ABSTRACT
Plasmacytoid dendritic cells (pDCs) are crucial in anti-viral immunity, acting as regulators in both adaptive and innate immunity. In this study, brief heat stress caused a decrease in splenic pDC activity in mice. Administration of Lactococcus lactis strain Plasma (LC-Plasma) significantly suppressed the decrease in pDC activity and IFN-α production.
Abbreviations: LC-Plasma: Lactococcus lactis strain Plasma; LAB: lactic acid bacteria; pDC: plasmacytoid dendritic cell; IFN: interferons; mDC: myeloid dendritic cells
Plasmacytoid dendritic cells (pDCs) are a crucial subset of cells in anti-viral immunity that act as proficient type I IFN producing cells [Citation1,Citation2]. pDC and pDC-derived type I IFNs act as regulators in both adaptive and innate immunity by controlling various immune factors, such as T cells [Citation3–Citation6], B cells [Citation7,Citation8], and NK cells [Citation9]. We previously discovered invaluable lactic acid bacteria (LAB): Lactococcus lactis strain Plasma (LC-Plasma, a synonym of Lactococcus lactis subsp. lactis JCM 5805), which stimulate murine pDCs in vitro and in vivo [Citation10]. Oral administration of LC-Plasma prevented parainfluenza virus and rotavirus infections in mice [Citation11,Citation12]. LC-Plasma also stimulated human pDCs in vitro and in vivo [Citation13]. Furthermore, oral administration of LC-Plasma suppressed the symptoms of an influenza-like illness in humans [Citation14]. The risk of seasonal influenza and the common cold are higher during the cold season, whereas the threat of recent emerging viruses, such as a novel influenza virus, dengue virus, and MERS coronavirus, can be spread during hot seasons or in hot regions. Thermal stress, such as extreme heat stress or rapid temperature change, is one of the factors which increases the risk of viral infection. Jin et al. reported that chronic heat stress retarded DC maturation and increased the virulence of highly pathogenic avian influenza virus H5N1 in mice [Citation15]; however, the influence of heat stress on pDC was unknown. In addition, foods which can improve decline of DCs activity have not been reported. In this study, we investigated the influence of heat stress on pDC activity in mice and the effect of LC-Plasma administration.
In the first study, we focused on the influence of thermal stress. Nine-week-old female C57BL/6J mice were obtained from Charles River Japan (Kanagawa, Japan). Eight mice were acclimatized for 1 week with free access to water and a basic diet, AIN93G (Oriental Yeast, Tokyo, Japan), then divided into two groups of 4 mice based on average body weight. The temperature of both groups was maintained at 23 ± 2°C, and 37 ± 2°C, respectively. Lighting was set at a 12 h/12 h light/dark cycle in both groups. Mice were housed individually in a cage and fed AIN93G. Twenty-four hours later, mice were sacrificed and the spleen (SPN) and mesenteric lymph nodes (MLN) were excised. To analyze the status of DCs, low density cell fractions were prepared as described previously with some modifications [Citation16]. SPN and MLN were minced in Mg2+- and Ca2+-free HBSS and digested with 1 mg/mL collagenase (Sigma) and 0.2 mg/mL DNase I for 30 min at 37 °C. EDTA concentration was adjusted to 30 mM and samples were incubated for 10 min at room temperature. Tissue lysates were filtered through a 100 mm nylon cell strainer and layered onto 15% Histodenz (Sigma) in RPMI1640 containing 10% FCS, and centrifuged at 450 × g for 20 min without braking. Low-density cells at the interface were collected, stained for CD11c, CD11b, mPDCA-1, MHC class II and CD86, and analyzed using the FACS Canto II system for CD86 and MHC class II expression on pDCs and mDCs. pDCs were defined as CD11c+ CD11b− mPDCA-1+ and mDCs were defined as CD11c+ CD11b+ mPDCA-1−. Results were evaluated using the standard Student’s t test. Animal procedures and experiments were approved by the Laboratory Animal Care Committee of Central Laboratories for Key Technologies, Kirin Co., Ltd. (approval ID YO12-00019). This study was conducted and completed in 2012. The results showed that the expression levels of MHC class II and CD86 on pDCs in SPN from the heat stress group were significantly lower than the control group (P < 0.05) ()). Expression levels of MHC class II on pDCs in MLN were also significantly lower in the heat stress group compared with the control (P < 0.01) (Supplemental Figure 1a). Interestingly, there were no significant differences in mDC activity between groups (Supplemental Figure 2a, 3a). These results indicated that heat stress could suppress pDC activity, but not mDC activity. In addition, heat-shock proteins (HSP) is known as activators of the innate immune system under heat stress condition [Citation17], however, the transcription level of HSP70 of DCs in heat stress group has been significantly lower than control group in this study (Supplemental Figure 4). It suggested that the decline of DCs activity might not be suppressed by function of HSPs.
