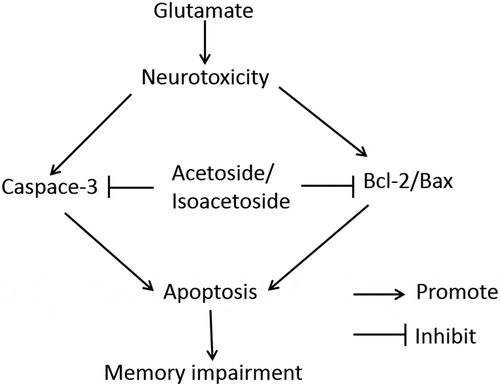
ABSTRACT
Exposure of PC12 cells to 10 mM glutamate caused significant viability loss, cell apoptosis, decreased activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) as well as increased levels of malondialdehyde (MDA). In parallel, glutamate significantly increased the intracellular levels of ROS and intracellular calcium. However, pretreatment of the cells with acteoside and isoacteoside significantly suppressed glutamate-induced cellular events. Moreover, acteoside and isoacteoside reduced the glutamate-induced increase of caspase-3 activity and also ameliorated the glutamate-induced Bcl-2/Bax ratio reduction in PC12 cells. Furthermore, acteoside and isoacteoside significantly inhibited glutamate-induced DNA damage. In the mouse model, acteoside significantly attenuated cognitive deficits in the Y maze test and attenuated neuronal damage of the hippocampal CA1 regions induced by glutamate. These data indicated that acteoside and isoacteoside play neuroprotective effects through anti-oxidative stress, anti-apoptosis, and maintenance of steady intracellular calcium.
Graphic Abstract
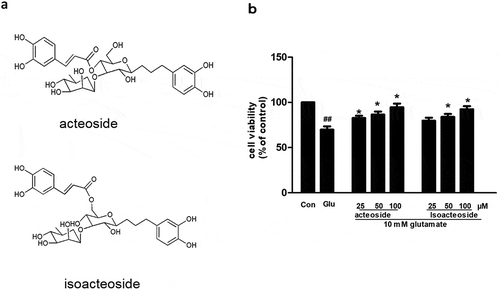
Protective effect of acteoside and isoacteoside on glutamate-induced cytotoxicity in PC12 cells.
Neurodegenerative diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD) and Huntington’s diseases (HD), are progressive neurological disorders characterized by typical protein assemblies and cell death or apoptosis [Citation1,Citation2]. Glutamate is the principal excitatory neurotransmitter in the nervous system and its concentration is strictly controlled in the brain. However, excessive release of glutamate into extracellular spaces leads to excitotoxic neuronal damages [Citation3,Citation4]. Excitotoxicity induced by excessive activation of glutamate receptors has been suggested to underlie the neuronal death in stroke, traumatic brain injury and neurodegenerative disorders [Citation5].
Recently, an increasing number of studies have found that mitochondria, organelles that are vitally important for controlling cell life and death, are involved in glutamate-induced excitotoxicity because they possess a large capacity for calcium uptake in response to pathological conditions, finally resulting in mitochondrial Ca2+ overload. Mitochondrial Ca2+ overload may activate neuronal cell death through the release of pro-apoptotic factors and increased generation of reactive oxygen species (ROS) [Citation6,Citation7]. The main mechanisms of glutamate-induced neuronal toxicity are associated with excessive influx of calcium and formation of ROS, induction of apoptosis, mitochondrial dysfunction and translocation of apoptosis-inducing factor (AIF) from the mitochondria to the cytosol and nucleus [Citation8].
Acteoside and isoacteoside are phenylethanoid glycosides extracted from a traditional herbal medicine Monochasma savatieri Franch ()). Previous studies have shown that both acteoside and isoacteoside have antioxidative effects and may be promising therapeutic agents against neurodegenerative diseases [Citation9]. Recently, acteoside and isoacteosdie were found to be neuroprotective in amyloid β induced cytotoxicity and cognition impairment induced by amyloid-β [Citation10]. Acteoside and isoacteoside are thought to attenuate glutamate-induced calcium influx [Citation11]. However, the detailed mechanism remains to be further studied. In the present study, we used an in vitro model of glutamate-induced excitotoxicity in PC12 cells which a good cell line for studying of glutamate-induced neurotoxicity [Citation12] and an in vivo dementia model of perinatal glutamate exposure [Citation13] to investigate the neuroprotective functions of acteoside and isoacteoside and the possible mechanisms.
Figure 1. Protective effect of acteoside and isoacteoside on glutamate-induced cytotoxicity in PC12 cells. (a) Chemical structure of acteoside and isoacteoside. (b) Effects of acteoside and isoacteoside on the cell viability measured by MTT assay. Values represent mean ± SD of 3 independent experiments. #p < 0.05 vs control, *p < 0.05, **p < 0.01 vs glutamate group.
