentages of the secondary structures are close to 100%, but not necessarily become 100%. After the incubation with BFP at 1000 µg/mL, the α-helix content of α-amylase was increased from 15.8% to 29.0%, while the β-sheet and random coil contents were decreased from 21.3% to 8.1% and from 37.9% to 31.8%, respectively. BFP also caused a slight increase in α-helix of α-glucosidase with decline of β-sheet (). These results suggest that BFP can affect the secondary structures of these enzymes with different extents depending on the enzymes. Although the exact reason for this is still unclear, affinity or strength of interaction of BFP with protein molecules may vary depending on the protein structures. Such differences can lead to the different enzyme inhibition pattern such as competitive and non-competitive inhibition.
Table 2. Effects of BFP on the secondary structures of α-amylase and α-glucosidase.
Figure 4. Effects of BFP on the CD spectrum of α-amylase or α-glucosidase.
CD spectra of α-amylase (a) or α-glucosidase at 5.0 mg/mL (b) in the presence (dotted line) or absence (solid line) of BFP (1000 µg/mL) were measured. Measurements were carried out after 15 min incubation of the mixture of enzyme and BFP at 25°C.
Intrinsic fluorescence of tryptophan residues in a protein is a sensitive probe to estimate conformational changes of protein molecule [Citation47]. To investigate the effects of BFP on the entire conformation of α-amylase or α-glucosidase, intrinsic fluorescence spectra of the enzymes in the absence or the presence of BFP (final 1000 µg/mL) were measured. As shown in , when excited at 295 nm, the α-amylase and α-glucosidase exhibited the specific fluorescence spectra with emission peak at near 355 nm ()) and 340 nm ()), respectively. BFP itself had no significant fluorescence around these wavelengths ()). In the presence of BFP, the fluorescence intensities of both α-amylase and α-glucosidase at each peak wavelength changed (), suggesting that BFP may slightly influence the microenvironment of the tryptophan residues in the protein molecules [Citation48], although the exact changes caused by BFP are still unclear.
Figure 5. Effects of BFP on the fluorescence spectrum of α-amylase or α-glucosidase.
Fluorescence emission spectra of α-amylase (a) or α-glucosidase (b) at 1.0 mg/mL in the presence (dotted line) or absence (solid line) of BFP (1000 µg/mL) were measured. Measurements were carried out after 15 min incubation of the mixture of enzyme and BFP at 25°C. Fluorescence emission spectrum of BFP alone (c) at 1000 µg/mL.
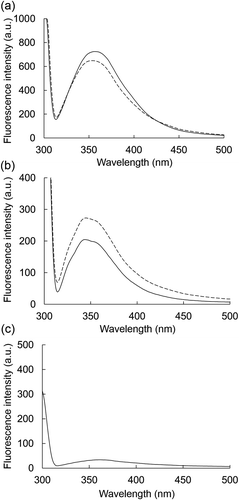
The stereochemical structural analyses suggested that BFP inhibits the enzyme activities of both α-amylase and α-glucosidase via inducing the changes of the conformational structures of these enzymes with non-covalent interaction. Furthermore, one can speculate that the different effects of BFP on the secondary structures of α-amylase and α-glucosidase may be a reason for the different inhibitory behaviors of BFP on these enzymes. Although there are several reports in which α-amylase and α-glucosidase are merely inhibited by seaweed-derived polysaccharides [Citation3,Citation6–Citation9], there is no available information for the underlying inhibitory mechanisms of the polysaccharides on these enzymes especially from the viewpoint of the interaction between polysaccharide and enzymes. Our findings provide some valuable tips for the understanding of inhibitory mechanisms of seaweed polysaccharides. Further studies are required to elucidate the exact chemical structure of BFP, which can contribute to the clarification of the exact action mechanisms of BFP on the enzymes, especially in terms of structure-activity relationship.