Figure 1. Administration of LC-Plasma inhibited the decrease in splenic pDC activity caused by heat stress. (a) Shown is the influence of heat stress on the expression of activation markers on pDCs from SPN. Both control and heat stress groups consisted of 4 mice. (b) Shown is the effect of the administration of LC-Plasma on the expression of activation markers on pDCs of SPN under heat stress conditions. Heat stress and heat stress + LC-Plasma groups consisted of 5 mice and 6 mice, respectively. The expression levels of MHC class II, CD86 and CD40 are shown as median fluorescence intensities (M.F.I.). Short lines represent the mean values. Statistical comparisons were performed using the Student’s t-test.
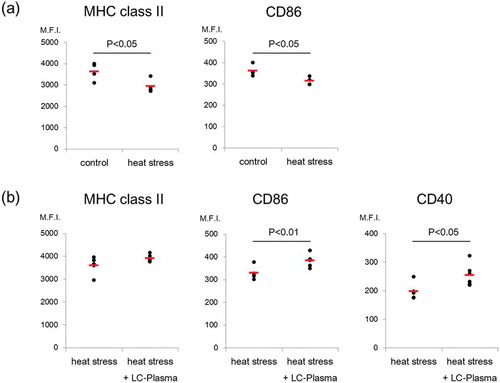
In the second study, we assessed whether LC-Plasma administration inhibited the reduction in pDC activity caused by heat stress. Twelve 5-week-old female C57BL/6J mice were acclimatized for 1 week with free access to water and AIN93G, before being divided into two groups: the heat stress group was fed AIN93G, and the heat stress + LC-Plasma group was fed AIN93G plus 1 mg/mouse/day of LC-Plasma for two weeks, at 23 ± 2°C. Both groups were then transferred to 37 ± 2°C conditions for 24 h before being sacrificed for SPN and MLN excision. FACS analysis was performed, as described in the first study, in addition, CD40 was evaluated. SPN cells were cultured at a density of 1 × 105 cells/0.5 mL RPMI medium in 48 well plates for 48 h at 37°C, with or without 1 μM of CpG-A (Invitrogen). One of the heat stress groups was excluded from the analysis due to an insufficient number of SPN cells. The concentration of IFN-α in culture supernatants was analyzed by ELISA (PBL Biomedical Laboratories). Animal procedures and experiments were approved by the Laboratory Animal Care Committee of Central Laboratories for Key Technologies, Kirin Co., Ltd. (approval ID YO12-00060). This study was conducted and completed in 2012. The expression levels of CD86 and CD40 on pDCs in SPN from the heat stress + LC-Plasma group were significantly higher when compared with the heat stress group (P < 0.01, P < 0.05) ()). There were no significant differences in pDC activity in MLN or mDC activities in both SPN and MLN between the two groups (Supplemental Figure 1b, 2b, 3b). In addition, we evaluated CpG stimulated IFN-α production in SPN cells: IFN-α production was significantly higher in the heat stress + LC-Plasma group compared with the heat stress group (P < 0.05) (Supplemental Figure 5). Our results suggest that LC-Plasma maintains IFN-α production in SPN by inhibiting the reduction in pDC activity.
In our previous clinical study, conducted during a hot season, pDC activity was maintained in the LC-Plasma group but significantly decreased in the placebo group after the 2 weeks intake period [Citation13]. It was suggested that heat stress had a negative influence on pDC activity; administration of LC-Plasma suppressed the negative influence. In this study, pDC activity in mice was significantly suppressed by heat stress for 24 h; LC-Plasma administration inhibited the reduction in pDC activity. The results from the mouse study support the previous clinical study. It was reported that heat stress reduced CD11c+ cell maturation in SPN [Citation15]; however, only pDC (CD11c+ CD11b− mPDCA-1+) activity was suppressed in this study: mDC (CD11c+ CD11b+ mPDCA-1−) activity was not suppressed. Any deterioration in the function of pDCs seriously affects the whole immune system because they are responsible for, not only innate immune functions such as phagocytosis and cytokine secretion in response to antigens, but also acquired immunity by means of antigen presentation and priming T cells and B cells [Citation18,Citation19]. Increased pDC activity was observed in SPN in particular in this study. We previously reported that 2 weeks administration of LC-Plasma stimulated pDC activity in MLN but not in SPN [Citation10]. These results suggest that 2 weeks administration of LC-Plasma does not activate pDC of SPN however it can suppress the decrease of pDC activity in SPN due to heat stress. We consider that LC-Plasma might have different effects depending on the environmental conditions. On the one hand, administration of LC-Plasma under normal condition may activate local immune cells, on the other hand, that under heat stressed-condition may inhibit the acute decline of systemic immunity system and contribute to maintain the overall immune homeostasis. Furthermore, we also reported that long-term administration for 82 weeks increased pDC activity in SPN but not in MLN [Citation20]. We suggest that stresses like heat and aging negatively influence systemic immunity systems, and administration of LC-Plasma may be able to improve it. IFN-α production was also increased in SPN cells in the presence of CpG. In our previous study, the transcription level of IFN genes and some interferon regulatory factor (IRF) genes