Materials and methods
Chemicals and reagents
L-monosodium glutamate (MSG), 20,7-dichlorofluorescein diacetate (DCFH-DA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Hoechst33342, Annexin V-FITC/PI apoptosis detection kit were purchased from Nanjing KeyGen BioTech. Co. Ltd. (China). The kits for determination of glutathione peroxidase (GSH-Px), MDA, and SOD were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Acteoside and isoacteoside were provided by the department of natural medicinal chemistry in Soochow University, with a high purity (more than 99% at HPLC chromatogram), as evidenced by NMR analysis carried out according to the method previously described [Citation14]. All other chemicals used were of the highest grade commercially available.
Cell culture and treatments
The rat adrenal pheochromocytoma cell line PC12 was obtained from Cell Bank of Shanghai Institute of Cell Biology (Shanghai, China), maintained in DMEM plus 5% fetal bovine serum and 10% heat-inactivated horse serum. All cell lines were cultured with 100 U/mL penicillin, 100 μg/mL streptomycin at 37°C with 5% CO2. Cells in logarithmic growth phase were used for further experiments. Adherent cells were detached by trypsin at 70–80% confluence and plated onto poly-d-lysine coated 96-well plates for the measurements of cell viability, six-well plates for the measurements of apoptosis and protein expression, coverslips for the measurements of intracellular Ca2+ concentration ([Ca2+]i). Samples were dissolved in culture medium without serum to make a stock solution of 1 mM, and cells at the confluence of 70–80% were treated with vehicle, glutamate (10 mM for 24 h), acteoside (or isoacteoside, for 24 h prior to glutamate) plus glutamate (10 mM for 24 h), respectively. The concentration of acteoside and isoacteoside changed as 25, 50 and 100 µM, according to our preliminary data.
Cell viability assessment
The viability of PC12 cells was measured by a colorimetric MTT assay, according to previous study [Citation15]. The concentration of glutamate used to treat PC12 cells was 10 mM according to the preliminary experiment. PC12 cells were incubated with various concentrations of acteoside and isoacteoside (25, 50 and 100 µM) for 24 h prior to treatment with glutamate. Then, PC12 cells were lysed in DMSO after incubation with MTT (0.5 mg/mL) for 4 h and the amount of MTT formazan was quantified by determining the absorbance at a wavelength of 490nm, using a microplate reader (ELx800; Bio-Tek, Winooski, VT, USA). Each experiment was performed at least three times.
Measurement of cell apoptosis
Cell apoptosis was measured by Hoechst 33342 staining after glutamate or acteoside treatment for the chromosomal condensation and morphological changes. PC12 cells were plated in six-well plates for 24 h and treated with glutamate and acteoside, the cells were treated with Hoechst 33342 for 15 min at 37°C, followed by rinsing with PBS for 3 times. Finally, the cells were rinsed and detected under an inverted microscope (Nikon, Japan).
Flow cytometry was performed using the Annexin V-FITC/PI Apoptosis Detection kit, according to the manufacturer’s instructions. PC12 cells were seeded in six-well plates (1 × 105cells/well), treated with glutamate and/or acteoside/isoacteoside (as above) for 24 h, collected by trypsinization and washed once with PBS (pH 7.4). After centrifugation, the cells were stained with annexin-V and propidiun iodide (PI) using the annexin V-FITC apoptosis detection kit for 15 min at room temperature, followed by translocation of phosphatidylserine (PS) from inner to outer leaflets of the plasma membrane. Then, the samples were immediately analyzed using FC500 flow cytometry (Beckman coulter Corporation, USA) with at least 10,000 events.
Detection of DNA fragment
The cells treated with glutamate and/or acteoside/isoacteoside were collected under the sterile condition and the fragmented DNA was isolated using a DNA Extraction kit (Beyotime Biotechnology, China) according to manufacturer instructions. DNA extracts were electrophoresed on 1.5% agarose gel containing ethidium bromide at 55 V/cm for 45 min. Ladder formation of oligonucleosomal DNA was detected under ultraviolet light.
Measurement of MDA content and antioxidant enzyme activities
After the treatments as described above, the cells were washed two times with PBS, disrupted with an ultrasonic disrupter and centrifuged. MDA, GSH, and SOD were measured as described by the manufacturer’s instructions.
Analysis of intracellular ROS
Intracellular formation of ROS was detected using the oxidation-sensitive dye DCFH-DA, as described previously [Citation16]. PC12 cells were seeded at 1 × 105 cells/well in six-well plates for 24 h and treated with various concentrations of acteoside and isoacteoside incubated for 1 h, followed by treatment with 10 mM glutamate for 6 h. After 30 min incubation at 37°C in dark, the cells were washed with PBS, centrifuged at 1200 r/min for 5 min, followed by addition of 0.5 mL PBS. Then, oxidation of DCFH in the presence of ROS was read at excitation wavelength of 488 nm and emission wavelength of 525 nm using flow cytometer. Data were analyzed and expressed as a percentage of the control and the experiment was repeated three times.
