ABSTRACT
Motile bacteria often exhibit chemotaxis toward favorable compounds. However, the diversity of bacteria that are attracted to a given substance is largely unknown. This study aimed to reveal the diversity of bacteria with natural chemotaxis towards methanol. We tried to enrich environmental chemotactic bacteria using a glass capillary that is half-filled with methanol solidified with agarose as a trap (“chemotaxis fishing”). The pilot experiment using methanol-chemotactic Methylobacterium aquaticum strain 22A enriched the cells by 46-fold. The method was then applied to bacterial suspensions from paddy water and plants. Depending on the isolation sources and the methods of motility induction, methylotrophic bacteria were enriched 1.2–330-fold. The fished isolates belong to 32 species in 18 genera, mainly containing Acinetobacter, Methylobacterium and Pseudomonas species. Our chemotaxis fishing unveiled a part of diversity of the bacteria with natural chemotaxis towards methanol.
GRAPHICAL ABSTRACT
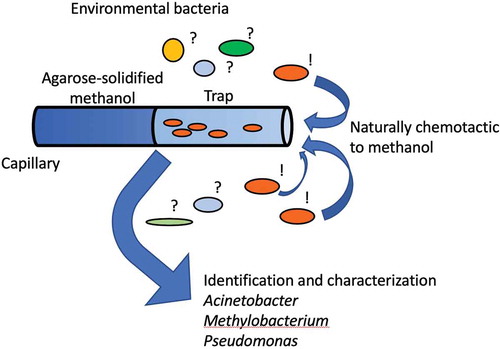
A glass capillary containing methanol was used as a trap to enrich methanol-chemotactic bacteria.
Chemotaxis is the movement of microorganisms towards higher concentrations of attractive substances [Citation1]. In bacteria, methyl-accepting chemotaxis proteins (MCPs) sense substances and transduce signals to the flagellar motor to control its direction of rotation, through a histidine kinase (CheA), a coupling protein (CheW) and a response regulator (CheY) [Citation2–Citation4]. A methyltransferase CheR and a methylesterase CheA reset the activity of MCPs. MCPs and Che components cooperatively regulate the swimming and tumbling of bacterial cells, depending on the concentration gradient of attractants, which enables bacterial chemotaxis [Citation5–Citation7].
In Escherichia coli, Tar and Tsr are major MCPs whereas Trg, Tap and Aer are minor MCPs [Citation4]. The ligand specificities of these MCPs have been extensively studied [Citation8–Citation10]. E. coli contains five MCPs and the other environmental bacteria may contain many more. For example, amongst plant-associated bacteria, Agrobacterium species have 20 to 40 MCPs and Rhizobium species have 15 to 30 MCPs, and Bradyrhizobium species have 30 to 60 MCPs [Citation11]. For example, McfR, McfS and McfQ receptors detect organic acids (succinate, malate, citrate and fumarate) in Pseudomonas putida F1 [Citation12,Citation13] and HemAT (MCP-GCS amino acids/protein) senses the O2 gradient in Halobacterium salinarum and Bacillus subtilis [Citation14]. In addition to these large numbers of MCPs, they also have multiple chemotaxis systems, which are important for both their survival and their competition for root colonization. Although the intracellular signaling domain in different MCPs is highly conserved, the extracellular domains for ligand recognition are highly variable and the ligand specificity of most MCPs is unknown. To examine this, disruption of MCP genes or the heterologous expression of MCP genes in another host and the chemotaxis of the mutants (transformants) to the given substance is often examined. Even though the number of MCPs in a genome may be limited, the substances possibly recognized by an MCP are unlimited. This is the main reason for the limited knowledge on MCP ligand specificity.
Plants emit large amounts of methanol that originated from pectin demethylation in the cell wall [Citation15,Citation16]. The surfaces of plants host various kinds of bacteria and the genus Methylobacterium is one of the ubiquitous colonizers [Citation17–Citation19]. They are facultative methylotrophs and their mutual relationship with plants can encourage plant growth [Citation20–Citation22]. M. aquaticum strain 22A was isolated from a hydroponic culture of a moss, Racomitrium japonicum, and is a potent plant growth promoter [Citation23]. We found that strain 22A exhibits strong chemotaxis to methanol. Its genome contains all the genes necessary for methylotrophy as well as 50 MCPs and at least two Che systems [Citation24]. Though MCPs for methanol have not been identified yet, they may be of primary importance for plant-symbiotic bacteria to find their hosts. We hypothesized that it is possible to “fish” the bacteria gathering towards the attractants and furthermore, we sought to examine the diversity of bacteria chemotactic towards methanol.
