ABSTRACT
An irregular C11 homoterpene, (E)-4,8-dimethylnona-1,3,7-triene (DMNT) was identified as a major component of the volatile compounds emitted from Basella alba leaves induced by herbivore. The terpenes including DMNT were not detected from the leaves infected by Botrytis cinerea. These results suggested that volatile emission from B. alba leaves was induced by herbivory but not by a fungal infection.
Volatile organic compound emitted by plants mediate a variety of interactions between plants and insects. In response to herbivory attack, plants produce volatile compounds which act to repel pest and attract their natural enemies. Damaged plants also produce volatiles that warns other plants of impending danger so that they can prepare themselves.
Basella alba, known as Malabar spinach, is often less damaged by insect pests. However, its chemical nature has not yet been elucidated in detail. We showed flavonoids in B. alba leaves inhibited the growth of Spodoptera litura larvae in the previous study [Citation1]. Plant can protect themselves from herbivore feeding by producing volatile as well as nonvolatile compounds. Thus, we examined volatile compounds emitted from B. alba leaves by using the solid phase absorbent Mono Trap DCC18 (GL Science Inc.).
shows that leaves damaged by S. litura larvae produced three volatile compounds (1–3), while the leaves without damage emitted very little. The relative content of each volatile compound was calculated by a ratio of the peak area of each component to total area of all volatile compound peaks in TIC. The major component of the volatiles was identified as an irregular C11 homoterpene (E)-4,8-dimethylnona-1,3,7-triene (DMNT, 3, relative content 87.21%). The second most abundant was linalool (2, 10.83%), followed by (Z)-3-hexenyl acetate (1, 1.95%). As shown in , mechanically damaged leaves also produced DMNT, but its amount was much lower than that produced by insect herbivory. Infection with Botrytis cinerea did not induce any volatile emission.
Figure 1. GC/MS chromatograms of volatile compounds emitted from undamaged leaves of B. alba and damaged leaves by S. litura larvae.
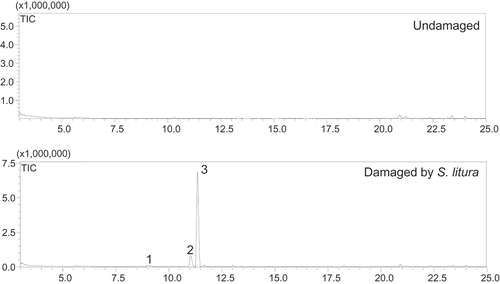
Table 1. Volatile compounds emitted from B. alba (mean±SEM, n= 5).
DMNT and linalool have been implicated in attracting natural enemies of insect herbivores when emitted from damage leaves. DMNT was found to be attractive to the predatory mite Phytoseiulus persimilis [Citation2,Citation3]. Linalool was shown to be effective in attracting female parasitic wasps Cotesia marginiventris [Citation4]. DMNT emission has been reported from numerous angiosperms, including maize [Citation5], potato [Citation6], apple [Citation7], rice [Citation8] and Medicago [Citation9,Citation10], but it is rare that relative content of DMNT is more than 80% of total volatile compounds like B. alba.
Plants also produce a blend of volatile compounds in response to pathogenic fungi [Citation11–Citation13]. In this study, no volatile compound was detected from B. alba leaves infected with B. cinerea. Volatile emission from B. alba leaves was induced by herbivory but not by a fungal infection. Herbivore-induced volatile emission has been reported to be mediated by insect-derived cues from saliva or regurgitant [Citation14,Citation15]. Further investigation is necessary to know what extent the volatile emission induced by herbivory contributes to B. alba defense to herbivore.
Plant material and volatile induction. B. alba plants were grown on soil containing fertilizer in pots outside. After plants were grown up to approximately 1 m tall, young leaves were detached for experiments. B. alba can be propagated from cuttings and detached leaves usually grow. Five third instar larvae of S. litura and a detached leaf with wet cotton wool were placed in a plastic petri dish for a night. Then, leaves are placed in a vial of water. A Monotrap DCC18 disc was hung in a glass vial using a thread and the vial was sealed with a screw cap at 28°C for 24 h. The trap was then transferred into another vial, and the trapped volatiles were extracted with 200 μL of dichloromethane for 24 h. The extracts were subjected to GC/MS analysis. To produce mechanical damage, leaves were cut with scissors (1 cm cuts) along the long axis of the leaf. B. cinerea was cultured on potato dextrose agar (PDA) incubated at 25°C. A 1 cm-square mycelial agar of B. cinerea taken from 7 d-old cultures on PDA was placed in the middle of the leaf and incubated for a week at 28°C. After removing the agar, the leaf was placed in a vial with a Monotrap for 24 h.