Measurement of intracellular calcium concentration
The PC12 cells were cultured at 1 × 105 cells/well in six-well plates in the presence or absence of glutamate and acteoside, for 24 h and then incubated with 5 μmol/L Fluo-3 AM (Beyotime Ins. Bio, China) at 37°C for 30 min. Subsequently, the mixed solution was removed and cells were washed twice with PBS. Fluorescent changes in response to [Ca2+]i levels were recorded at excitation wavelength of 488 nm and emission wavelength of 525 nm at 37°C with FC500 flow cytometry (Beckman Coulter Corporation, USA). Meanwhile, four fields in each group were randomly selected and the mean of fluorescence intensity was obtained and calculated for statistical analysis.
Western blot analysis
After treatment, the PC12cells were lysed in a buffer (50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% Triton X-100, and a protease inhibitor mixture). The sample was centrifuged at 10,000 × g at 4°C for 10 min and the supernatant was preserved at −80°C. Protein samples were analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis and incubated with antibodies against Bcl-2, Bax, Caspase-3, β-actin (1:500, Cell Signaling Technologies, Beverly, MA) at 4°C overnight. The membranes were washed and incubated with anti-mouse IgG (H + L) HSA, dyLight 680 labeled second antibody or anti-rabbit IgG (H + L), dyLight 800 labeled second antibody (Kirkegaard & Perry Laboratories, Inc., USA) in TBST containing 3% non-fat dry milk for 2 h. The signal intensity of primary antibody binding was quantitatively scanned and analyzed with dyssey two-color-infrared imaging system.
Behavioral study
Institute of Cancer Research (ICR) mice (male, 4–5 weeks, 18–22 g) were purchased from the Experimental Animal Center of Soochow University and housed in the animal room with temperature of 22 ± 2°C, 12/12 light/dark cycle and humidity of 50 ± 10%. Care and handling of these animals were approved by the Institutional Animal Care and Use Committee of Soochow University and were in accordance with the national guidelines for laboratory animal care. After several days of habituation, the mice were randomly divided into four groups: control group, glutamate group, and glutamate + acteoside group. The animals in glutamate group were treated with glutamate (6.0 g/kg/d, i.g.) for 10 days while animals in glutamate + acteoside group were treated with acteoside (100 or 200 mg/kg/d, i.g.) for 7 days prior to glutamate treatment (6.0 g/kg/d, i.g.) for 10 days. The animals in control group received same dose of saline (i.g.) for 10 days. On the 18th day, all the animals were tested with Y-maze test for three days. After behavior test, the CA1 area of the dorsal hippocampus was taken out under anesthesia with chloral hydrate (i.p., 0.4%, 0.1 mL/10 g), then three samples were randomly selected from each group.
To evaluate the non-spatial learning and memory effects of acteoside, step-through-type passive avoidance tests were performed three days after the last drug treatment, using the Y-maze test system. The apparatus (MG-3, Zhang jia gang biomedical Instrument Factory, China) consisted of a Y-shaped maze with three white, opaque plastic arms (length 35, width 5, wall height 10; fitted with a 15W signal lamp) at a 120° angle from each other and a wire mesh floor. The stimulator turned one of the lights on while the other two were off. The bright arm was a safe district, and the two dark arms provided 50 V (could be accommodated) pulse electric stimulation. The tests consisted of acquisition and memory retention sessions. Before the formal test, all mice performed a training trial in which they were alternately habituated to the light and the dark chambers for 3 min.
In the learning test, the mouse was placed in the light part and inescapable electric shock was provided (50 V, delay 5 s) when the hind paws of the mouse were completely placed in the dark part. Right reflection was defined as the correct running to the safe area within 10 s after the muse was shocked, and the study ability was determined according to the correct rate of 90% or above of arrival at the safe area in 20 times.
The consolidation of memory retention test was performed 24 h or 48 h after the single training trial. The mouse was again placed in the light part and the method employed was identical to those of the acquisition trial. The numbers of times the mice stepped into the illuminated chamber (rights) were recorded within 20 times to evaluate the memory retention abilities of the mice.
Hippocampal tissue were detected by HE staining
The animals (n = 6 mice/group) were deeply anesthetized with chloral hydrate (i.p., 0.4%, 0.1 mL/10 g) and perfused with ice-cold 0.9% saline solution. The brains were post-fixed in 4% paraformaldehyde (PFA) overnight at 4°C, dehydrated in 30% sucrose in 0.1 M in phosphate-buffered saline (PBS, pH 7.2) until sank to the container bottom and embedded in paraffin. Coronal hippocampal sections were cut into 4 µm of thickness with a freezing microtome, stained with HE for 8 min and 1 min, respectively, and dehydrated again with a series concentrations of ethanol and mounted with coverslip. Moreover, some hippocampal tissues were stained with 1% toluidine blue (Nissl staining) to observe the distribution of neurons in hippocampal CA1 area in the same way.