In this study, we demonstrated that “chemotaxis fishing” indeed works for enrichment of M. aquaticum strain 22A. We then applied this method to bacterial mixture from plants and paddy water. The isolated bacteria were characterized by whole-cell matrix laser-assisted desorption/ionization mass spectrometry (WC-MS) and the representatives were identified by 16S rRNA gene sequencing. We further characterized their methylotrophy and chemotaxis towards methanol. This paper reveals the diversity of bacteria with natural chemotaxis towards methanol.
Materials and methods
Bacterial strain and growth conditions
Methylobacterium aquaticum strain 22A (accession number: FERM-BP11078) [Citation23] was used as a model bacterium with chemotaxis towards methanol. It was cultured in mineral medium (MM) containing 0.5% methanol [Citation25] or R2A (Beckton and Dickinson and Company, Sparks, MD) overnight and cultivated at 28°C.
Sampling and motility induction
Environmental samples of plants (Conyza bonariensis and Plantago virginica) and paddy water were collected from the Institute of Plant Sciences and Resources, Okayama University campus in June 2015. Plant leaves were put in sterile 50 mL tubes containing 10 mM HEPES buffer (pH 7.0). The tubes were then vigorously shaken to release the bacterial cells. Paddy water (50 mL) was centrifuged (1000 x g for 5 min) to remove soil particles and filtered with a funnel and a filter paper (mesh size, 5 µm, No. 2 filter paper, Advantec, Tokyo, Japan). We recognized bacterial cells in the filtrate samples microscopically, but most were not motile.
To activate motility, methanol (final concentration 0.1%) or a sterile Arabidopsis thaliana Col-0 seedling were added to the cell suspension. A. thaliana seeds were sterilized and sown on 1/2 MS agar, and 3-weeks-old seedlings were used [Citation25]. The suspensions were incubated at 23°C for one night and subjected to the following experiments. These treatments activated bacterial motility. The suspension before and after induction was appropriately diluted and spread over plate media of R2A or MM containing 0.5% methanol for bacterial colony forming unit (CFU) determination.
Chemotaxis fishing
Chemotaxis fishing depends on the successful manufacture of a capillary, one side of which is filled with an attractant solidified with agarose and the other filled with water (buffer) that serves as a trap for gathering bacterial cells. The attractant solution (2% methanol) was solidified with 1.5% NuSieveTM GTGTM Agarose (Lonza Japan, Tokyo). Capillaries were made with a spirit lamp and a micropipette (100 µL, 12.5 cm × 1.2 mm, BRAND® disposable BLAUBRAND® micropipettes, Merck, Tokyo), usually approximately 7 to 10 mm (length) × 30 to 90 µm (inner dia.). The tip of a tube was put in the attractant solution warmed to 75°C. The entire tube was then put in a 5-mL HEPES buffer prepared in a 4-cm diameter glass plate. The plate was vacuumed in a desiccator for 15 min. This procedure was used to fill the trap with the buffer.
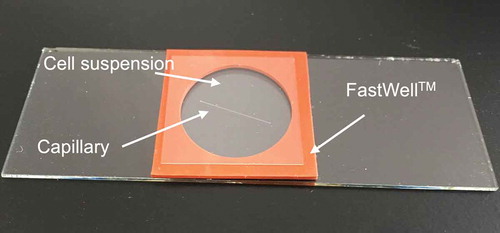
The activated cell suspension (300 µL) was put in a chamber made with FastWellTM (1 mm thick and 20 mm diameter, Grace Biolabs, Bend, OR) on a slide glass (). The capillary was soaked in the suspension and a cover slip was put on. Bacterial cells gathering into the tube were observed for 25 to 30 min microscopically. The tube was then removed with tweezers and washed with a flow of sterile water to remove adhering cells. The tube was then crushed in 100 µl HEPES buffer with a pestle in a 1.5 mL tube. Ten microliters of the suspension were spread over plate media (R2A or MM containing 0.5% methanol). The plates were incubated at 28°C for 3 to 7 days to determine CFU.