Author contributions
Tatsuya Oda and Zedong Jiang designed this study and wrote the manuscript. Zedong Jiang, Gang Yu, Yan Liang, and Tianyuan Song performed the experiments and analyzed data. Yanbing Zhu, Hui Ni, and Kenichi Yamaguchi critically revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Supplemental Material
Download MS Word (16.1 KB)Figure S2. HPLC analysis of monosaccharide compositions of BFP. Elution profiles of PMP-derived monosaccharide standards (a) and PMP-derived acid-hydrolyzed BFP (b).
Download TIFF Image (474.9 KB)Figure S1. The calibration curve of dextrans by HPLC on a TSK-gel G4000 PW<sub>XL</sub> column (7.8 mm I.D. × 300 mm) eluted with 0.1 M Na<sub>2</sub>SO<sub>4</sub> solved in PBS (pH = 6.8) at a flow rate of 1.0 mL/min.
Download TIFF Image (113.9 KB)Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 31501441) and supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Lahaye M. Marine algae as sources of fibers: determination of soluble and insoluble dietary fiber contents in some ‘sea vegetables’. J Sci Food Agric. 1991;54:587–594.
- Mabeau S, Fleurence J. Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci Tech. 1993;4:103–107.
- Kim KT, Rioux LE, Turgeon SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27–33.
- Gupta S, Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Tech. 2011;22:315–326.
- Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–597.
- Lakshmana Senthil S, Vinoth Kumar T, Geetharamani D, et al. Fucoidan–an α-amylase inhibitor from Sargassum wightii with relevance to NIDDM. Int J Biol Macromol. 2015;81:644–647.
- Cho ML, Han JH, You SG. Inhibitory effects of fucan sulfates on enzymatic hydrolysis of starch. LWT-Food Sci Technol. 2011;44:1164–1171.
- Vinoth Kumar T, Lakshmana Senthil S, Geetharamani D, et al. Fucoidan–a α-D-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int J Biol Macromol. 2015;72:1044–1047.
- Okimura T, Jiang Z, Liang Y, et al. Suppressive effect of ascophyllan HS on postprandial blood sugar level through the inhibition of α-glucosidase and stimulation of glucagon-like peptide-1 (GLP-1) secretion. Int J Biol Macromol. 2019;125:453–458.
- Geng P, Bai G. Two novel aminooligosaccharides isolated from the culture of Streptomyces coelicoflavus ZG0656 as potent inhibitors of α-amylase. Carbohydr Res. 2008;343:470–476.
- Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years. Diabetes Care. 1999;22:960–964.
- Su CH, Hsu CH, Ng LT. Inhibitory potential of fatty acids on key enzymes related to type 2 diabetes. Biofactors. 2013;39:415–421.
- Dehghan-kooshkghazi M, Mathers JC. Starch digestion, large-bowel fermentation and intestinal mucosal cell proliferation in rats treated with the alpha-glucosidase inhibitor acarbose. Br J Nutr. 2004;91:357–365.
- Wang WJ, Zhu JY, Xu P, et al. Characterization of the life history of Bangia fuscopurpurea, (Bangiaceae, rhodophyta) in connection with its cultivation in china. Aquaculture. 2008;278:101–109.
- Zheng J, Gao Y, Wang W, et al. Separation of multiple-phycobiliproteins and its subunits from Bangia fusco-purpurea. J Fishery Sci China. 2003;10:277–281.
- Fu X, Zhou Q, Weng L, et al. Purification and characterization of phycoerythrin from Bangia fusco-purpurea. Food Sci. 2006;8:845–851.
- Jiang Z, Hama Y, Yamaguchi K, et al. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J Biochem. 2012;151:65–74.
- Chen R, Liu Z, Zhao J, et al. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127:434–440.
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356.
- Sawant SS, Salunke BK, Kim BS. A rapid, sensitive, simple plate assay for detection of microbial alginate lyase activity. Enzyme Microb Technol. 2015;77:8–13.
- Huang W, Deng H, Jin S, et al. The isolation, structural characterization and anti-osteosarcoma activity of a water soluble polysaccharide from Agrimonia pilosa. Carbohydr Polym. 2018;187:19–25.