Transmission electron microscopy
For transmission electron microscopy (TEM), the CA1 area of hippocampal tissue were cut into pieces (0.5–1 mm) and fixed in 2.5% (v/v) glutaraldehyde polyoxymethylene solution for 6–8 h at 4°C. The tissues were washed and post-fixed in 1% OsO4 for 1 h at 4°C, dehydrated through ascending grades of acetone and embedded in araldite CY212. Then, the samples were dropped onto carbon-coated copper grids (200 mesh) and stained with uranyl acetate and alkaline lead citrate. Sections were visualized under a Hitachi H-600 transmission electron microscope.
Statistical analysis
All statistical analyses were performed using SPSS16.0 and data are presented as mean ± SD. Analysis of variance (ANOVA) accompanied with Tukey’s tests was performed and p < 0.05 was considered as the significance level for all analyses.
Results
Effect of acteoside/isoacteoside on glutamate-induced cytotoxicity
As shown in , the viability of PC12 cells was significantly decreased by glutamate treatment at concentration of 10 mM for 24 h (p < 0.05; )), when compared with control group. Pretreatment of the cells with different concentration of acteoside (25–100 μM) prevented the decrease of cell viability induced by exposure to glutamate; there was significant difference between glutamate group and acteoside group (p < 0.05, )) but no significant difference between control group and acteoside group. Pretreatment of the cells with isoacteoside at 50 and 100 μM prevented the decrease of cell viability induced by exposure to glutamate; there was significant difference between control group and isoacteoside group (p < 0.05, )) but no significant difference between control group and isoacteoside group. However, 25 μM isoacteoside had no significant effect on the decrease of viability induced by glutamate (p > 0.05).
Effect of acteoside/isoacteoside on glutamate-induced cell apoptosis
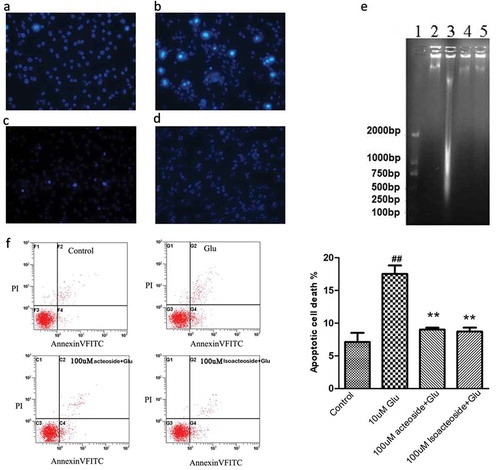
We first used Hoechst 33342 staining and flow cytometry to examine the effects of acteoside and isoacteoside on cell apoptosis induced by glutamate. The Hoechst33342 staining indicated that 10 mM glutamate-induced significant DNA degeneration and that this effect was prevented by pretreatment of cells with acteoside and isoacteoside (100 µM; ). Meanwhile, Annexin V and PI double staining were applied to further verify anti-apoptotic effects of acteoside and isoacteoside. When cells were incubated with 10 mM glutamate for 24 h, the percentage of Annexin V-FITC+ apoptotic cells was observed to increase from 6.84% ± 1.96% for control group to 17.95% ± 2.07% for glutamate group (p < 0.05, )). However, when cells were pre-treated with acteoside or isoacteoside prior to glutamate treatment for 24 h, the percentages of apoptotic cells was 8.96% ± 0.31% for acteoside and 6.92% ± 1.28% for isoacteoside, respectively ()). There was significant difference between glutamate group and acteoside/isoacteoside group (p < 0.05) while there was no significant difference between control group and acteoside/isoacteoside group.
Figure 2. Effects of acteoside and isoacteoside on glutamate-induced apoptosis in PC12 cells. Cells were incubated with indicated concentrations of acteoside and isoacteoside for 12 h before exposure to 10 mM glutamate for 24 h. (a-d), Nuclear morphological changes in the cells were observed after Hoechst 33342 staining in control (a), glutamate (b), glutamate+acteoside (c) and glutamate+isoacteoside (d) groups. (e), DNA degradation with agarose gel electrophoresis analysis. Lane 1: DNA marker; Lane 2: normal; Lane 3: glutamate; Lane 4, 5: pre-incubated 12 h with 100 μM acteoside and isoacteoside and then treated with glutamate for 24 h. (f), Representative patterns of flow cytometric distribution of PC12 cells after Annexin V-PI double staining and the quantitative analysis of apoptotic cells. ##p < 0.01 vs the control group. **p < 0.01 vs glutamate group.