Figure 1. An experimental setup component for chemotaxis fishing.
A FastWellTM (2.5 cm square, 1 mm thickness) is laid on a slide glass (2.6 cm × 7.6 cm), and a capillary and bacterial suspension are put in the chamber. The capillary is half-filled with methanol solidified with agar. The bacterial cells are allowed to swim into the capillary under microscopic observation.
Characterization of the isolates
Bacterial colonies obtained as above on MM were isolated through successive streaking onto MM containing 0.5% methanol. The isolates were subjected to whole-cell protein profiling, with a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Ultraflex, Bruker Daltonics, Bremen, Germany) [Citation23]. The colony of bacteria was smeared directly onto the MALDI target and 2 µl matrix solution (a saturated solution of sinapinic acid in 50% acetonitrile and 2.5% trifluoroacetic acid) was added. The mass spectrum data were analyzed to make a dendrogram based on their similarity using BioTyper software (Bruker Daltonics) with a default setting [Citation24].
The representatives in the WC-MS dendrogram were selected for 16S rRNA gene sequencing. A portion (ca. 1.4 kbp) of the gene was polymerase chain reaction (PCR)-amplified with Eu8f and Eu1492r primers and sequenced [Citation26]. Phylogenetic identification of the isolates was done at EZtaxon [Citation27]. The motility and chemotaxis of the isolates was checked qualitatively in the same manner as above, using capillaries filled with 2% methanol and cells grown overnight on solid MM containing 0.5% methanol. Their growth on 0.5% methanol in 100 µL MM in a 96-well plate was monitored for 14 days by measuring OD600 using a microplate reader. Isolates that showed more than OD600 > 0.4 and OD600 > 0.1 in 14 days were regarded as positive and weakly positive for methylotrophy, respectively.
Nucleotide sequence accession numbers
The DDBJ accession numbers for the 16S rRNA gene sequences reported in this study are LC191518-LC191558.
Results
Chemotaxis fishing using a model strain
Chemotaxis fishing trials were performed using M. aquaticum strain 22A as a model. The cells in overnight or two-day culture on solid MM containing 0.5% methanol were motile but further cultivation resulted in weaker motility. The result of chemotaxis fishing using strain 22A is summarized in . In this experiment, we used a cell suspension containing 1.3 × 106 cells/300 µL. Successful manufacturing of the glass tube trap was carried out as described above. Since it was difficult to control the volume of agarose in the capillary, we microscopically measured the diameter and length of the trap to calculate the trap volume, which differed in different trials. Given that the cells are not motile and not chemotactic, the “expected” number of cells in the trap as a result of simple diffusion, could be calculated. In the range of 25 to 30 min, we could observe that the cells actively moved into the trap (Supplementary movie 1). The fished cells were subjected to CFU determination. We could thus calculate how much the cells could be enriched against the expectation (fished cells per expected cells in the trap) and how many of the cells from the fishing suspension could be fished. Through nine trials, on average, the number of expected cells in the trap was 195 and the number of fished cells was around 6900, thus showing a 46-fold enrichment in cells. On average, less than 1% of cells were fished from the suspension. This primary experiment prompted us to apply the method for environmental samples.
Table 1. Chemotaxis fishing using M. aquaticum strain 22A as a model with methanol.
Chemotaxis fishing method
When we first attempted to isolate chemotactic bacteria directly from the environment, we found that most of the collected bacteria were not motile under microscopic observation. Incubation with either 0.1% methanol or an Arabidopsis seedling overnight significantly activated their motility. It remains unknown what substance exuded from the plant was effective at inducing the motility. Since the treatment increased the cell population, we measured CFU before and after the treatment.