were increased in pDCs activated by LC-Plasma in vitro [Citation13]. It suggests that pDC activation is ready condition against TLR ligands like CpG, and can immediately react to produce IFNs. pDC-derived type I IFNs can induce cellular antiviral responses to restrict viral replication and spread [Citation21], suggesting that administration of LC-Plasma could improve the reactivity to viral stimulation under stress conditions. In this study, just 24 h of heat stress had a negative influence. We also evaluated the influence of heat stress over 7 days: there was no significant difference between the control group and test group (data not shown). Our results suggested that continuous heat stress resulted in acclimatization to the environment, that is, abrupt temperature changes cause the suppression in pDC activity. The influence of cold stress on DCs is also crucial. Preliminary tests have shown that DC activities tend to partially increase in response to cold stress (data not shown). Cold stress promoted activation of macrophages, which secrete catecholamines to induce thermogenic gene expression in brown adipose tissue and lipolysis in white adipose tissue in order to sustain adaptive thermogenesis [Citation22]. DCs might also be activated for adaptation to cold environments. Further studies exploring the mechanisms underlying the relationship between cold stress and DC activities are required.
In conclusion, we have shown that brief heat stress causes a decrease in pDC activity, and administration of LC-Plasma for two weeks increased pDC activity under heat stressed-conditions. pDCs are an important immune cell subset which protect against viral infection; reductions in their activity can increase the risk of emerging viral infection in hot seasons and hot regions. We propose that intake of LC-Plasma is an effective solution to maintain pDC activity under heat stress conditions.
Author Contribution
Tetsu Sugimura and Kenta Jounai designed the study protocols, conducted the experiments and analyzed data. Tetsu Sugimura, Kenta Jounai and Konomi Ohshio conducted the experiments and analyzed data. Tetsu Sugimura and Daisuke Fujiwara wrote and reviewed the manuscript. All authors discussed the data and reviewed the manuscripts.
BBB-190217.R2_Supplemental_data_Sugimura.docx
Download MS Word (183.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978;147:1314–1333.
- Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837.
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436.
- Le Bon A, Etchart N, Rossmann C, et al. Crosspriming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015.
- Mescher MF, Curtsinger JM, Agarwal P, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92.
- Takagi H, Fukaya T, Eizumi K, et al. Plasmacytoiddendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971.
- Jego G, Palucka AK, Blanck JP, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234.
- Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064.
- Gerosa F, Gobbi A, Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734.
- Jounai K, Ikado K, Sugimura T, et al. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS One. 2012;7:e32588.
- Jounai K, Sugimura T, Ohshio K, et al. Oral administration of Lactococcus lactis subsp. lactis LC-Plasma enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLoS One. 2015;10:e0119055.
- Jounai K, Sugimura T, Morita Y, et al. Administration of Lactococcus lactis strain Plasma induces maturation of plasmacytoid dendritic cells and protection from rotavirus infection in suckling mice. Int Immunopharmacol. 2018;56:205–211.
- Sugimura T, Jounai K, Ohshio K, et al. Immunomodulatory effect of Lactococcus lactis LC-Plasma on human plasmacytoid dendritic cells. Clin Immunol. 2013;149:509–518.
- Sugimura T, Takahashi H, Jounai K, et al. Effects of oral intake of pDC-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br J Nutr. 2015;114:727–733.
- Jin Y, Hu Y, Han D, et al. Chronic heat stress weakened the innate immunity and increased the virulence of highly pathogenic avian influenza virus H5N1 in mice. J Biomed Biotechnol. 2011;367846:2011.
- Fujiwara D, Wei B, Presley LL, et al. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J Immunol. 2008;180:5843–5852.
- Wallin RP, Lundqvist A, Moré SH, et al. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135.
- Gigley JP, Khan IA. Plasmacytoid DC from aged mice down-regulate CD8 T cell responses by inhibiting cDC maturation after Encephalitozoon cuniculi infection. PLoS One. 2011;6:e20838.
- Gupta S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp Gerontol. 2014;54:47–52.
- Sugimura T, Jounai K, Ohshio K, et al. Long-term administration of pDC-Stimulative Lactococcus lactis strain decelerates senescence and prolongs the lifespan of mice. Int Immunopharmacol. 2018;58:166–172.
- Theofilopoulos AN, Baccala R, Beutler B, et al. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336.
- Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108.