Effect of acteoside/isoacteoside on the activities of antioxidant enzyme and MDA content
To further investigate the neuroprotective effects of acteoside and isoacteoside, the activities of SOD, GSH-Px, and MDA were measured by using a commercial assay kit. After incubation of cells with glutamate for 24 h, the MDA production markedly increased (137.2% of the control values). However, pretreatment with acteoside and isoacteoside (50 and 100 µM) markedly attenuated the activities of MDA induced by glutamate (29.6% and 52.0% for 50 µM acteoside and isoacteoside, respectively; 46.2% and 43.9% for 100 µM acteoside and isoacteoside, respectively). Further, exposure to 10 mM glutamate for 24 h significantly decreased the activities of SOD (73.5%) and GSH-Px (62.6%) as compared to control group. However, pretreatment with acteoside and isoacteoside (50 or 100 µM) showed a significant increasing tendency in the activities of SOD and GSH-Px when compared to cells treated with glutamate (). These results indicated that pretreatment with acteoside and isoacteoside (50–100 µM) prevent lipid peroxidation and attenuated the changes in SOD and GSH-Px activities induced by the treatment of glutamate.
Table 1. Effects of acteoside and Isoacteoside on lipid peroxidation and antioxidant enzyme activities in PC12 cells.
Effect of acteoside/isoacteoside on intracellular ROS and calcium
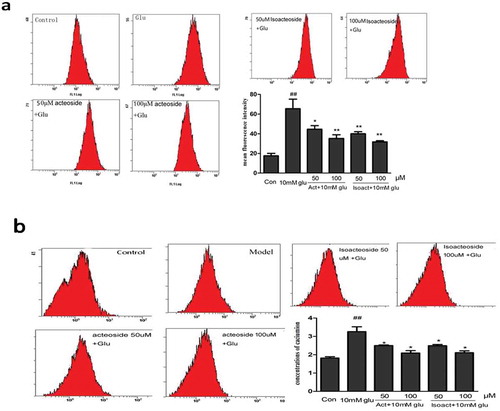
Oxidative stress and calcium overload were believed to play two distinct equally important roles in the pathogenesis of AD. In order to examine the effect of acteoside and isoacteoside in decreasing glutamate-induced ROS generation and calcium overload, and the resulting associated oxidative stress, we examined the intracellular level of ROS by adding the fluorescence DCFH-DA. Exposure of cells to 10 µM glutamate for 24 h significantly increased DCF signal as compared with the control group. However, pretreatment with acteoside/isoacteoside significantly decreased the generation and accumulation of ROS ()). Moreover, similar to the changes of ROS, the concentration of intracellular Ca2+ was enhanced by treatment of glutamate (10 mM) when compared to control group (p < 0.05), which was prevented by pretreatment with acteoside and isoacteoside (p < 0.05; )).
Figure 3. Effect of acteoside and isoacteoside on glutamate-induced changes of ROS level and concentration of Ca2+ in PC12 cells. (a), Effects of acteoside and isoacteoside on production of intracellular ROS. (b), Effect of acteoside and isoacteoside on the concentration of Ca2+ in glutamate-treated PC12 cells. Values represent mean ± SD; ##P < 0.01 vs control group; *P < 0.05, **P < 0.01 vs glutamate group.
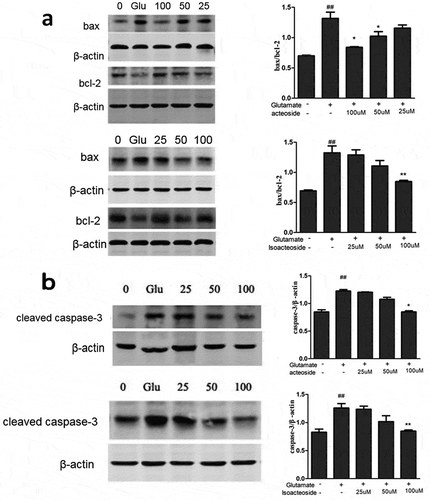
Effects of acteoside/isoacteoside on glutamate-induced changes of apoptosis-related proteins in PC12 cells
The expression of Bax, Bcl-2 and cleaved caspase-3, was detected by Western blot. The results showed that the treatment of PC12 cells with glutamate decreased the expression of Bcl-2 and simultaneously increased Bax expression. The ratio of Bax/Bcl-2 in the glutamate-treated cells markedly increased as compared with the untreated cells in control group. However, acteoside/isoacteoside (25–100 µM) significantly suppressed these changes ()). Also, glutamate caused an increase of the level of cleaved caspase-3 in PC12 cells, which was prevented by pretreatment with acteoside/isoacteoside ()).
Figure 4. Effects of acteoside and isoacteoside on the changes of protein expression induced by glutamate in PC12 cells. (a), Assessment of Bcl-2, Bax protein levels and the ratio of Bax/Bcl-2 by western blotting. (b), Relative level of activated caspase-3 subunit. ##P < 0.01 vs control; *P < 0.05, **P < 0.01 vs glutamate group.