Chemotaxis fishing from environmental samples
presents the chemotaxis fishing results for bacteria from Plantago verginica and Conyza banariensis (Supplementary movie 2). The fished cells were spread on MM plates containing 0.5% methanol and R2A plates to count methylotrophs and heterotrophs, respectively. We define here “methylotrophs” as microorganisms that grow on methanol and “heterotrophs” as those grow on R2A; but “heterotrophs” may contain heterotrophic methylotrophs (facultative methylotrophs). R2A contains glucose, pyruvate, starch, peptone, and yeast extract that serve as carbon and energy sources. When methanol was used for induction for the cells collected from Plantago verginica (trial 1), fishing enrichment of heterotrophs was 11- and 40-fold whereas that for methylotrophs was 17- and 26-fold. Although fishing worked to concentrate the bacteria, the ratio of methylotrophs to heterotrophs did not increase. The suspension was occupied by heterotrophic methylotrophs, judged from CFUs in the fishing suspension, due to the use of methanol for induction. When an A. thaliana seedling was used for induction (trial 2), the fishing suspension was occupied by heterotrophic bacteria, and the fishing resulted in high enrichment for methylotrophs (180- and 330-fold) whereas those for heterotrophs was 3.5- and 4.3-fold. In addition, the fished cells from the suspension were 5.4% and 9.4%, suggesting a high recovery of methylotrophs. Next, we used another plant, C. banariensis, and used an A. thaliana seedling for induction (trial 3). The fishing suspension contained comparable numbers of heterotrophs and methylotrophs and the fishing resulted in comparable enrichment in both. This result suggests that even when the seedling was used for induction, the overnight induction resulted in a different composition of heterotrophic and methylotrophic bacteria ratio, probably due to the different microbial composition of the different plants. Overall, these results suggested that depending on the original bacterial composition of the samples and induction methods, fishing may result in different enrichment efficiencies for methylotrophic bacteria and that even so the fishing was effective for enrichment.
Table 2. Chemotaxis fishing using plant leaf washes with methanol.
Next, we used paddy water samples, the results of which are summarized in . We used seedlings (trials 1–3) and methanol (trials 4–6) for induction. Regardless of the manner of induction, induction resulted in a 1,000- to 10,000-fold increase in CFU and comparable cell numbers for heterotrophic and methylotrophic bacteria. Depending on the trials, enrichment resulted in low values (1- to 2-fold for seedling induction and 3.8- to 10-fold for methanol induction, on average). Since we could observe the cells trapped for all samples (Supplementary movie 3), these low efficiencies might be caused by bacterial adhesion to glass or agarose in the tube-crushing process. It was also possible that the chemotactic cells cannot form colonies when spread on the plate media.
Table 3. Chemotaxis fishing using paddy water samples with methanol and inducted with A. thaliana seedlings and methanol.
Characterization of the isolates
The colonies of different morphotypes formed on MM containing methanol from chemotaxis fishing were isolated by several passages on the same media. Seventy-five bacterial strains (53 from paddy water, 19 from P. verginica, and 3 from C. banariensis) were isolated. The isolates were subjected to WC-MS analysis directly using bacterial cells as samples that can discriminate bacteria at the species level by clustering the mass spectrum data. We selected representative one or several isolates from groups showing similar morphotypes and close dendrogram positions. The identification and characterization of these bacterial strains are summarized in .
Figure 2. Identification and characterization of the isolates, associated with a MALDI dendrogram based on whole-cell protein profiles of bacterial isolates.
Results of identification, growth on methanol, motility, chemotaxis toward methanol, and sources of isolation are summarized. Abbreviations: +, positive; -, negative; W, weak. Pw, paddy water; Pv, P. virginica; and Cb, C. bonariensis. Blanks are not tested.

Identification resulted in 32 species in 18 genera. Many isolates (33 isolates) belonged to Acinetobacter species (A. baumannii, A. brisouli, A. oleivorans, A. oryzae, A. soli, and A. tandoii). They were all from paddy water samples, except strain 45w from P. verginica. The genera are ubiquitous in nature, including soil, plants, water, and animals [Citation28,Citation29]. Several species among them are known to be methylotrophic [Citation30–Citation32], and there are many reports on their association with plants [Citation33–Citation39], although the genus is generally known as non-motile [Citation33,Citation40,Citation41]. Many of the isolates could grow weakly on methanol in liquid medium, but almost all were non-motile and therefore not chemotactic to methanol. The frequent isolation of the genera was thus considered not to be due to their chemotaxis, but to their high abundance in the activated samples or to their strong adhesion to substrates leading to biofilm formation [Citation42].