- Wang HX, Zhao J, Li DM, et al. Structural investigation of a uronic acid-containing polysaccharide from abalone by graded acid hydrolysis followed by PMP-HPLC-MSn, and NMR analysis. Carbohydr Res. 2015;402:95–101.
- Knutson CA, Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968;24:470–481.
- Yaphe W, Arsenault GP. Improved resorcinol reagent for the determination of fructose, and of 3,6-anhydrogalactose in polysaccharides. Anal Biochem. 1965;13:143–148.
- Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962;84:106–110.
- Schanderl SH. Tannins and related phenolics. In: Joslyn MA, editor. Methods for Analysis. New York (NY): Academic Press; 1970. p. 701–724.
- Teixeira R, Sant’Ana Da Silva A, Ferreira-Leitão VS, et al. Amino acids interference on the quantification of reducing sugars by the 3,5-dinitrosalicylic acid assay mislead carbohydrase activity measurements. Carbohydr Res. 2012;363:33–37.
- Kalita D, Holm D, LaBarbera D, et al. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS One. 2018;13:e0191025.
- Wang S, Dong S, Zhang R, et al. Effects of proanthocyanidins on porcine pancreatic lipase: conformation, activity, kinetics and thermodynamics. Process Biochem. 2014;49:237–243.
- Wang S, Sun Z, Dong S, et al. Molecular interactions between (-)-epigallocatechin gallate analogs and pancreatic lipase. PLoS One. 2014;9:e111143.
- Puzer L, Barros NM, Oliveira V, et al. Defining the substrate specificity of mouse cathepsin P. Arch Biochem Biophys. 2005;435:190–196.
- de Andradea SA, Pedrosaa MF, de Andradea RG, et al. Conformational changes of Loxosceles venom sphingomyelinases monitored by circular dichroism. Biochem Biophys Res Commun. 2005;327:117–123.
- Barros FC, Da Silva DC, Sombra VG, et al. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr Polym. 2013;92:598–603.
- Yu P, Sun H. Purification of a fucoidan from kelp polysaccharide and its inhibitory kinetics for tyrosinase. Carbohydr Polym. 2014;99:278–283.
- Malagoli BG, Cardozo FT, Gomes JH, et al. Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnion muelleri. Int J Biol Macromol. 2014;66:332–337.
- Bilan MI, Grachev AA, Ustuzhanina NE, et al. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag.. Carbohydr Res. 2002;337:719–730.
- Jiao G, Yu G, Zhang J, et al. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–223.
- Wu YC, Ho HK. Studies of water-soluble polysaccharides from red seaweeds (Pterocladia Tenuis Okam. and Bangia Fusco-Purpurea Lyngb). J Chin Chem Soc. 1959;6:84–94.
- Usov AI. Polysaccharides of the red algae. Adv Carbohydr Chem Biochem. 2011;65:115–217.
- Morrice LM, McLean MW, Long WF, et al. Porphyran primary structure. Hydrobiologia. 1984;116:572–575.
- Gretz MR, Sommerfeld MR, Aronson JM. Cell wall composition of the generic phase of Bangia atropurpurea (Rhodophyta). Bot Mar. 1982;25:529–536.
- Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40R.
- Mabeau S, Kloareg B, Joseleau JP. Fractionation and analysis of fucans from brown algae. Phytochemistry. 1990;29:2441–2445.
- Huang YL, Chow CJ, Tsai YH. Composition, characteristics, and in vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem. 2012;134:1967–1972.
- Xu J, Nie X, Hong Y, et al. Synthesis of water soluble glycosides of pentacyclic dihydroxytriterpene carboxylic acids as inhibitors of α-glucosidase. Carbohydr Res. 2016;424:42–53.
- Chai J, Xu Q, Dai J, et al. Investigation on potential enzyme toxicity of clenbuterol to trypsin. Spectrochim Acta A. 2013;105:200–206.
- Eftink MR. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys J. 1994;66:482–501.
- Papadopoulou A, Green RJ, Frazier RA. Interaction of flavonoids with bovine serum albumin: a fluorescence quenching study. J Agric Food Chem. 2005;53:158–163.