Effect of acteoside on behavioral tests
Then, we tested the effects of acteoside on learning and memory in vivo using the glutamate-induced amnesia mode which is well known as a measure of spatial memory. After being treated with acteoside for 18 days, Y-maze test was used to test the ability of spatial acquisition of learning and memory consolidation. As shown in , the mice with intragastrical administration of glutamate (6.0 g/kg) showed significant impairment of learning capacity and memory at 24 h and 48 h (p < 0.05) when compared to mice treated with vehicle. However, pretreatment with acteoside (0.2 g/kg) significantly increased the learning capacity and memory (all p < 0.05) but lower dosage of acteoside (0.1 g/kg) only significantly increased the memory (p < 0.05).
Table 2. Protective effect of acteoside against Y-maze test in glutamate-induced amnesia.
Effect of acteoside on hippocampal morphology
As shown in ), there was no remarkable neuronal abnormality in control group, CA1 pyramidal cells were arranged neatly and tightly with no noticeable cell loss. However, obvious edema and loss of neurons in the CA1 were observed in the AD model group ()). The pyramidal layer was disordered and accompanied with neuronal loss. The changes were significantly reduced in the group treated with acteoside ().
Figure 5. Effect of acteoside on morphological changes of CA1 neuron in hippocampus. (a-d), representative images obtained by HE staining (400×) of control group (a), glutamate group (6 g/kg) (b), glutamate+ acteoside (0.1 g/kg) group (c), and glutamate+ acteoside (0.2 g/kg) group (d). (e), ultrastructural images of nuclei (upper), organells (middle) and synapse (below) in CA1 region of mice from control group, glutamate group (6 g/kg), glutamate+ acteoside (0.1 g/kg) group, and glutamate+ acteoside (0.2 g/kg) group (from left to right).
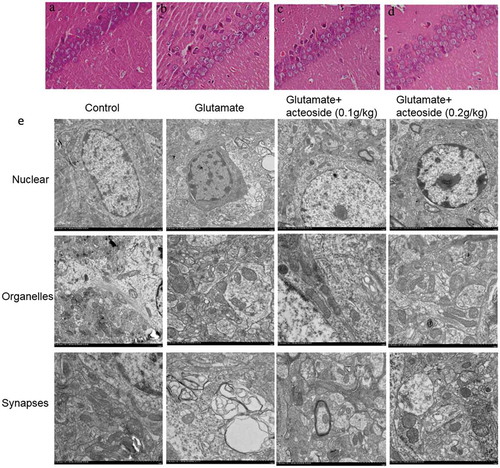
The ultrastructural changes of hippocampus were analyzed by using TEM. As shown in ), the control group showed normal neurons, nuclei, uniform chromatin distribution, abundant cytoplasm organelles, and synaptic structure and presynaptic membrane. However, in glutamate group, the neurons demonstrated varying degrees of damage, irregular nuclei, partially condensed chromatin, apoptotic nuclear marginalization, Golgi complex vesicle dilation in the perinuclear cytoplasm, decreased synaptic density, smaller unclear synaptic gap, reduced quantity of synaptic vesicles and less free ribosomes. Low dose of acteoside (0.1 g/kg) slightly improved these changes induced by glutamate, showing normal construction, clear presynaptic membrane, synaptic cleft, and mitochondrial cristae. High dose group of acteoside (0.2 g/kg) significantly improved the change of morphology, showing similar neuronal structure with the control group.
Discussion
In the present study, we demonstrate that the exposure of cultured PC12 cells to glutamate caused decrease of cell viability, apoptotic cell death, decreases of antioxidant enzymes of SOD and GSH-Px and lipid peroxide formation, disruption of morphology and cognitive behavior. All these changes induced by glutamate could be blocked by pretreatment with phenylethanoid glycosides acteoside/isoacteoside. These results showed that acteoside and isoacteoside could be useful neuroprotective agents through alleviating apoptosis mediated by oxidative stress.
More and more evidences suggest that most neurodegenerative disorders are closely correlated with glutamate neurotoxicity and oxidative stress [Citation17,Citation18]. The activation of glutamate receptors is responsible for many neural activities, including synaptic plasticity, sensation, and movement [Citation19,Citation20]. However, excessive glutamate can also cause excitotoxicity in neural cells [Citation21]. In previous study, we demonstrated that acteoside and isoacteoside have very commendable antioxidative activity, resulting from a balance of enzymatic and non-enzymatic oxygenation of free radical production, reduction of lipid peroxidation, protein oxidation and DNA damage [Citation22,Citation23]. Consistently, other experiments showed that glutamate-induced mitochondrial dysfunction as evidenced by increased ROS accumulation and up-regulation of Ca2+ concentration [Citation24,Citation25]. In the present study, we demonstrated that acteoside and isoacteoside could attenuate the apoptosis of PC12 cells induced by glutamate, suggesting attenuation of neurotoxicity by acteoside and isoacteoside.