The second most frequently isolated genera were Methylobacterium (M. oryzae, M. adhaesivum and M. brachiatum). The genus is well known to be methylotrophic and motile. Indeed, the M. oryzae isolates could grow well on methanol and were chemotactic to methanol. The isolates of M. adhaesivum and M. brachiatum were motile but not chemotactic. These results suggested that the chemotaxis to methanol is not conserved across the species in the genera, even if they can grow on methanol. One of the reasons for non-chemotactic isolates in fishing would be their abundance in the activated samples.
The third most frequent genera were Pseudomonas (P. nitroreducens, P. oryzihabitans, and P. putida). The genera are generally regarded to be motile and to contain some methylotrophic species [Citation43–Citation46]. In this study, the P. nitroreducens and P. oryzihabitans isolates were motile and chemotactic to methanol, but they could not grow on methanol. Bacterial chemotaxis to a substance is usually associated with growth capability on it, but this was not the case. Since they are members of the plant-associated Pseudomonas species [Citation47–Citation50], their chemotaxis to methanol might be advantageous in that it enables them to reach plants for their colonization. Future research is needed to determine what kind of chemotaxis sensor is present in the species.
As other fished species, we isolated strains belonging to Curvibacter gracilis, Delfitia lacustris and Herbaspirillum seropedicae. Interestingly, they did not grow on methanol. Although C. gracilis is known to be non-motile [Citation51], our result suggested that C. gracilis was motile. D. lacustris is known to be motile and capable of degradation of peptidoglycan [Citation52] and this study revealed its chemotaxis to methanol. The genomes of different species of the genus, D. acidovorans SPH-1 (Accession: CP000884.1 [Citation53],) and Delftia sp. Cs1-4 (Accession: NC_015563.1 [Citation54],), encode methanol dehydrogenase genes that are essential for methylotrophy in gram-negative bacteria. Thus, it is possible that the D. lacustris isolate is methylotrophic, and the growth condition was not suited for the isolate. H. seropedicae is a nitrogen-fixing plant-associated bacterium [Citation55] and the genome of H. seropedicae SmR1 (Accession: CP002039.1 [Citation56],) does not encode methanol dehydrogenase. In our study the isolate was found to be chemotactic but not methylotrophic.
Discussion
The aim of this study was to reveal the diversity of bacterial cells with natural chemotaxis towards methanol using a capillary as a trap for the gathering cells (chemotaxis fishing). As practical examples, we used A. thaliana seedlings and methanol for taxis induction, and plants or paddy water samples as sources for chemotactic bacteria. We found that environmental bacteria were not motile when collected and did not show any chemotaxis to methanol. This might be due to the low endogenous energy in the cells that is necessary for their motility [Citation57] or the temporal loss of flagella that are not necessary when they are attaching to substrates [Citation58]. We found that induction using methanol or A. thaliana seedlings that emit methanol was effective at inducing their motility and chemotaxis. It has been reported that various compounds are contained in plant root exudates [Citation59], many of which can be nutrient sources for microorganisms. Sugars, amino acids and organic acids are superior growth substances for microorganisms and are also recognized in chemotaxis [Citation60].
We found that many of the fished bacteria did not grow well on methanol in liquid media, which we have previously reported [Citation23]. One possible explanation is that the solid medium contains some impurities that help bacterial growth, especially in agar. When this takes place, it leads to the overestimation of methylotrophic bacteria. The use of a purer solidifying material such as agarose or gellan gum could solve this problem. Another explanation is that they utilize gaseous substances in the air as nutrient sources. These gaseous substances could include carbon monoxide, hydrogen, and aldehydes, all of which are present in laboratories, and carbon dioxide as a carbon source. This is known to occur in Rhodococcus species [Citation61], which exhibit very oligotrophic growth and are dependent on gaseous substances in the air.
Some isolates exhibited chemotaxis to methanol but no growth on methanol in liquid culture (e.g., C. gracilis, D. lacustris, H. seropedicae, P. oryzihabitans and P. nitroreducens). This finding is intriguing since most chemotaxis substrates are considered preferable substrates like growth substances for bacteria. These bacteria may be recognizing methanol as a chemotaxis substance to find plants to inhabit. It would be interesting to investigate whether the MCPs are conserved in the methanol-chemotactic bacteria of methylotrophs and non-methylotrophs, or if they would not be able to grow on methanol in the liquid medium as described above.