GC/MS condition. Volatiles were analyzed by a GC/MS-QP2010 (Shimadzu, Kyoto) equipped with Rtx-5MS (30 m × 0.25 mm i.d., 0.25 μm film thickness; Shimadzu GLC, Tokyo). Helium was used as the carrier gas at a flow rate of 1 mL/min, with a split-less mode. The oven temperature program was as follow: 60°C for 5 min, rate 10°C/min to 290°C, rate 15°C/min to 320°C, and final 1 min hold. Electron impact ionization was employed for the mass spectrometry at 70 eV. The injector and the ion source temperatures were set at 230°C and 200°C, respectively. GC/MS data were processed using GC/MS solution (Shimadzu), with reference to an MS database (NIST 11 library).
Volatile identification. All volatile compounds were identified by GC/MS by using authentic compounds commercially available or prepared by synthesis. Linalool and (Z)-3-hexenyl acetate were purchased from Tokyo Chemical Industry (Tokyo). DMNT was prepared as described in Organic Syntheses procedure with a modification [Citation16]. Geraniol was oxidized with activated MnO2 to geranial, which was converted to DMNT with the Wittig reagent methyltriphenylphosphonium iodide.
Author Contribution
T. A. designed the research. T. A. and A. T. performed the experiments with the aid of T. A. and T. M. T. A. wrote the paper.
Acknowledgments
We thank Dr. K Izumitsu, The University of Shiga Prefecture, for providing the strain of B. cinerea.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aboshi T, Ishiguri S, Shiono Y, et al. Flavonoid glycosides in Malabar spinach Basella alba inhibit the growth of Spodoptera litura larvae. Biosci Biotechnol Biochem. 2017;82:9–14.
- Dicke M, Van Beek TA, Posthumus MA, et al. Isolation and identification of volatile kairomone that affects acarine predatorprey interactions Involvement of host plant in its production. J Chem Ecol. 1990;16:381–396.
- De Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol. 2004;30:2215–2230.
- Yuan JS, Köllner TG, Wiggins G, et al. Elucidation of the genomic basis of indirect plant defense against insects. Plant Signal Behav. 2008; 3: 720–721.
- Turlings TCJ, Tumlinson JH, Heath RR, et al. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol. 1991;17:2235–2251.
- Bolter CJ, Dicke M, vanLoon JJA, et al. Attraction of colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J Chem Ecol. 1997;23:1003–1023.
- Bengtsson M, Bäckman AC, Liblikas I, et al. Plant odor analysis of apple: antennal response of codling moth females to apple volatiles during phenological development. J Agric Food Chem. 2001;49:3736–3741.
- Lou YG, Hua XY, Turlings TCJ, et al. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. J Chem Ecol. 2006;32:2375–2387.
- Arimura G, Garms S, Maffei M, et al. Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta 2008; 227: 453–464.
- Blackmer JL, Rodriguez-Saona C, Byers JA, et al. Behavioral response of Lygus hesperus to conspecifics and headspace volatiles of alfalfa in a Y-tube olfactometer. J Chem Ecol. 2004;30:1547–1564.
- Piesik D, Lemńczyk G, Skoczek A, et al. Fusarium infection in maize: volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. J Plant Physiol. 2011;168:1534–1542.
- Castelyn HD, Appelgryn JJ, Mafa MS, et al. Volatiles emitted by leaf rust infected wheat induce a defence response in exposed uninfected wheat seedlings. Australas. Plant Pathol. 2015; 44: 245–254.
- Patt JM, Robbins PS, Niedz R, et al. Exogenous application of the plant signalers methyl jasmonate and salicylic acid induces changes in volatile emissions from citrus foliage and influences the aggregation behavior of Asian citrus psyllid (Diaphorina citri), vector of Huanglongbing. PLoS One 2018; 13: e0193724.
- Mattiacci L, Dicke M, Posthumus MA. beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci U S A. 1995;92:2036–2040.
- Alborn T, Turlings TCJ, Jones TH, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997; 276: 945–949.
- Leopold EJ Selective hydroboration of a 1,3,7-triene: homogeraniol. Org Synth. 1986; 64: 164.