The neuroprotection of acteoside and isoacteoside is related with antioxidative stress. Several studies have demonstrated that ROS could lead to neuronal apoptosis in neurodegenerative disorders including AD, PD, and HD [Citation26,Citation27]. The activities of the antioxidant enzymes SOD and GSH-Px were found to be significantly reduced in AD brains while MDA was elevated. The present study showed that PC12 cells exposed to 10 mM glutamate significantly decreased activities of SOD and GSH-Px, as well as increased levels of MDA production, which was blocked by acteoside and isoacteoside pretreatments. The results are consistent with previous studies showing that acteoside and isoacteoside represent effective antioxidants that inhibit the association of lipid peroxidation products [Citation16,Citation28]. Oxidative stress can elicit apoptotic death of neurons [Citation21,Citation29] and ROS may be the initiators committing neurons to apoptosis or adjuvant playing additive roles in the subsequent cascade of apoptosis. There is now considerable evidence that increased production of ROS in cells leading to oxidative stress is implicated in synaptic failure in AD. In particular, ROS-induced dysfunction of synaptic plasticity contributes to early memory loss [Citation30,Citation31]. Therefore, searching for neuroprotective drugs of natural origin against ROS-induced neuronal apoptosis has thereby attracted more attentions. The present study indicated that acteoside and isoacteoside could decrease the increase of bax/bcl-2 induced by glutamate in PC12 cells, suggesting anti-oxidative effect of these two chemicals.
The mechanism of oxidative stress-induced neuronal death and apoptosis has not been fully elucidated so far. However, dysfunction of Ca2+ homeostasis and imbalance of apoptosis-related protein expression may play a pivotal role. Several researches have pointed to the fact that glutamate could adversely affect Ca2+ homeostasis through the activation of certain receptors such as the NMDA receptors and calcium membrane channels [Citation32]. Increase of the Ca2+ concentration could lead to the formation and accumulation of ROS and vice versa, suggesting that these two key elements observed in glutamate toxicity are strongly correlated to each other [Citation33,Citation34]. In particular, given that the Ca2+ overload contributes to cell toxicity and cell death [Citation35,Citation36], our results showed that acteoside and isoacteoside markedly attenuated the elevation of Ca2+ concentration induced by glutamate, suggesting that acteoside and isoacteoside can relieve the neuronal toxicity induced by free radicals via preventing the Ca2+ overload. These results are consistent with previous studies showing that acteoside and isoacteoside could attenuate glutamate-induced calcium influx [Citation11].
We explored mouse spatial memory by analyzing the object location in the Y-maze task after glutamate treatment (i.p. for about three weeks). Glutamate-treated mice displayed impaired short-term spatial memory in the object location test, a task dependent on CA1 region of the hippocampus [Citation37], which was improved by acteoside. Consistently, there are studies verifying the effects of acteoside in improving learning and memory, using a mouse model of senescence induced by a combination of D-galactose and AlCl3 [Citation23,Citation38,Citation39]. But, this Y-maze task is not comprehensive enough to evaluate cognitive deficit in this test, it could be related to the fact that this task evaluates working memory more related to the prefrontal cortex function [Citation40,Citation41]. Our results confirmed that the mouse AD model produced cognitive deficits in the Y-maze test, demonstrated as a reduction in memory of context. Administration of acteoside attenuated cognitive impairment as evidenced by the extended latency of step-down and decreased number of errors. In addition, the ultrastructural changes analyzed by using TEM in the hippocampus of the AD model group were improved by acteoside, suggesting acteoside has neuroprotective effects.
In summary, the present study indicated that acteoside and isoacteoside could attenuate the oxidative stress and apoptosis induced by glutamate. Acteoside also attenuated the ultrastructure changes of hippocampus and improved the cognitive impairment induced by glutamate. These results suggested that acteoside and isoacteoside may be promising neuroprotective agent in treating neurodegenerative diseases.
Authors’ contributions
Yan-li Liu, Qiong-ming Xu, Shi-lin Yang, and Cheng Wang were involved in the design of the study and performed the majority of the analyses. Shi-liang Ji drafted the manuscript. Shi-liang Ji, Ke-ke Cao, Xing-xing Zhao, Nai-xin Kang, and Ying Zhang conceived and coordinated the study. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rubinsztein D. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786.
- Bredesen D, Rao R, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802.
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695.
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381.
- Lai T, Zhang S, Wang Y. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188.
- Nicholls D. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta. 2009;1787:1416–1424.
- Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. Febs J. 2010;277:3622–3636.
- Zhang Y, Bhavnani B. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49.
- Chou YS, Ho YL, Ding CW, et al. New antioxidant phenylethanol glycosides from Torenia concolor. J Asian Nat Prod Res. 2009;11:110–115.