It is understandable that Methylobacterium species are one of the most frequently isolated bacteria in this study, since they are major plant-associated methylotrophic bacteria. Methylobacterium genomes contain as many as 40 to 50 MCP genes, probably because they have different habitats (both plant and water systems, dust, soil, and other habitats) [Citation62,Citation63], and they need to adapt to many different environments. We are currently attempting to identify the MCP for methanol in strain 22A.
As noted in the introduction, the identification of ligands for MCPs is not easy. Although MCPs conserve some structurally common features, the ligands recognized by them are not known at all, except in a few cases like those in E. coli and Pseudomonas [Citation64]. The fishing method established here will help identify MCPs for screening of the gene library. Our method is still primitive and needs further improvement. For example, the conditions for motility induction (e.g., methanol concentration, time of incubation, and temperature) and the manufacturing of the capillary (uniform size and length, volume of agarose, and the method to fill it with buffer) are to be optimized and improved. A technique to limit the non-specific adhesion of bacterial cells is also needed. The cells gathering around the capillary could also be directly isolated using a sophisticated micromanipulator system, which we did not utilize in this study.
Author contribution
YHT and YF carried out major experiments. YHT and AT designed the research, and wrote the manuscript.
Movie_3.mov
Download QuickTime Video (405 KB)Movie2.mov
Download QuickTime Video (14.4 MB)movie_1.mov
Download QuickTime Video (351.8 KB)Acknowledgments
We gratefully acknowledge JSPS-AASPP for the fund support. Our thanks also go to Dr. J. Yamashita (IPSR) for plant identification and Faith Obange (icipe) for English grammar revision.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Engelmann TW. Bacterium photometricum Pflugers. Arch Eur J Physiol. 1883;30(1):95–124.
- Falke JJ, Hazelbauer GL. Transmembrane signaling in bacterial chemoreceptors. Tr Biochem Sci. 2001;26:257–265.
- Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: high-performance signaling in networked arrays. Tr Biochem Sci. 2008;33(1):9–19.
- Sourjik V, Armitage JP. Spatial organization in bacterial chemotaxis. Embo J. 2010;29(16):2724–2733.
- Parkinson JS, Ames P, Studdert CA. Collaborative signaling by bacterial chemoreceptors. Curr Opin Microbiol. 2005;8(2):116–121.
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69(1):183–215.
- Li M, Hazelbauer GL. Core unit of chemotaxis signaling complexes. Proc Natl Acad Sci. 2011;108(23):9390–9395.
- Antommattei FM, Munzner JB, Weis RM. Ligand-specific activation of Escherichia coli chemoreceptor trans-methylation. J Bacteriol. 2004;186(22):7556–7563.
- Laca A, Sáenz MC, Paredes B, et al. Rheological properties, stability and sensory evaluation of low-cholesterol mayonnaises prepared using egg yolk granules as emulsifying agent. J Food Eng. 2010;97(2):243–252.
- McKellar JL, Minnell JJ, Gerth ML. A high‐throughput screen for ligand binding reveals the specificities of three amino acid chemoreceptors from Pseudomonas syringae pv. actinidiae. Mol Microbiol. 2015;96(4):694–707.
- Scharf BE, Hynes MF, Alexandre GM. Chemotaxis signaling systems in model beneficial plant–bacteria associations. Plant Mol Biol. 2016;90(6):549–559.
- García V, Reyes-Darias JA, Martín-Mora D, et al. Identification of a Chemoreceptor for C2 and C3 Carboxylic Acids. Appl Environ Microbiol. 2015;81(16):5449–5457.
- Parales RE, Luu RA, Chen GY, et al. Pseudomonas putida F1 has multiple chemoreceptors with overlapping specificity for organic acids. Microbiology. 2013;159(6):1086–1096.
- Hou S, Belisle C, Lam S, et al. Globin-coupled oxygen sensor from the facultatively alkaliphilic Bacillus halodurans C-125. Extremophiles. 2001;5(5):351–354.
- Fall R, Benson AA. Leaf methanol the simplest natural product from plants. Trends Plant Sci. 1996;1(9):296–301.
- Harholt J, Suttangkakul A, Scheller HV. Biosynthesis of pectin. Plant Physiol. 2010;153(2):384–395.
- Delmotte N, Knief C, Chaffron S, et al. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci. 2009;106(38):16428–16433.
- Kutschera U. Plant-associated methylobacteria as co-evolved phytosymbionts: a hypothesis. Plant Signal Behav. 2007;2(2):74–78.
- Schauer S, Kutschera U. Methylotrophic bacteria on the surfaces of field-grown sunflower plants: a biogeographic perspective. Theory Biosci. 2008;127(1):23–29.
- Dourado MN, Bogas AC, Pomini AM, et al. Methylobacterium-plant interaction genes regulated by plant exudate and quorum sensing molecules. Braz J Microbiol. 2013;44(4):1331–1339.
- Madhaiyan M, Poonguzhali S, Lee HS, et al. Pink-pigmented facultative methylotrophic bacteria accelerate germination, growth and yield of sugarcane clone Co86032 (Saccharum officinarum L.). Biol Fertil Soils. 2005;41(5):350–358.
- Tani A, Sahin N, Matsuyama Y, et al. Methylobacterium oxalidis sp. nov., isolated from leaves of Oxalis corniculata. Int J Syst Evol Microbiol. 2012;62:1647–1652.
- Tani A, Takai Y, Suzukawa I, et al. Practical application of methanol-mediated mutualistic symbiosis between Methylobacterium species and a roof greening moss, Racomitrium japonicum. PloS ONE. 2012;7(3):e33800–e33800.
- Tani A, Ogura Y, Hayashi T, et al. Complete genome sequence of Methylobacterium aquaticum strain 22A, isolated from Racomitrium japonicum moss. Genome Announc. 2015;3(2):e00266–15.
- Alamgir KM, Masuda S, Fujitani Y, et al. Production of ergothioneine by Methylobacterium species. Front Microbiol. 2015;6:1185.
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester: John Wiley and Sons; 1991. p. 115–175.
- Chun J, Lee JH, Jung Y, et al. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57(10):2259–2261.
- Al Atrouni A, Joly-Guillou ML, Hamze M, et al. Reservoirs of non-baumannii Acinetobacter species. Front Microbiol. 2016;7:49.
- Nemec A, Musílek M, Šedo O, et al. Acinetobacter bereziniae sp. nov. and Acinetobacter guillouiae sp. nov., to accommodate Acinetobacter genomic species 10 and 11, respectively. Int J Syst Evol Microbiol. 2010;60(4):896–903.
- Duine JA, Van Zeeland JK. Glucose dehydrogenase from Acinetobacter calcoaceticus. FEBS Lett. 1979;108(2):443–446.
- Duine JA, Jzn JF. Quinoprotein alcohol dehydrogenase from a non-methylotroph, Acinetobacter calcoaceticus. Microbiology. 1981;122(2):201–209.
- Ghosh NN, Kiskan B, Yagci Y. Polybenzoxazines—new high-performance thermosetting resins: synthesis and properties. Prog Polym Sci. 2007;32(11):1344–1391.
- Álvarez-Pérez S, Lievens B, Jacquemyn H, et al. Acinetobacter nectaris sp. nov. and Acinetobacter boissieri sp. nov., isolated from floral nectar of wild Mediterranean insect-pollinated plants. Int J Syst Evol Microbiol. 2013;63(4):1532–1539.
- Indiragandhi P, Anandham R, Madhaiyan M, et al. Characterization of plant growth–promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: plutellidae). Curr Microbiol. 2008;56(4):327–333.
- Kang SM, Joo GJ, Hamayun M, et al. Gibberellin production and phosphate solubilization by newly isolated strain of Acinetobacter calcoaceticus and its effect on plant growth. Biotechnol Lett. 2009;31(2):277–281.
- Kuklinsky‐Sobral J, Araújo WL, Mendes R, et al. Isolation and characterization of soybean‐associated bacteria and their potential for plant growth promotion. Environ Microbiol. 2004;6(12):1244–1251.
- Peix A, Lang E, Verbarg S, et al. Acinetobacter strains IH9 and OCI1, two rhizospheric phosphate solubilizing isolates able to promote plant growth, constitute a new genomovar of Acinetobacter calcoaceticus. Syst Appl Microbiol. 2009;32(5):334–341.
- Rokhbakhsh-Zamin F, Sachdev D, Kazemi-Pour N, et al. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol. 2011;21(6):556–566.
- Sachdev D, Nema P, Dhakephalkar P, et al. Assessment of 16S rRNA gene-based phylogenetic diversity and promising plant growth-promoting traits of Acinetobacter community from the rhizosphere of wheat. Microbiol Res. 2010;165(8):627–638.