- Shen C, Ding Y, Tang J, et al. An ameliorated prediction of drug-target interactions based on multi-scale discrete wavelet transform and network features. Int J Mol Sci. 2017;18(8):pii: E1781.
- Koo KA1, Kim SH, Oh TH, et al. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 2006;79(7):709–716.
- Kritis AA, Stamoula EG, Paniskaki KA, et al. Researching glutamate - induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci. 2015;9:91.
- Ishikawa K, Kubo T, Shibanoki S, et al. Hippocampal degeneration inducing impairment of learning in rats: model of dementia? Behav Brain Res. 1997;83:39–44.
- Shi M, He W, Liu Y, et al. Protective effect of total phenylethanoid glycosides from Monochasma savatieri Franch on myocardial ischemia injury. Phytomedicine. 2013;20:1251–1255.
- Sun Z, Zhang L, Zhu S, et al. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci Bull. 2010;26:8–16.
- Wang H, Xu Y, Yan J, et al. Acteoside protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell injury. Brain Res. 2009;1283:139–147.
- Kim HN, Jang JY, Choi BT. A single fraction fromUncaria sinensisexerts neuroprotective effects against glutamate-induced neurotoxicity in primary cultured cortical neurons. Anat Cell Biol. 2015;48:95.
- Cassano T, Pace L, Bedse G, et al. Glutamate and mitochondria: two prominent players in the oxidative stress-induced neurodegeneration. Curr Alzheimer Res. 2016;13:185–197.
- Lewerenz J, Maher P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front Neurosci. 2015;9:469.
- Lisman J. Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philos Trans R Soc Lond, B, Biol Sci. 2017;372.
- Prentice H, Modi JP, Wu J-Y. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:1–7.
- Di Giancamillo A, Rossi R, Pastorelli G, et al. The effects of dietary verbascoside on blood and liver oxidative stress status induced by a high n-6 polyunsaturated fatty acids diet in piglets. J Anim Sci. 2015;93:2849–2859.
- Xiong L, Mao S, Lu B, et al. Osmanthus fragrans flower extract and acteoside protect against d-galactose-induced aging in an ICR mouse model. J Med Food. 2016;19:54–61.
- Wang R, Reddy P. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57:1041–1048.
- Wei Y, Liu D, Zheng Y, et al. Neuroprotective effects of kinetin against glutamate-induced oxidative cytotoxicity in HT22 cells: involvement of Nrf2 and heme oxygenase-1. Neurotox Res. 2018;33:725–737.
- Kallarackal AJ, Kvarta MD, Cammarata E, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci. 2013;33:15669–15674.
- Kim GH, Kim JE, Rhie SJ, et al. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24:325–340.
- Davinelli S, Sapere N, Zella D, et al. Pleiotropic protective effects of phytochemicals in Alzheimer’s disease. Oxid Med Cell Longev. 2012;2012:386527.
- Annunziato L, Amoroso S, Pannaccione A, et al. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett. 2003;139:125–133.
- Netto MB, de Oliveira Junior AN, Goldim M, et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun. 2018;73:661–669.
- Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57:1105–1121.
- Baptiste D, Hartwick A, Jollimore C, et al. An investigation of the neuroprotective effects of tetracycline derivatives in experimental models of retinal cell death. Mol Pharmacol. 2004;66:1113–1122.
- Sousa S, Maciel E, Vercesi A, et al. Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett. 2003;543:179–183.
- Zhang Y, Soboloff J, Zhu Z, et al. Inhibition of Ca2+ influx is required for mitochondrial reactive oxygen species-induced endoplasmic reticulum Ca2+ depletion and cell death in leukemia cells. Mol Pharmacol. 2006;70:1424–1434.
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565.
- Maher P, van Leyen K, Dey PN, et al. The role of Ca(2+) in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium. 2018;70:47–55.
- Si D, Yang P, Jiang R, et al. Improved cognitive outcome after progesterone administration is associated with protecting hippocampal neurons from secondary damage studied in vitro and in vivo. Behav Brain Res. 2014;264:135–142.
- Peng X, Gao L, Huo S, et al. The mechanism of memory enhancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of D-gal and AlCl3. Phytother Res. 2015;29:1137–1144.
- Li H, Song J, Zhang J, et al. Ginseng protein reverses amyloid beta peptide and H2 O2 cytotoxicity in neurons, and ameliorates cognitive impairment in AD rats induced by a combination of D-galactose and AlCl3. Phytother Res. 2017;31:284–295.
- Heo HJ, Kim MJ, Lee JM, et al. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement Geriatr Cogn Disord. 2004;17:151–157.
- Ha JS, Jin DE, Park SK, et al. Antiamnesic effect of actinidia arguta extract intake in a mouse model of TMT-induced learning and memory dysfunction. Evid Based Complement Alternat Med. 2015;2015:876484.