- Nemec A, Musilek M, Maixnerova M, et al. Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int J Syst Evol Microbiol. 2009;59(1):118–124.
- Vaz-Moreira I, Novo A, Hantsis-Zacharov E, et al. Acinetobacter rudis sp. nov., isolated from raw milk and raw wastewater. Int J Syst Evol Microbiol. 2011;61(12):2837–2843.
- Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009;4(3):273–278.
- Green PN, Gillis M. Classification of Pseudomonas aminovorans and some related methylated amine utilizing bacteria. Microbiology. 1989;135(7):2071–2076.
- Jenkins O, Jones D. Taxonomic studies on some gram-negative methylotrophic bacteria. Microbiology. 1987;133(2):453–473.
- Pacheco CC, Passos JF, Moradas-Ferreira P, et al. Strain PM2, a novel methylotrophic fluorescent Pseudomonas sp. FEMS Microbiol Lett. 2003;227(2):279–285.
- Wallace PL, Hollis DG, Weaver RE, et al. Biochemical and chemical characterization of pink-pigmented oxidative bacteria. J Clin Microbiol. 1990;28(4):689–693.
- Elasri M, Delorme S, Lemanceau P, et al. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl Environ Microbiol. 2001;67(3):1198–1209.
- Loper JE, Hassan KA, Mavrodi DV, et al. Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 2012;8(7):e1002784.
- Raaijmakers JM, de Bruijn I, de Kock MJ. Cyclic lipopeptide production by plant-associated Pseudomonas spp.: diversity, activity, biosynthesis, and regulation. Mol Plant Microbe Interact. 2006;19(7):699–710.
- Rosenberg C, Casse-Delbart F, Dusha I, et al. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982;150(1):402–406.
- Ding L, Yokota A. Curvibacter fontana sp. nov., a microaerobic bacteria isolated from well water. J Gen Appl Microbiol. 2010;56(3):267–271.
- Jørgensen NO, Brandt KK, Nybroe O, et al. Delftia lacustris sp. nov., a peptidoglycan-degrading bacterium from fresh water, and emended description of Delftia tsuruhatensis as a peptidoglycan-degrading bacterium. Int J Syst Evol Microbiol. 2009;59(9):2195–2199.
- Schleheck D, Knepper TP, Fischer K, et al. Mineralization of individual congeners of linear alkylbenzenesulfonate by defined pairs of heterotrophic bacteria. Appl Environ Microbiol. 2004;70(7):4053–4063.
- Shetty AR, de Gannes V, Obi CC, et al. Complete genome sequence of the phenanthrene-degrading soil bacterium Delftia acidovorans Cs1-4. Stand Genomic. Sci. 2015;10(1):1.
- James EK, Gyaneshwar P, Mathan N, et al. Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant Microbe Interact. 2002;15(9):894–906.
- Pedrosa FO, Monteiro RA, Wassem R, et al. Genome of Herbaspirillum seropedicae strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLoS Genet. 2011;7(5):e1002064.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74(1):77–91.
- Adler J, Templeton B. The effect of environmental conditions on the motility of Escherichia coli. Microbiology. 1967;46(2):175–184.
- Chaparro JM, Badri DV, Bakker MG, et al. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE. 2013;8(2):e55731.
- Nihorimbere V, Ongena M, Smargiassi M, et al. Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotech Agro Soci Environ. 2011;15(2):327.
- Yoshida N, Inaba S, Takagi H. Utilization of atmospheric ammonia by an extremely oligotrophic bacterium, Rhodococcus erythropolis N9T-4. J Biosci Bioeng. 2014;117(1):28–32.
- Gallego V, García MT, Ventosa A. Methylobacterium variabile sp. nov., a methylotrophic bacterium isolated from an aquatic environment. Int J Syst Evol Microbiol. 2005;55(4):1429–1433.
- Hiraishi A, Furuhata K, Matsumoto A, et al. Phenotypic and genetic diversity of chlorine resistant Methylobacterium strains isolated from various environments. Appl Environ Microbiol. 1995;61(6):2099–2107.
- Kort EN, Goy MF, Larsen SH, et al. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Nat Acad Sci. 1975;72:3939–3943.
